Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation
Abstract
1. Introduction
2. Materials and Methods
- A—control (seeds without coating),
- B—inoculated seeds: HiStick® Soy,
- C—coated seeds: chitosan + alginate/PEG
- D—B + C.
3. Results
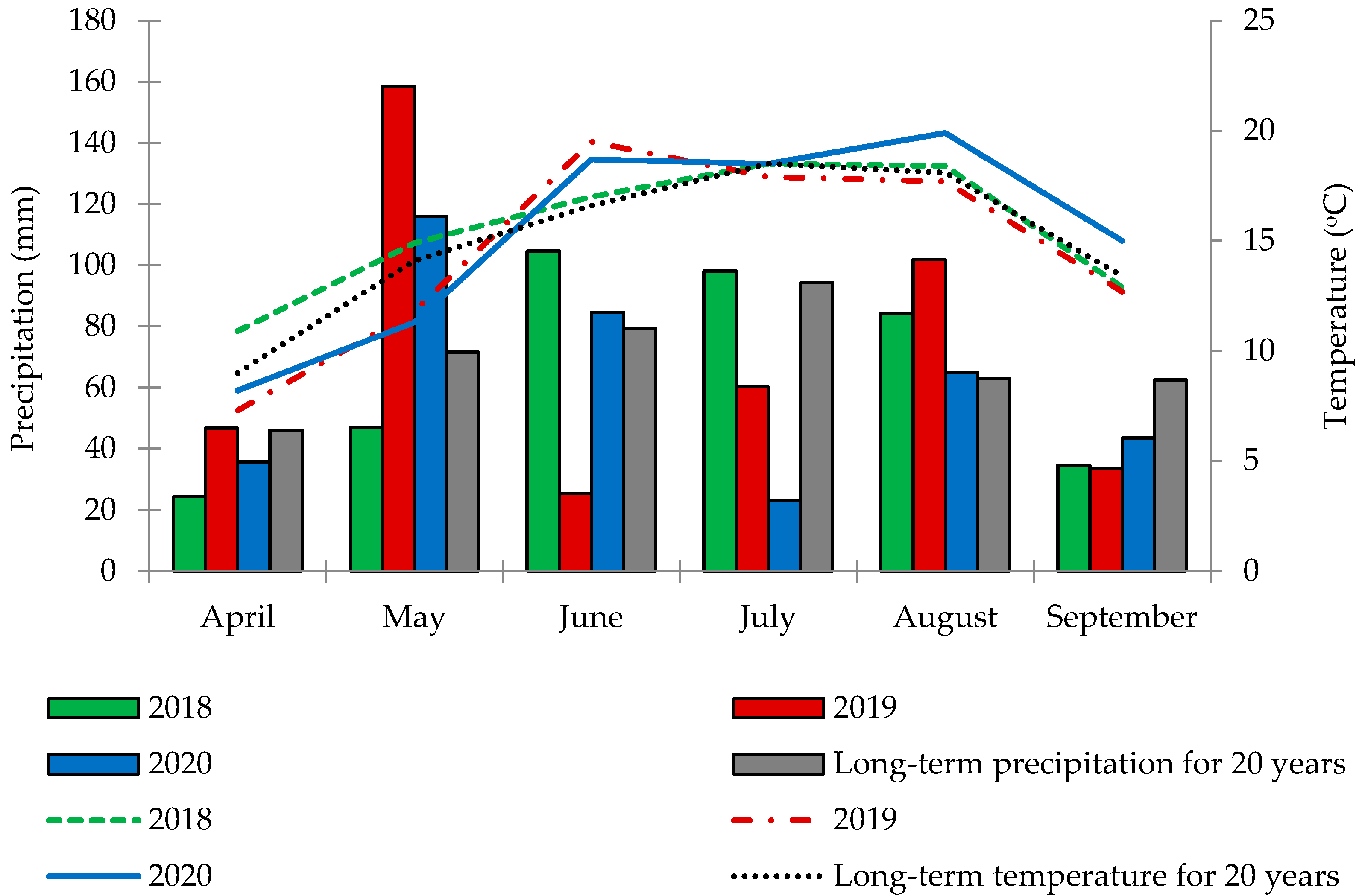
3.1. Weather Conditions
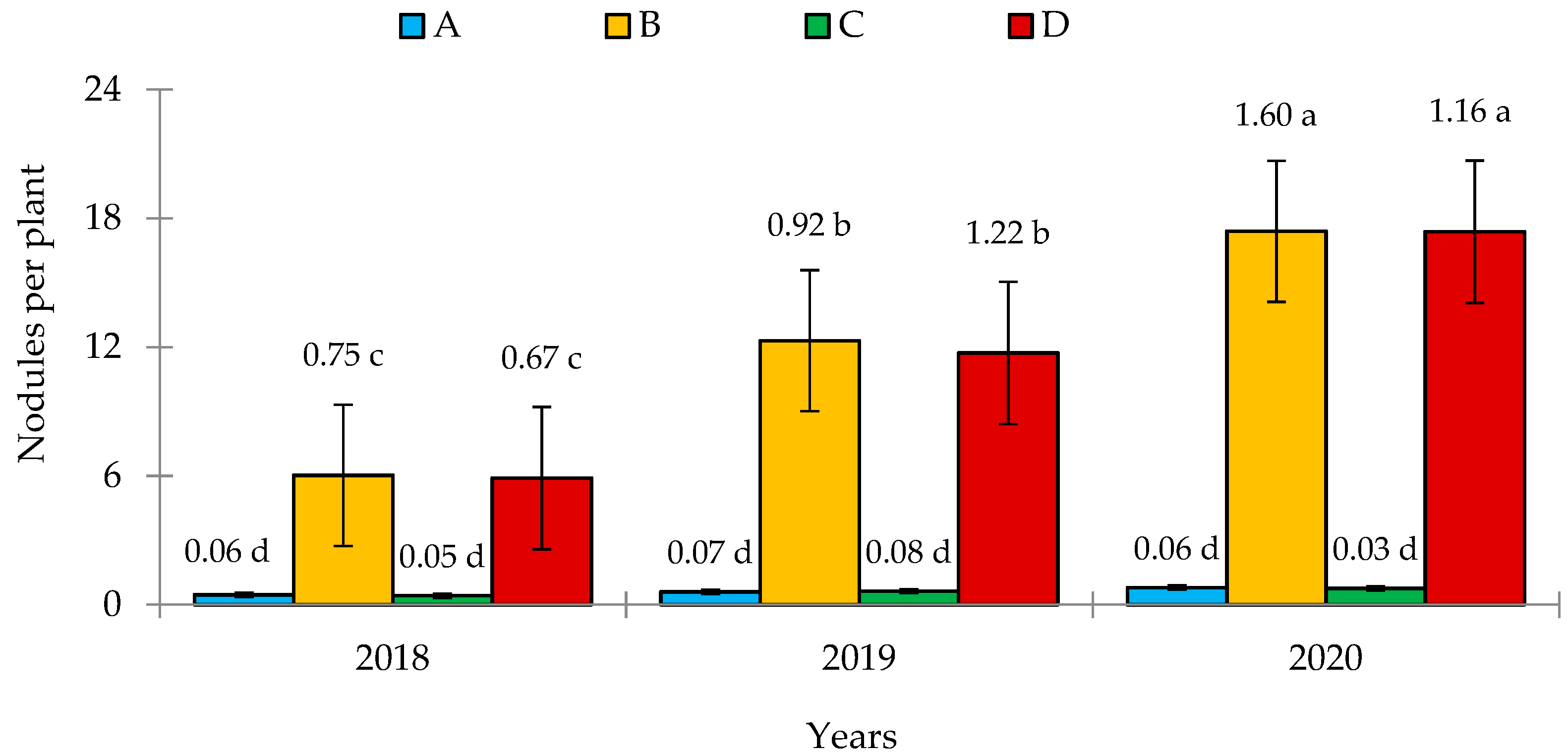
3.2. Field and Biometric Measurements
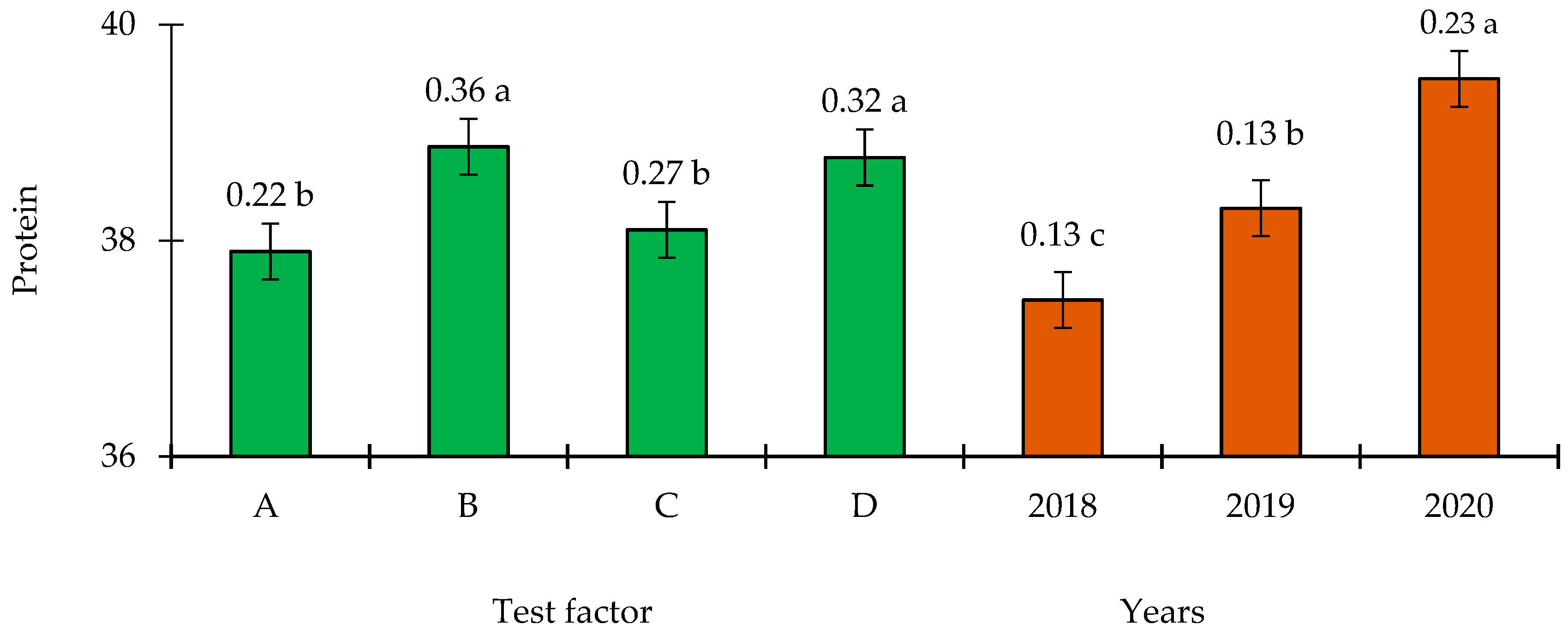
3.3. Protein Content
4. Discussion
4.1. Influence of Weather Conditions
4.2. Inoculation and Nodulation
4.3. Effect of Inoculation on Seed Quality
4.4. Effect of Seed Coating
4.5. Measurements of the Condition of the Plants
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Gs | leaf stomatal conductance |
| LAI | leaf area index |
| NDVI | normalized difference vegetation index |
| PEG | polyethylene glycol |
| SE | standard error |
| SPAD | soil plant analysis development |
| TSW | thousand-seed weight |
References
- Watson, C.A.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindström, K.; Nemecek, T.; Topp, C.F.E.; Vanhatalo, A.; et al. Chapter four—Grain legume production and use in European agricultural systems. Adv. Agron. 2017, 144, 235–303. [Google Scholar] [CrossRef]
- Zimmer, S.; Messmer, M.; Haase, T.; Piepho, H.P.; Mindermann, A.; Schulz, H.; Habekuß, A.; Ordon, F.; Wilbois, K.P.; Heß, J. Effects of soybean variety and Bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in Germany. Eur. J. Agron. 2016, 72, 38–46. [Google Scholar] [CrossRef]
- Bandara, A.Y.; Weerasooriya, D.K.; Bell, T.H.; Esker, P.D. Prospects of alleviating early planting-associated cold susceptibility of soybean using microbes: New insights from microbiome analysis. J. Agron. Crop Sci. 2021, 207, 171–185. [Google Scholar] [CrossRef]
- Madias, A.; Di Mauro, G.; Vitantonio-Mazzini, L.N.; Gambin, B.L.; Borrás, L. Environment quality, sowing date, and genotype determine soybean yields in the Argentinean Gran Chaco. Eur. J. Agron. 2021, 123, 126217. [Google Scholar] [CrossRef]
- Gwata, E.T.; Wofford, D.S.; Pfahler, P.L.; Boote, K.J. Genetics of promiscuous nodulation in soybean: Nodule dry weight and leaf color score. J. Hered. 2004, 95, 154–157. [Google Scholar] [CrossRef]
- Iturralde, E.T.; Covelli, J.M.; Álvarez, F.; Pérez-Giménez, J.; Arrese-Igor, C.; Lodeiro, A.R. Soybean-nodulating strains with low intrinsic competitiveness for nodulation, good symbiotic performance, and stress-tolerance isolated from soybean-cropped soils in Argentina. Front. Microbiol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Vargas-Díaz, A.A.; Ferrera-Cerrato, R.; Silva-Rojas, H.V.; Alarcón, A. Isolation and evaluation of endophytic bacteria from root nodules of Glycine max L. (Merr.) and their potential use as biofertilizers. Span. J. Agric. Res. 2019, 17, e1103. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Sahrawat, K.L.; Upadhyaya, H.D.; Mengoni, A.; Galardini, M.; Bazzicalupo, M.; Biondi, E.G.; Hungria, M.; Kaschuk, G.; Blair, M.W.; et al. Chapter one—Advances in host plant and Rhizobium genomics to enhance symbiotic nitrogen fixation in grain legumes. Adv. Agron. 2015, 129, 1–116. [Google Scholar] [CrossRef]
- Marinković, J.B.; Bjelić, D.Đ.; Tintor, B.B.; Ignjatov, M.V.; Nikolić, Z.T.; Đukić, V.H.; Balešević-Tubić, S.N. Molecular identification of Bradyrhizobium japonicum strains isolated from root nodules of soybean (Glycine max L.). Matica Srp. J. Nat. Sci. 2017, 132, 49–56. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol. Biochem. 2017, 105, 177–196. [Google Scholar] [CrossRef]
- Solomon, T.; Pant, L.M.; Angaw, T. Effects of inoculation by Bradyrhizobium japonicum strains on nodulation, nitrogen fixation, and yield of soybean (Glycine max L. Merill) varieties on Nitisols of Bako, western Ethiopia. Int. Sch. Res. Not. 2012, 2012, 261475. [Google Scholar] [CrossRef][Green Version]
- Wongphatcharachai, M.; Staley, C.; Wang, P.; Moncada, K.M.; Sheaffer, C.C.; Sadowsky, M.J. Predominant populations of indigenous soy-bean nodulating Bradyrhizobium japonicum strains obtained from organic farming systems in Minnesota. J. Appl. Microbiol. 2015, 118, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Kaschuk, G.; Saridakis, G.P.; Hungria, M. Genetic variability in Bradyrhizobium japonicum strains nodulating soybean [Glycine max (L.) Merrill]. World J. Microbiol. Biotechnol. 2012, 28, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Giongo, A.; Ambrosini, A.; Vargas, L.K.; Freire, J.R.J.; Bodanese-Zanettini, M.H.; Passaglia, L.M.P. Evaluation of genetic diversity of bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl. Soil Ecol. 2008, 38, 261–269. [Google Scholar] [CrossRef]
- Flajšman, M.; Šantavec, I.; Kolmanič, A.; Kocjan Ačko, D. Bacterial seed inoculation and row spacing affect the nutritional composition and agronomic performance of soybean. Int. J. Plant Prod. 2019, 13, 183–192. [Google Scholar] [CrossRef]
- Pannecoucque, J.; Goormachtigh, S.; Ceusters, J.; Debode, J.; Van Waes, C.; Van Waes, J. Temperature as a key factor for successful inoculation of soybean with Bradyrhizobium spp. under cool growing conditions in Belgium. J. Agric. Sci. 2018, 156, 493–503. [Google Scholar] [CrossRef]
- Jarecki, W.; Bobrecka-Jamro, D. Influence of seed inoculation with commercial bacterial inoculants (Bradyrhizobium japonicum) on growth and yield of soybean. Legume Res. 2019, 42, 688–693. [Google Scholar] [CrossRef]
- Althabegoiti, M.J.; López-García, S.L.; Piccinetti, C.; Mongiardini, E.J.; Perez-Gimenez, J.; Quelas, J.I.; Perticari, A.; Lodeiro, A.R. Strain selection for improvement of Bradyrhizobium japonicum competitiveness for nodulation of soybean. FEMS Microbiol. Lett. 2008, 282, 115–123. [Google Scholar] [CrossRef]
- Pereira, C.E.; de Souza Moreira, F.M.; Oliveira, J.A.; Caldeira, C.M. Compatibility among fungicide treatments on soybean seeds through film coating and inoculation with Bradyrhizobium strains. Acta Sci. Agron. 2010, 32, 585–589. [Google Scholar] [CrossRef]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume seed inoculation technology—A review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- Yamakawa, T.; Fukushima, Y. Low inoculum densities of Bradyrhizobium japonicum USDA 110 is effective on production of soybean (Glycine max L. Merr.) cultivar Fukuyutaka. J. Fac. Agric. Kyushu Univ. 2014, 59, 45–53. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Alternative methods of soybean inoculation to overcome adverse conditions at sowing. Afr. J. Agric. Res. 2015, 10, 2329–2338. [Google Scholar] [CrossRef][Green Version]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Kaschuk, G.; Nogueira, M.A.; de Luca, M.J.; Hungria, M. Response of determinate and indeterminate soybean cultivars to basal and topdressing N fertilization compared to sole inoculation with Bradyrhizobium. Field Crop. Res. 2016, 195, 21–27. [Google Scholar] [CrossRef]
- Prusiński, J.; Baturo-Cieśniewska, A.; Borowska, M. Response of soybean (Glycine max (L.) Merrill) to mineral nitrogen fertilization and Bradyrhizobium japonicum seed inoculation. Agronomy 2020, 10, 1300. [Google Scholar] [CrossRef]
- Leggett, M.; Diaz-Zorita, M.; Koivunen, M.; Bowman, R.; Pesek, R.; Stevenson, C.; Leister, T. Soybean response to inoculation with in the United States and Argentina. Agron. J. 2017, 109, 1031–1038. [Google Scholar] [CrossRef]
- Lacerda, M.P.; Umburanas, R.C.; Martins, K.V.; Rodrigues, M.A.T.; Reichardt, K.; Dourado-Neto, D. Vigor and oxidation reactions in soybean seedlings submitted to different seed chemical treatments. J. Seed Sci. 2021, 43, e202143012. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.; Wiśniewska-Wrona, M.; Kopania, E. The use of natural polymers for treatments enhancing sowing material. Polimery 2021, 66, 11–20. [Google Scholar] [CrossRef]
- Sarreta, Y.; de Castro Neto, J.C. Effects of 660 nm laser irradiation of soybean seeds on germination, emergence and seedling growth. Acta Agroph. 2021, 28, 5–18. [Google Scholar] [CrossRef]
- Hara, Y. Comparison of the effects of seed coating with tungsten and molybdenum compounds on seedling establishment rates of rice, wheat, barley, and soybean under flooded conditions. Plant Prod. Sci. 2017, 20, 406–411. [Google Scholar] [CrossRef][Green Version]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 104565. [Google Scholar] [CrossRef]
- Avelar, S.A.G.; Baudet, L.; de Oliveira, S.; Ludwig, M.P.; Crizel, R.L.; Rigo, G.A. Soybean seed treatment and coating with liquid and powdered polymer. Interciencia 2015, 40, 133–137. [Google Scholar]
- Ludwig, E.J.; Nunes, U.R.; Prestes, O.D.; Fagundes, L.K.; Fernandes, T.S.; Saibt, N. Polymer coating in soybean seed treatment and their relation to leaching of chemicals. Rev. Ambient. Água 2020, 15, e2602. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration ready. Restor. Ecol. 2020, 28, 266–275. [Google Scholar] [CrossRef]
- Poliserpi, M.B.; Cristos, D.S.; Brodeur, J.C. Imidacloprid seed coating poses a risk of acute toxicity to small farmland birds: A weight-of-evidence analysis using data from the grayish baywing Agelaioides badius. Sci. Total Environ. 2021, 763, 142957. [Google Scholar] [CrossRef]
- Lentola, A.; Giorio, C.; Petrucco Toffolo, E.; Girolami, V.; Tapparo, A. A new method to assess the acute toxicity toward honeybees of the abrasion particles generated from seeds coated with insecticides. Environ. Sci. Eur. 2020, 32, 93. [Google Scholar] [CrossRef]
- Lentola, A.; Giorio, C.; Bogialli, S.; Roverso, M.; Marzaro, M.; Girolami, V.; Tapparo, A. Methiocarb metabolites are systemically distributed throughout corn plants grown from coated seeds. Environ. Chem. Lett. 2021, 19, 1887–1892. [Google Scholar] [CrossRef]
- Han, R.; Wu, Z.; Huang, Z.; Man, X.; Teng, L.; Wang, T.; Liu, P.; Wang, W.; Zhao, X.; Hao, J.; et al. Tracking pesticide exposure to operating workers for risk assessment in seed coating with tebuconazole and carbofuran. Pest Manag. Sci. 2021, 77, 2820–2825. [Google Scholar] [CrossRef]
- Gesch, R.W.; Archer, D.W.; Spokas, K. Can using polymer-coated seed reduce the risk of poor soybean emergence in no-tillage soil? Field Crops Res. 2012, 125, 109–116. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.R.E.; Oliveira, J.A.; Botelho, F.J.E.; Oliveira, R.M.E.; Pereira, C.E. Performance of film coated soybean seeds in soil different water contents. Ciênc. Agrotecnologia 2007, 31, 994–999. [Google Scholar] [CrossRef]
- Zeng, D.-F.; Zhang, L. A novel environmentally friendly soybean seed-coating agent. Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 545–551. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Kuchlan, P.; Kuchlan, M.K.; Ansari, M.M. Efficient application of Trichoderma viride on soybean [Glycine max (L.) Merrill] seed using thin layer polymer coating. Legume Res. 2019, 42, 250–259. [Google Scholar] [CrossRef]
- Zerpa, M.; Mayz, J.; Mendez, J. Effects of Bradyrhizobium japonicum inoculants on soybean (Glycine max (L.) Merr.) growth and nodulation. Ann. Biol. Res. 2013, 4, 193–199. [Google Scholar]
- Adjetey, J.A.; Mbotho, K. Evaluation of Bradyrhizobium formulations on performance of soybean grown on soil without a long-term history of the crop. Bots. J. Agric. Appl. Sci. 2019, 13, 66–70. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; Wongphatcharachai, M.; Sheaffer, C.C.; Orf, J.C.; Sadowsky, M.J. Competition between introduced Bradyrhizobium japonicum strains and indigenous bradyrhizobia in Minnesota organic farming systems. Symbiosis 2017, 73, 155–163. [Google Scholar] [CrossRef]
- Coskan, A.; Dogan, K. Symbiotic nitrogen fixation in soybean. In Soybean Physiology and Biochemistry; El-Shemy, H., Ed.; InTech: London, UK, 2011; Volume 307, pp. 167–182. [Google Scholar] [CrossRef][Green Version]
- Jarecki, W.; Buczek, J.; Bobrecka-Jamro, D. Response of soybean (Glycine max (L.) Merr.) to bacterial soil inoculants and foliar fertilization. Plant Soil Environ. 2016, 62, 422–427. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Fontoura, S.M.V.; de Moraes, R.P.; Tamagno, S.; Ciampitti, I.A.; Bayer, C. Soybean yield response to Bradyrhizobium strains in fields with inoculation history in Southern Brazil. J. Plant Nutr. 2019, 42, 1941–1951. [Google Scholar] [CrossRef]
- Kühling, I.; Hüsing, B.; Bome, N.; Trautz, D. Soybeans in high latitudes: Effects of Bradyrhizobium inoculation in northwest Germany and southern west Siberia. Org. Agric. 2018, 8, 159–171. [Google Scholar] [CrossRef]
- Duzan, H.M.; Zhou, X.; Souleimanov, A.; Smith, D.L. Perception of Bradyrhizobium japonicum Nod factor by soybean [Glycine max (L.) Merr.] root hairs under abiotic stress conditions. J. Exp. Bot. 2004, 55, 2641–2646. [Google Scholar] [CrossRef]
- Suzuki, Y.; Adhikari, D.; Itoh, K.; Suyama, K. Effects of temperature on competition and relative dominance of Bradyrhizobium japonicum and Bradyrhizobium elkanii in the process of soybean nodulation. Plant Soil. 2014, 374, 915–924. [Google Scholar] [CrossRef]
- Albareda, M.; Rodriguea-Navarro, D.N.; Temprano, F.J. Soybean inoculation: Dose, N fertilizer supplementation and rhizobia persistence in soil. Field Crops Res. 2009, 113, 352–356. [Google Scholar] [CrossRef]
- Narożna, D.; Pudełko, K.; Króliczek, J.; Golińska, B.; Sugawara, M.; Mądrzak, C.J.; Sadowsky, M.J. Survival and competitiveness of Bradyrhizobium japonicum strains 20 years after introduction into field locations in Poland. Appl. Environ. Microbiol. 2015, 81, 5552–5559. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A. Symbiotic effectiveness of inoculation with Bradyrhizobium isolates on soybean [Glycine max (L.) Merrill] genotypes with different maturities. SpringerPlus 2014, 3, 753. [Google Scholar] [CrossRef]
- Căpățână, N.; Bolohan, C.; Marin, D.I. Research regarding the influence of mineral fertilization along with Bradyrhizobium japonicum on soybean grain yield (Glycine max (L.) Merrill), under the conditions of south-east Romania. Sci. Pap. Ser. A Agron. 2017, 60, 207–214. [Google Scholar]
- Thuita, M.; Pypers, P.; Herrmann, L.; Okalebo, R.J.; Othieno, C.; Muema, E.; Lesueur, D. Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol. Fertil. Soils 2012, 48, 87–96. [Google Scholar] [CrossRef]
- López-García, S.L.; Perticari, A.; Piccinetti, C.; Ventimiglia, L.; Arias, N.; De Battista, J.J.; Althabegoiti, M.J.; Mongiardini, E.J.; Pérez-Giménez, J.; Quelas, J.I.; et al. In-Furrow inoculation and selection for higher motility enhances the efficacy of Bradyrhizobium japonicum nodulation. Agron. J. 2009, 101, 357–363. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Rosso, L.H.M.; Secchi, M.A.; Torres, A.R.; Naeve, S.; Casteel, S.N.; Kovács, P.; Davidson, D.; Purcell, L.C.; Archontoulis, S.; et al. Soybean yield, biological N2 fixation and seed composition responses to additional inoculation in the United States. Sci. Rep. 2019, 9, 19908. [Google Scholar] [CrossRef]
- Ludwig, M.P.; Filho, O.A.L.; Baudet, L.; Dutra, L.M.C.; Avelar, S.A.G.; Crizel, R.L.; de Oliveira, S. Coating efficiency of soybean seeds in equipment with spray system. Ciência Rural 2011, 41, 557–563. [Google Scholar] [CrossRef]
- Averitt, B.J.; Welbaum, G.E.; Li, X.; Prenger, E.; Qin, J.; Zhang, B. Evaluating genotypes and seed treatments to increase field emergence of low phytic acid soybeans. Agriculture 2020, 10, 516. [Google Scholar] [CrossRef]
- Thuita, M.; Vanlauwe, B.; Mutegi, E.; Masso, C. Reducing spatial variability of soybean response to rhizobia inoculants in farms of variable soil fertility in Siaya County of western Kenya. Agric. Ecosyst. Environ. 2018, 261, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.J.; Alam, P.; Alyemeni, M.N.; Ahmad, P. Interactive effects of nutrients and Bradyrhizobium japonicum on the growth and root architecture of soybean (Glycine Max, L.). Front. Microbiol. 2018, 9, 1000. [Google Scholar] [CrossRef]
- Stecca, J.D.L.; Martin, T.N.; Dall’Coll Lúcio, A.; Deak, E.A.; Fipke, G.M.; Bruning, L.A. Inoculation of soybean seeds coated with osmoprotector in diferents soil pH’s. Acta Scientiarum. Agronomy 2019, 41, e39482. [Google Scholar] [CrossRef]
- Cafaro La Menza, N.; Monzon, J.P.; Specht, J.E.; Grassini, P. Is soybean yield limited by nitrogen supply? Field Crop. Res. 2017, 213, 204–212. [Google Scholar] [CrossRef]
- Wiatrak, P. Effect of polymer seed coating with micronutrients on soybeans in Southeastern Coastal Plains. Am. J. Agric. Biol. Sci. 2013, 8, 302–308. [Google Scholar] [CrossRef][Green Version]
- Rocha, I.D.S.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Fron. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Santos, V.M.; Oliveira, T.C.; Mendes, M.G.; Yamanaka, C.H.; Macedo, W.R. Soybean seed chemical treatment associated with inoculants: Physiological and agronomical analyses. Plant Physiol. Rep. 2021, 26, 247–255. [Google Scholar] [CrossRef]
- Chachalis, D.; Smith, M.L. Hydrophobic-polymer application reduces imbibition rate and partially improves germination of emergence of soybean seedlings. Seed Sci. Technol. 2001, 29, 91–98. [Google Scholar]
- Ambika, S.; Manonmani, V.; Bhaskaran, M.; Deepika, S. Influence of polymer coated KSL 441 (op) soybean seed on productivity under moisture stress conditions. Legume Res. 2017, 40, 150–154. [Google Scholar] [CrossRef]
- Sharratt, B.S.; Gesch, R.W. Emergence of polymer-coated corn and soybean influenced by tillage and sowing date. Agron. J. 2008, 100, 585–590. [Google Scholar] [CrossRef]
- Tripathi, B.; Pandey, A.; Bhatia, R.; Walia, S.; Yadav, A.K. Improving soybean seed performance with natural colorant-based novel seed-coats. J. Crop Improv. 2015, 29, 301–318. [Google Scholar] [CrossRef]
- Jeyabal, A.; Kuppuswamy, G.; Lakshmanan, A. Effect of seed coating on yield attributes and yield of soybean (Glycine max L.). J. Agron. Crop Sci. 1992, 169, 145–150. [Google Scholar] [CrossRef]
- Macák, M.; Candráková, E. The effect of fertilization on yield components and quality parameters of soybeans [(Glycine max (L.) Merr.)] seeds. J. Cent. Eur. Agric. 2013, 14, 1232–1242. [Google Scholar] [CrossRef]
- Jarecki, W.; Wietecha, J. Effect of seed coating on the yield of soybean Glycine max (L.) Merr. Plant Soil Environ. 2021, 67, 468–473. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schweitzer, L.E.; Nelson, R.L. Association of specific leaf weight, an estimate of chlorophyll, and chlorophyll concentration with apparent photosynthesis in soybean. Photosynth. Res. 1996, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, F.B.; Ray, J.D. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Vollmann, J.; Walter, H.; Sato, T.; Schweiger, P. Digital image analysis and chlorophyll metering for phenotyping the effects of nodulation in soybean. Comput. Electron. Agric. 2011, 75, 190–195. [Google Scholar] [CrossRef]
- Yu, M.; Ding, G.; Gao, G.; Zhao, Y.; Sai, K. Leaf temperature fluctuations of typical psammophytic plants and their application to stomatal conductance estimation. Forests 2018, 9, 313. [Google Scholar] [CrossRef]
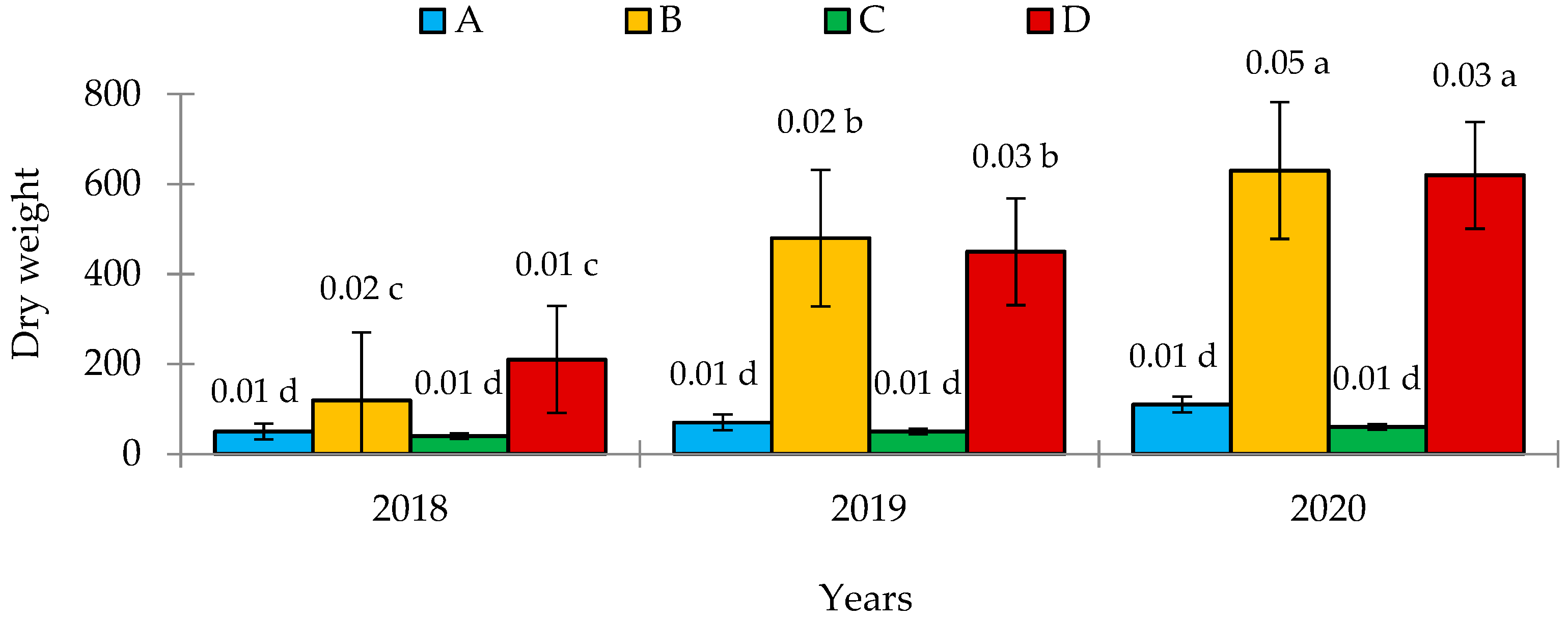
| Years | Decade of the Month (Days of the Month) | ||
|---|---|---|---|
| I (1–10) | II (11–20) | III (21–30) | |
| 2018 | 5.7 | 10.3 | 11.5 |
| 2019 | 6.0 | 6.1 | 10.3 |
| 2020 | 5.5 | 7.6 | 10.1 |
| Treatment (T) | Emergence (Days from the Date of Sowing) | Plant Population after Emergence (Plants·m−2) | Field Emergence (%) | Plant Population before Harvest (Plants·m−2) |
|---|---|---|---|---|
| Tested factor—TF | ||||
| A | 15.0 b | 47.5 b | 79.2 b | 43.4 b |
| B | 14.8 b | 47.3 b | 78.9 b | 42.9 b |
| C | 16.3 a | 50.8 a | 84.6 a | 46.4 a |
| D | 16.8 a | 50.0 a | 83.3 a | 45.7 a |
| Years—Y | ||||
| 2018 | 15.2 b | 54.6 a | 90.9 a | 49.9 a |
| 2019 | 16.6 a | 44.1 c | 73.5 c | 40.2 c |
| 2020 | 15.4 b | 48.0 b | 80.0 b | 43.7 b |
| ANOVA p value | ||||
| TF | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| Y | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| TFxY | n.s. | n.s. | n.s. | n.s. |
| Treatment (T) | SPAD | Gs (mmol m−2 s−1) | LAI |
|---|---|---|---|
| Tested factor—TF | |||
| A | 39.4 b | 367.7 ab | 3.29 a |
| B | 43.1 a | 371.5 a | 3.39 a |
| C | 39.4 b | 360.1 b | 3.30 a |
| D | 42.6 a | 363.1 ab | 3.39 a |
| Years—Y | |||
| 2018 | 42.9 a | 381.5 a | 3.56 a |
| 2019 | 40.0 b | 352.6 c | 3.06 c |
| 2020 | 40.4 b | 362.8 b | 3.41 b |
| ANOVA p value | |||
| TF | ≤0.001 | ≤0.01 | ≤0.05 |
| Y | ≤0.001 | ≤0.001 | ≤0.001 |
| TFxY | n.s. | n.s. | n.s. |
| Treatment (T) | Number of Pods per Plant | Number of Seeds in the Pod | TSW (g) | Seed Yield (t·ha−1) |
|---|---|---|---|---|
| Tested factor—TF | ||||
| A | 31.6 bc | 1.97 b | 138.9 b | 3.64 b |
| B | 34.2 a | 2.05 a | 145.0 a | 4.23 a |
| C | 30.1 c | 1.98 b | 140.0 b | 3.76 b |
| D | 33.0 ab | 2.02 ab | 146.2 a | 4.32 a |
| Years—Y | ||||
| 2018 | 27.7 c | 2.00 a | 149.3 a | 4.04 b |
| 2019 | 33.4 b | 2.02 a | 135.9 c | 3.61 c |
| 2020 | 35.6 a | 1.99 a | 142.3 b | 4.32 a |
| ANOVA p value | ||||
| TF | ≤0.001 | ≤0.01 | ≤0.01 | ≤0.001 |
| Y | ≤0.001 | n.s. | ≤0.001 | ≤0.001 |
| TFxY | n.s. | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarecki, W. Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation. Agronomy 2021, 11, 1737. https://doi.org/10.3390/agronomy11091737
Jarecki W. Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation. Agronomy. 2021; 11(9):1737. https://doi.org/10.3390/agronomy11091737
Chicago/Turabian StyleJarecki, Wacław. 2021. "Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation" Agronomy 11, no. 9: 1737. https://doi.org/10.3390/agronomy11091737
APA StyleJarecki, W. (2021). Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation. Agronomy, 11(9), 1737. https://doi.org/10.3390/agronomy11091737





