Use of a Biostimulant to Mitigate Salt Stress in Maize Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Pigments Determination
2.3. Na+ and K+ Concentrations in Shoot Maize
2.4. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Contents
2.5. Samples Methanolic Extract
2.6. Determination of Total Phenolic Content (TPC)
2.7. Antioxidant Activity Measurement
2.8. Statistical Analysis
3. Results
3.1. Effect of Salinity on Maize Growth and Pigment Content in Control Samples and Treated with NaCl Alone or in Combination with Meg
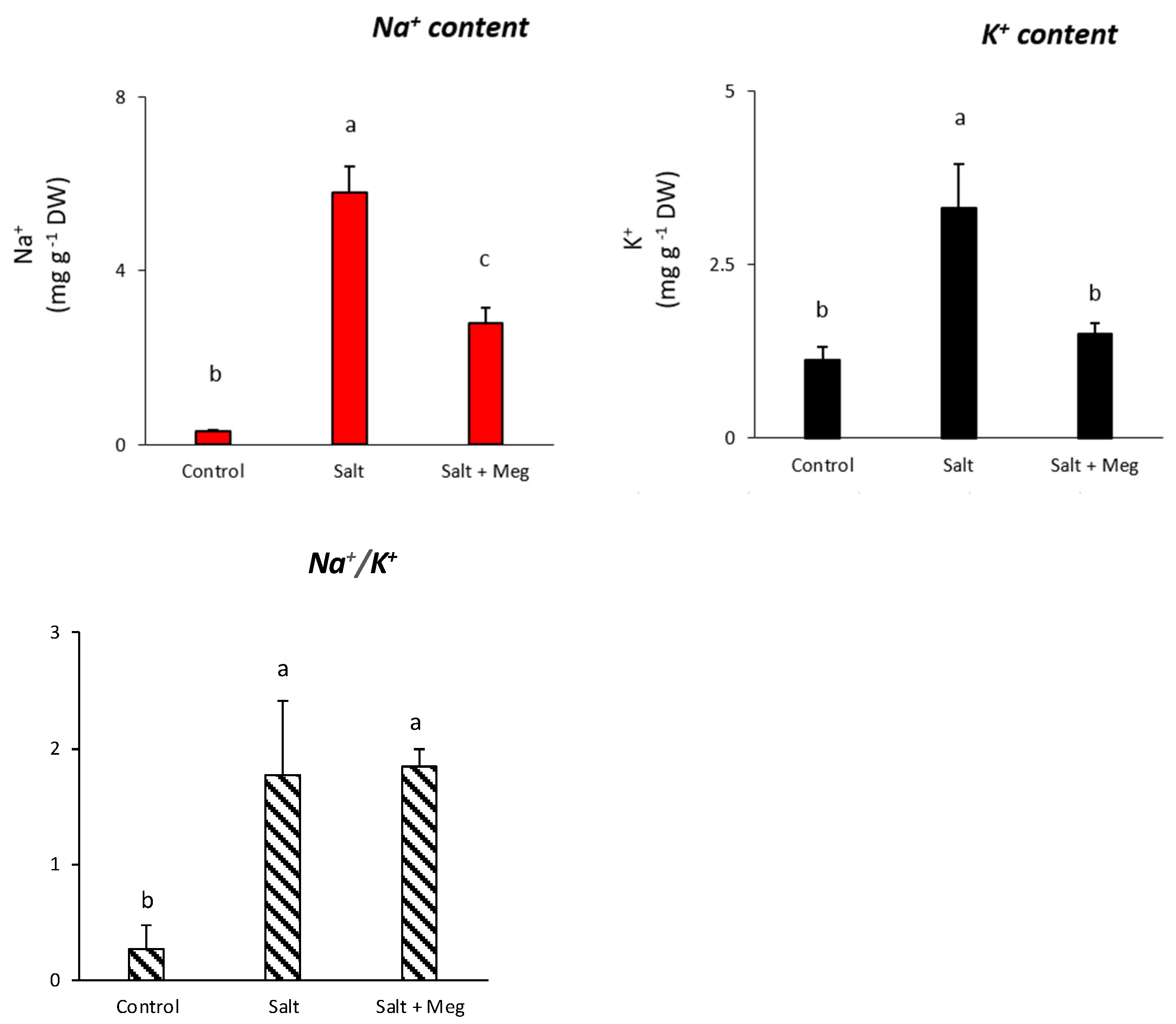
3.2. Na+ and K+ Content, and Relative Ratio, in Control Samples and in Maize Treated with NaCl Alone or in Combination with Meg
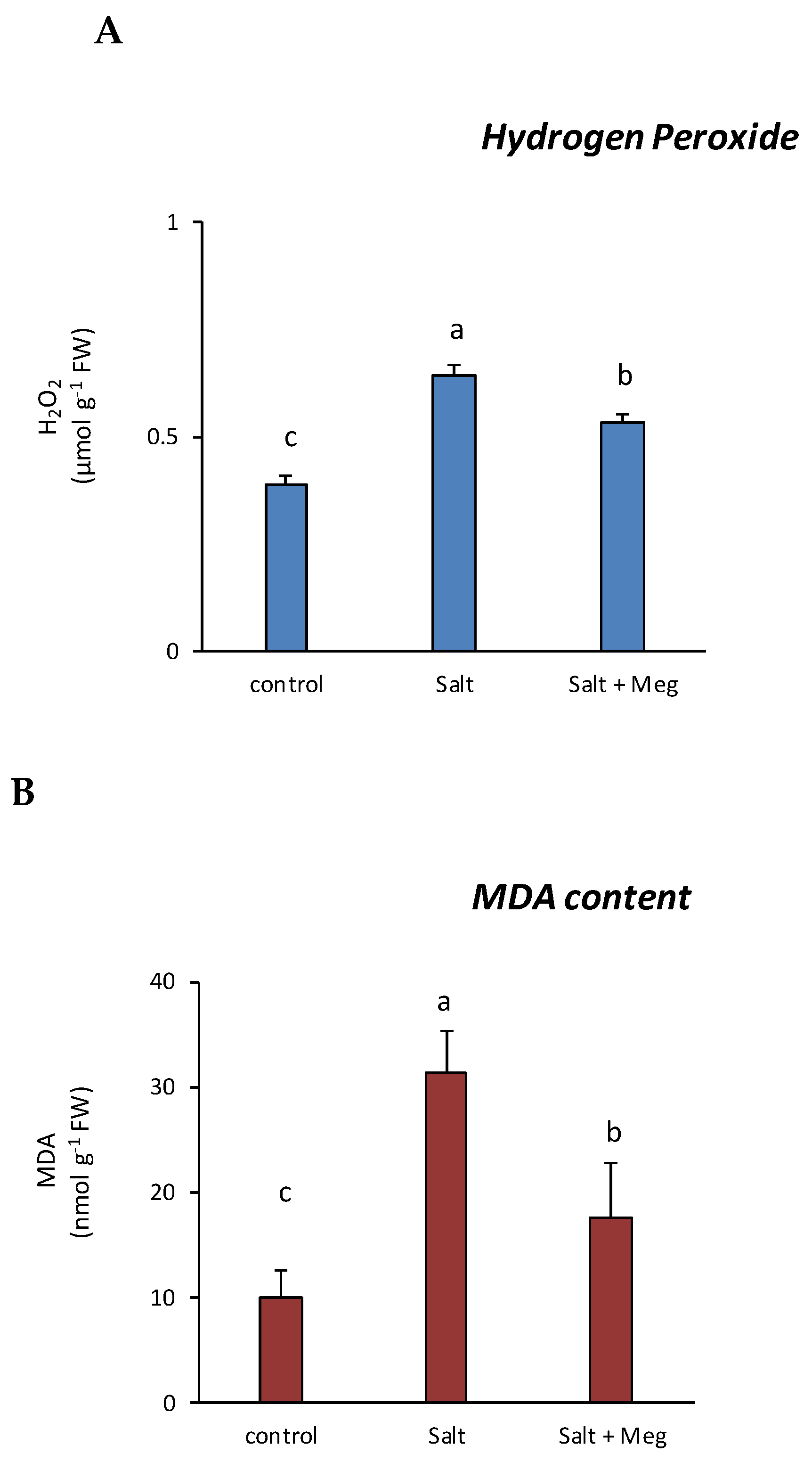
3.3. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content Samples and in Maize Treated with NaCl Alone or in Combination with Meg
3.4. TPC Content, DPPH, FRAP and ABTS Assays, in Control Samples and Maize Treated with NaCl Alone or in Combination with Meg
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.S.; Akther, T.; Hemalatha, S. Impact of Panchagavya on Oryza Sativa L. Grown Under Saline Stress. J. Plant Growth Regul. 2017, 36, 702–713. [Google Scholar] [CrossRef]
- Del Buono, D. Can Biostimulants be Used to Mitigate the Effect of Anthropogenic Climate Change on Agriculture? It Is Time to Respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Akbari, M.; Najafi Alamdarlo, H.; Mosavi, S.H. The Effects of Climate Change and Groundwater Salinity on Farmers’ Income Risk. Ecol. Indic. 2020, 110, 105893. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil Salinity under Climate Change: Challenges for Sustainable Agriculture and Food Security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Buono, D.; Regni, L.; Pino, A.M.D.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of Megafol on the Olive Cultivar ‘Arbequina’ Grown Under Severe Saline Stress in Terms of Physiological Traits, Oxidative Stress, Antioxidant Defenses, and Cytosolic Ca2. Front. Plant Sci. 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Abdo, A.I.E.; Abdel-Hamed, E.M.W.; Desoky, E.-S.M. Integrative Application of Licorice Root Extract or Lipoic Acid with Fulvic Acid Improves Wheat Production and Defenses under Salt Stress Conditions. Ecotoxicol. Environ. Saf. 2020, 190, 110144. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Zhang, L.; Zhou, Y.; Yang, R.; Ding, C.; Wang, X. The Physiological Response of Artemisia Annua L. to Salt Stress and Salicylic Acid Treatment. Physiol. Mol. Biol. Plants 2014, 20, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colantoni, A.; Recchia, L.; Bernabei, G.; Cardarelli, M.; Rouphael, Y.; Colla, G. Analyzing the Environmental Impact of Chemically-Produced Protein Hydrolysate from Leather Waste vs. Enzymatically-Produced Protein Hydrolysate from Legume Grains. Agriculture 2017, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. A Leaf Elongation Assay Detects an Unknown Growth Inhibitor in Xylem Sap From Wheat and Barley. Funct. Plant Biol. 1992, 19, 127–135. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.; Rady, M.M. Natural Bee-Honey Based Biostimulants Confer Salt Tolerance in Onion via Modulation of the Antioxidant Defence System. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Gomes, M.P.; Duarte, D.M.; Carneiro, M.M.L.C.; Barreto, L.C.; Carvalho, M.; Soares, A.M.; Guilherme, L.R.G.; Garcia, Q.S. Zinc Tolerance Modulation in Myracrodruon Urundeuva Plants. Plant Physiol. Biochem. 2013, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Bartucca, M.L.; Del Buono, D.; Cesco, S. Italian Ryegrass for the Phytoremediation of Solutions Polluted with Terbuthylazine. Chemosphere 2015, 119, 31–36. [Google Scholar] [CrossRef]
- Soothar, M.K.; Hamani, A.K.M.; Sootahar, M.K.; Sun, J.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of Acidic Biochar on the Growth, Physiology and Nutrients Uptake of Maize (Zea Mays L.) Seedlings under Salinity Stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The Application of a Biostimulant Based on Tannins Affects Root Architecture and Improves Tolerance to Salinity in Tomato Plants. Sci. Rep. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ckurshumova, W.; Fefer, M.; Liu, J.; Uddin, W.; Rosa, C. A Plant Based Modified Biostimulant (Copper Chlorophyllin), Mediates Defense Response in Arabidopsis Thaliana under Salinity Stress. Plants 2021, 10, 625. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon Mediated Improvement in the Growth and Ion Homeostasis by Decreasing Na+ Uptake in Maize (Zea Mays L.) Cultivars Exposed to Salinity Stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Adoko, M.Y.; Babalola, O.O.; Amogou, O.; Badé, F.T.; Noumavo, P.A.; Adjanohoun, A.; Baba-Moussa, L. Efficacy of Biostimulants Formulated With Pseudomonas Putida and Clay, Peat, Clay-Peat Binders on Maize Productivity in a Farming Environment in Southern Benin. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Yadav, S.; Khosla, B. Biostimulant Effect of Poultry Feather Hydrolysate on Maize (Zea Mays L.) Seedlings. Ann. Biol. 2020, 36, 508–512. [Google Scholar]
- Díaz-González, S.; Marín, P.; Sánchez, R.; Arribas, C.; Kruse, J.; González-Melendi, P.; Brunner, F.; Sacristán, S. Mutualistic Fungal Endophyte Colletotrichum Tofieldiae Ct0861 Colonizes and Increases Growth and Yield of Maize and Tomato Plants. Agronomy 2020, 10, 1493. [Google Scholar] [CrossRef]
- Kapela, K.; Sikorska, A.; Niewęgłowski, M.; Krasnodębska, E.; Zarzecka, K.; Gugała, M. The Impact of Nitrogen Fertilization and the Use of Biostimulants on the Yield of Two Maize Varieties (Zea Mays L.) Cultivated for Grain. Agronomy 2020, 10, 1408. [Google Scholar] [CrossRef]
- Alharby, H.F.; Alzahrani, Y.M.; Rady, M.M. Seeds Pretreatment with Zeatins or Maize Grain-Derived Organic Biostimulant Improved Hormonal Contents, Polyamine Gene Expression, and Salinity and Drought Tolerance of Wheat. Int. J. Agric. Biol. 2020, 24, 714–724. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A Novel Biostimulant, Belonging to Protein Hydrolysates, Mitigates Abiotic Stress Effects on Maize Seedlings Grown in Hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Panfili, I.; Bartucca, M.L.; Marrollo, G.; Povero, G.; Del Buono, D. Application of a Plant Biostimulant To Improve Maize (Zea Mays) Tolerance to Metolachlor. J. Agric. Food Chem. 2019, 67, 12164–12171. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica Napus Var Oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 17496. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Rea, E.; Cardarelli, M. Grafting Cucumber Plants Enhance Tolerance to Sodium Chloride and Sulfate Salinization. Sci. Hortic. 2012, 135, 177–185. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed Extracts Enhance Salam Turfgrass Performance during Prolonged Irrigation Intervals and Saline Shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; Desoky, E.-S.M.; Elrys, A.S.; Boghdady, M.S. Can Licorice Root Extract be Used as an Effective Natural Biostimulant for Salt-Stressed Common Bean Plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-Based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal Imaging of Soybean Response to Drought Stress: The Effect of Ascophyllum Nodosum Seaweed Extract. SpringerPlus 2016, 5, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, S.M.; Akladious, S.A. Application of Carrot Root Extract Induced Salinity Tolerance in Cowpea (Vigna Sinensis L.) Seedlings. Pak. J. Bot. 2013, 45, 795–806. [Google Scholar]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological Responses to Megafol® Treatments in Tomato Plants under Drought Stress: A Phenomic and Molecular Approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of Leaf Chlorophyll a, b and Carotenoid Contents and Their Ratios Using Hyperspectral Reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED Light Proportion Affects Vegetative Parameters, Pigment Content, and Oxidative Status of Einkorn (Triticum Monococcum L. Ssp. Monococcum) Wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Elsevier Inc. In Cooperation with AACC International: Amsterdam, The Netherlands, 2018; pp. 191–246. [Google Scholar]
- EL Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella Salina Exopolysaccharides: A Promising Biostimulant for Salt Stress Tolerance in Tomato (Solanum Lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Chartzoulakis, K.; Loupassaki, M.; Bertaki, M.; Androulakis, I. Effects of NaCl Salinity on Growth, Ion Content and CO2 Assimilation Rate of Six Olive Cultivars. Sci. Hortic. 2002, 96, 235–247. [Google Scholar] [CrossRef]
- Mousavi, S.; Regni, L.; Bocchini, M.; Mariotti, R.; Cultrera, N.G.M.; Mancuso, S.; Googlani, J.; Chakerolhosseini, M.R.; Guerrero, C.; Albertini, E.; et al. Physiological, Epigenetic and Genetic Regulation in Some Olive Cultivars under Salt Stress. Sci. Rep. 2019, 9, 1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, T.J. Improving Crop Salt Tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Cuin, T.A.; Svistunenko, D.; Smith, S.J.; Miller, A.J.; Shabala, S.; Sokolik, A.; Yurin, V. Arabidopsis Root K+-Efflux Conductance Activated by Hydroxyl Radicals: Single-Channel Properties, Genetic Basis and Involvement in Stress-Induced Cell Death. J. Cell Sci. 2010, 123, 1468–1479. [Google Scholar] [CrossRef] [Green Version]
- Chandra Rai, A.; Singh, M.; Shah, K. Effect of Water Withdrawal on Formation of Free Radical, Proline Accumulation and Activities of Antioxidant Enzymes in ZAT12-Transformed Transgenic Tomato Plants. Plant Physiol. Biochem. 2012, 61, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Desoky, E.-S.M.; ElSayed, A.I.; Merwad, A.-R.M.A.; Rady, M.M. Stimulating Antioxidant Defenses, Antioxidant Gene Expression, and Salt Tolerance in Pisum Sativum Seedling by Pretreatment Using Licorice Root Extract (LRE) as an Organic Biostimulant. Plant Physiol. Biochem. 2019, 142, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and Salinity Stress Alters ROS Accumulation, Water Retention, and Osmolyte Content in Sorghum Plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Lee, M.H.; Cho, E.J.; Wi, S.G.; Bae, H.; Kim, J.E.; Cho, J.-Y.; Lee, S.; Kim, J.-H.; Chung, B.Y. Divergences in Morphological Changes and Antioxidant Responses in Salt-Tolerant and Salt-Sensitive Rice Seedlings after Salt Stress. Plant Physiol. Biochem. 2013, 70, 325–335. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants 2021, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S.M. Estimation of Phytochemical Content and Antioxidant Activity of Some Selected Traditional Indian Medicinal Plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Shoot Length | Root Length | Shoot Fresh Weight | Root Fresh Weight | |
|---|---|---|---|---|
| (cm) | (cm) | (g) | (g) | |
| Control | 37.8 ± 8.8 a | 15.8 ± 5.8 a | 11.5 ± 1.3 a | 5.4 ± 1.4 a |
| Salt | 23.8 ± 6.9 c | 16.1 ± 4.2 a | 6.0 ± 1.8 b | 6.9 ± 1.4 a |
| Salt + Meg | 30.1 ± 5.3 b | 15.1 ± 4.4 a | 8.0 ± 0.3 b | 5.3 ± 0.4 a |
| ChlA mg g−1 FW | ChlB mg g−1 FW | TotChl mg g−1 FW | ChlA/ChlB | Car mg g−1 FW | ChlA/Car | |
|---|---|---|---|---|---|---|
| Control | 0.70 ± 0.05 c | 0.22 ± 0.02 a | 0.92 ± 0.06 a | 3.18 | 0.67 ± 0.02 a | 1.04 |
| Salt | 0.59 ± 0.01 a | 0.15 ± 0.04 b | 0.74 ± 0.05 b | 3.93 | 0.37 ± 0.05 b | 1.59 |
| Salt + Meg | 0.79 ± 0.04 b | 0.20 ± 0.02 ab | 0.99 ± 0.05 a | 3.65 | 0.74 ± 0.05 a | 1.07 |
| TPC | DPPH | FRAP | ABTS | |
|---|---|---|---|---|
| ɤ µg GAE g −1 FW | β µM TE g−1 FW | |||
| control | 549 ± 28 b | 9.2 ± 0.6 a | 14.7 ± 1.8 b | 23.1 ± 1.5 c |
| Salt | 821 ± 102 a | 13.6 ± 1.8 b | 20.6 ± 2.6 a | 35.0 ± 2.0 a |
| Salt + Meg | 697 ± 74 b | 14.0 ± 2.3 b | 14.2 ± 2.6 b | 28.8 ± 1.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato, R.; Del Buono, D. Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy 2021, 11, 1755. https://doi.org/10.3390/agronomy11091755
D’Amato R, Del Buono D. Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy. 2021; 11(9):1755. https://doi.org/10.3390/agronomy11091755
Chicago/Turabian StyleD’Amato, Roberto, and Daniele Del Buono. 2021. "Use of a Biostimulant to Mitigate Salt Stress in Maize Plants" Agronomy 11, no. 9: 1755. https://doi.org/10.3390/agronomy11091755
APA StyleD’Amato, R., & Del Buono, D. (2021). Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy, 11(9), 1755. https://doi.org/10.3390/agronomy11091755







