Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils
Abstract
:1. Introduction
- Fertilization regimes and varietal responses affect cassava growth and root yield performance.
- Fertilization increases RUE and modulates cassava varietal responses.
2. Materials and Methods
2.1. Study Area Description
2.2. Experimental Germplasm Description
2.3. Experimental Design and Trial Management
2.4. Data Collection
2.5. Parameter Estimation
2.6. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Properties
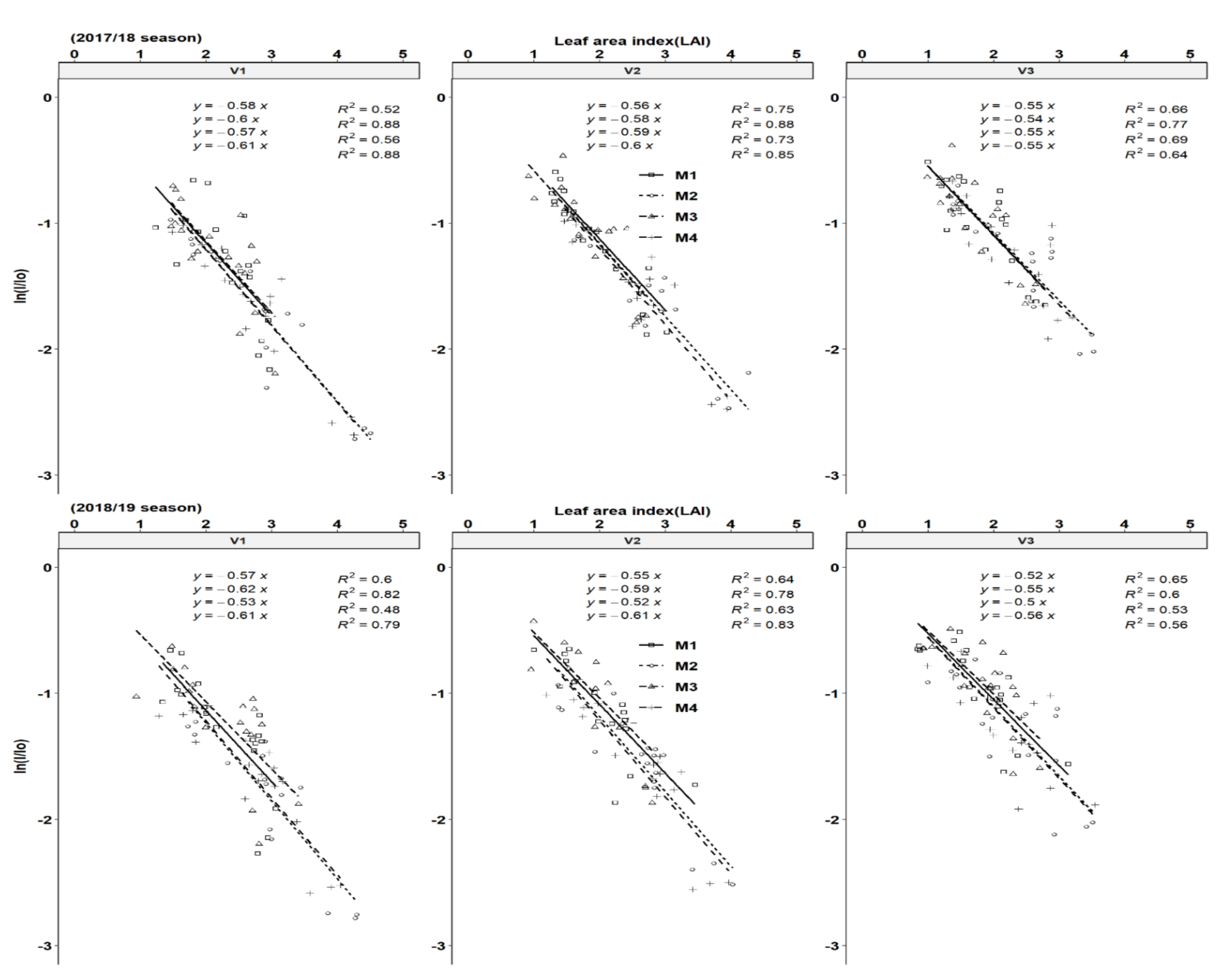
3.2. Leaf Area Index and Light Interception Dynamics
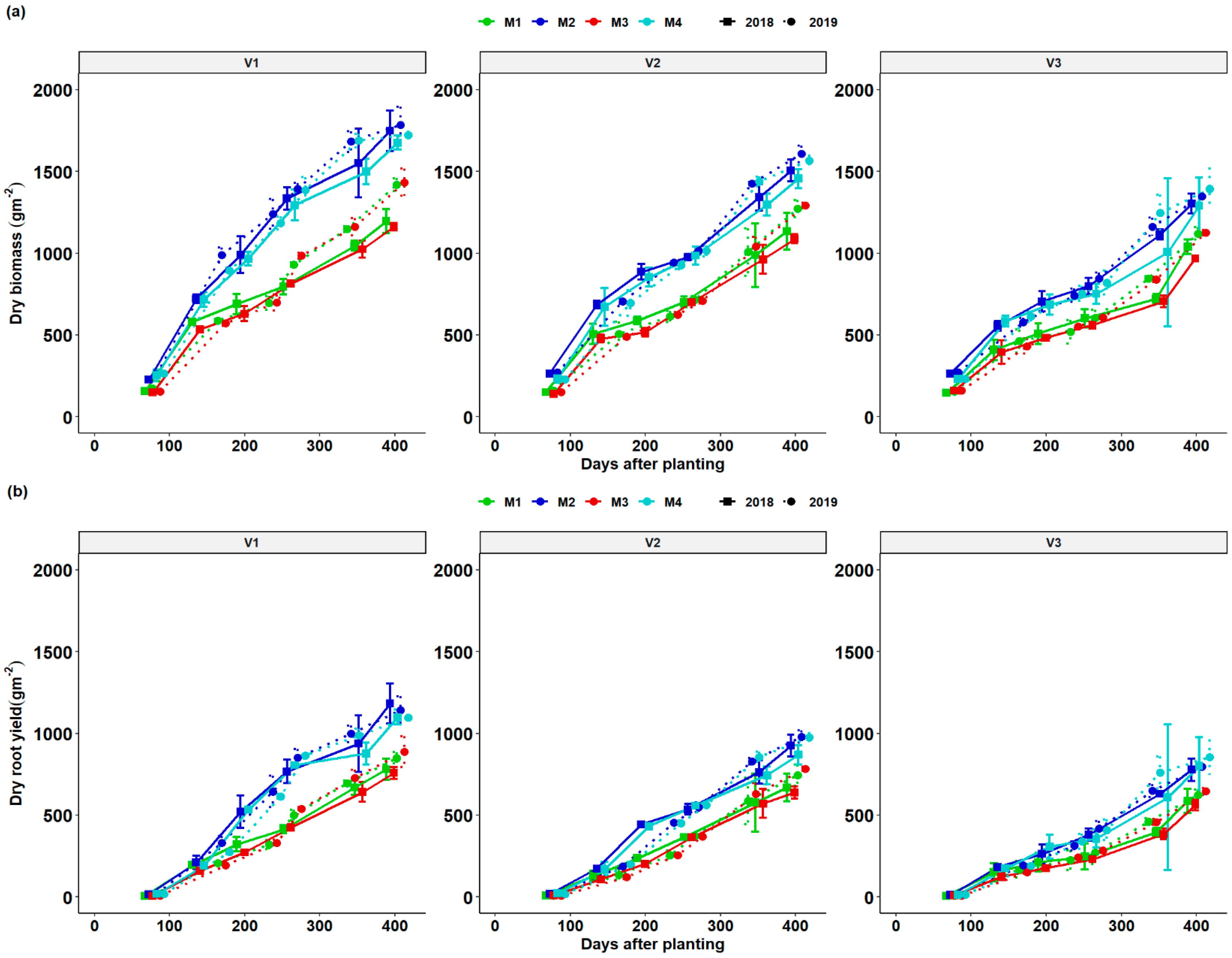
3.3. Dynamics of Total Dry Matter (TDM) and Root Dry Matter (RDM) as a Function of DAP and Season
3.4. Light Extinction Coefficient and Solar Radiation Use Efficiency
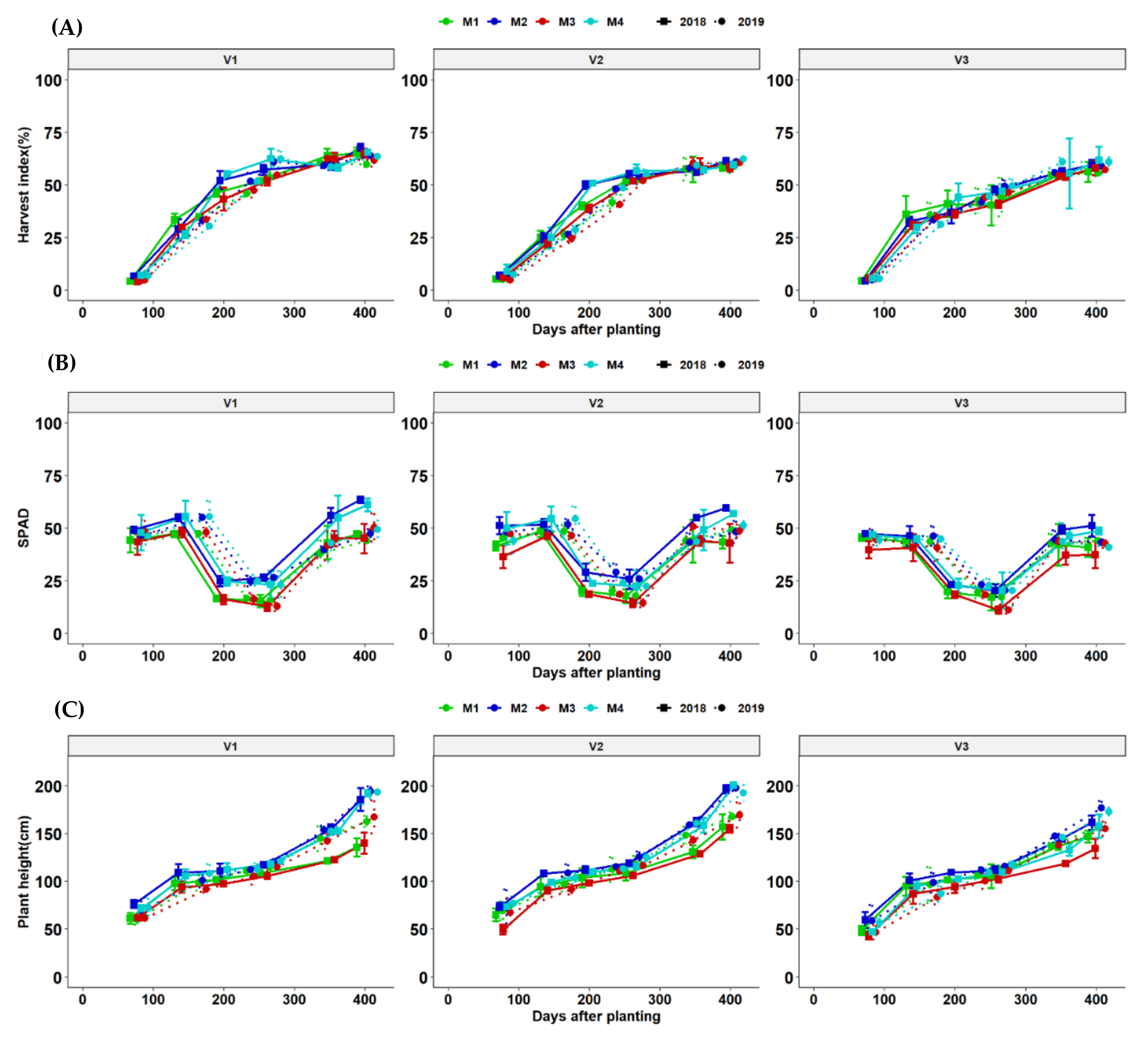
3.5. Effect of Fertilization on Yield, Yield Components and Source Traits of Different Cassava Genotypes across the Growing Seasons
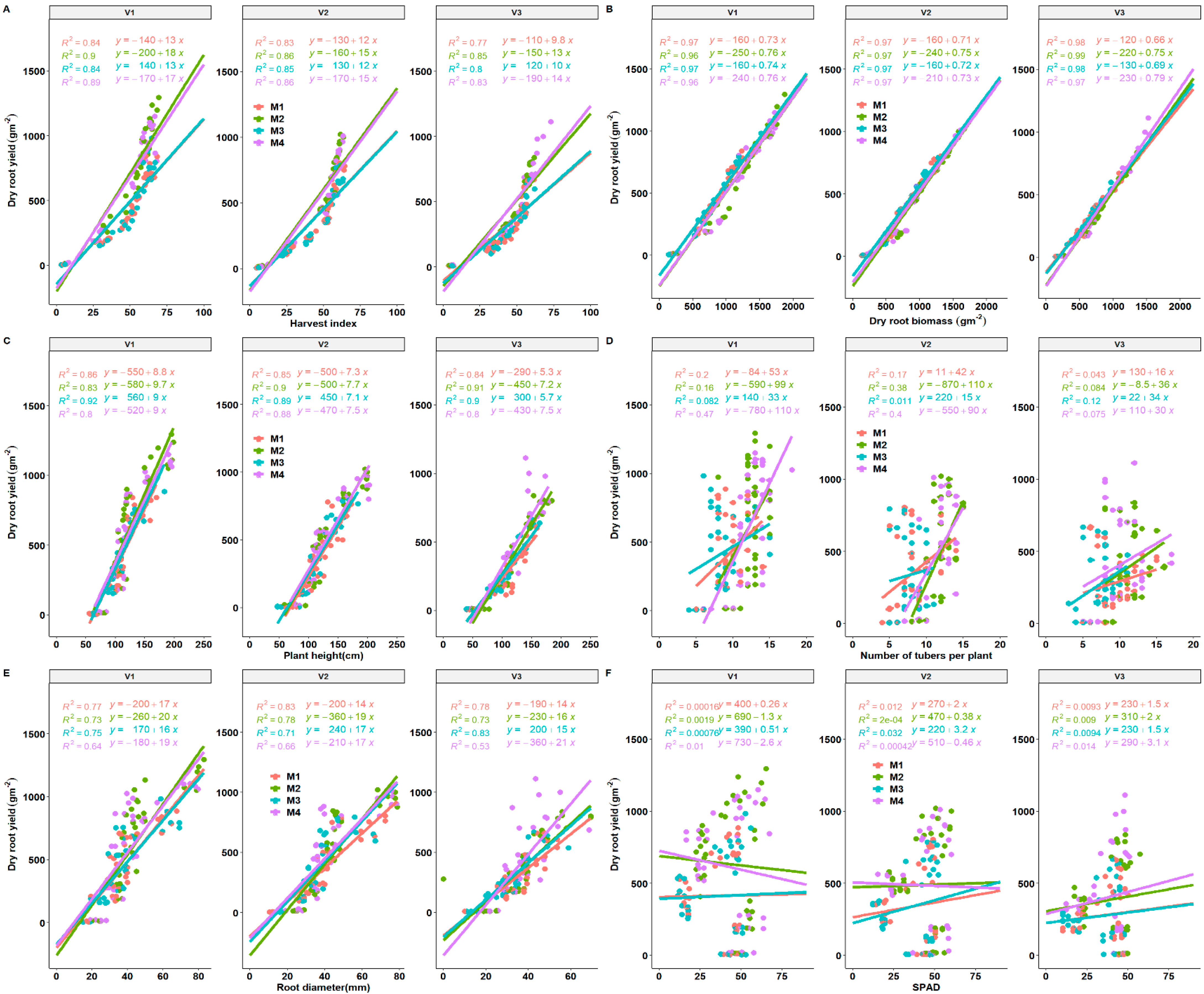
3.6. Important Traits for Cassava Yield, Stem Yield and Biomass
3.7. Growth Dynamics of Cassava Genotypes as Affected by Fertilization Regimes
3.8. Seasonal Yield Determinants of Cassava Storage Root Yield as Influenced by Fertilization Regimes and Variety
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaluba, P.; Mwamba, S.; Moualeu-Ngangue, D.P.; Chiona, M.; Munyinda, K.; Winter, E.; Stützel, H.; Chishala, B.H. Cropping Practices and Effects on Soil Nutrient Adequacy Levels and Cassava Yield of Smallholder Farmers in Northern Zambia. Int. J. Agron. 2021, 2021, 12. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Buresh, R.J.; Calhoun, F.; Shepherd, K.D.; Soule, M.J.; Place, F.M.; Buresh, R.J.; Izac, A.-M.N.; Mokwunye, A.U.; Kwesiga, F.R.; et al. Soil fertility replenishment in Africa: An investment in Natu Sanchez, Pedro A. Ral Resource Capital. In Replenishing Soil Fertility in Africa; American Society of Agronomy, Inc.: Madison, WI, USA, 1997; Volume 51. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Mejia de Tafur, S.; López, Y. Cassava productivity, photosynthesis, ecophysiology, and response to environmental stresses in the tropics: A multidisciplinary approach to crop improvement and sustainable production. In Cassava in the Third Millennium: Modern Production, Processing, Use, and Marketing Systems; Publicación CIAT No. 377; Bernardo, O.P., Hernán, C., Eds.; Centro Internacional de Agricultura Tropical (CIAT); Latin American and Caribbean Consortium to support Cassava Research and Development (CLAYUCA); Technical Centre for Agricultural and Rural Cooperation (CTA): Cali, CO, USA, 2012; pp. 29–88. [Google Scholar]
- Howeler, R.H. Cassava mineral nutrition and fertilization. Cassava Biol. Prod. Util. 2001, 1, 115–147. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. Effects of liming on phosphate availability in acid soils—A critical review. Plant Soil 1982, 68, 289–308. [Google Scholar] [CrossRef]
- Ezui, K.S.; Franke, A.C.; Leffelaar, P.A.; Mando, A.; van Heerwaarden, J.; Sanabria, J.; Sogbedji, J.; Giller, K.E. Water and radiation use efficiencies explain the effect of potassium on the productivity of cassava. Eur. J. Agron. 2017, 83, 28–39. [Google Scholar] [CrossRef]
- Howeler, R.H. Long-term effect of cassava cultivation on soil productivity. Field Crop. Res. 1991, 26, 1–18. [Google Scholar] [CrossRef]
- Kelly, V. Factors Affecting Demand for Fertilizers in SubSaharan Africa; Agriculture and Rural Development Discussion Paper 23; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Fening, J.O.; Gyapong, T.A.; Ababio, F.; Gaisie, E. Effect of site characteristics on the productivity and economic returns from cassava legume intercropping in ghana. Afr. J. Environ. Sci. Technol. 2009, 3, 326–331. [Google Scholar]
- Pypers, P.; Sanginga, J.M.; Kasereka, B.; Walangululu, M.; Vanlauwe, B. Increased productivity through integrated soil fertility management in cassava-legume Intercropping Systems in the Highlands of Sud-Kivu, DR Congo. Field Crop. Res. 2010, 120, 76–85. [Google Scholar] [CrossRef]
- Howeler, R. Sustainable Soil and Crop Management of Cassava in Asia: A Reference Manual; International Center for Tropical Agriculture: Cali, CO, USA, 2014. [Google Scholar]
- Vanlauwe, B.; Descheemaeker, K.; Giller, K.E.; Huising, J.; Merckx, R.; Nziguheba, G.; Wendt, J.; Zingore, S. Integrated soil fertility management in sub-Saharan africa: Unravelling local adaptation. Soil 2015, 1, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Alamu, E.O.; Ntawuruhunga, P.; Chibwe, T.; Mukuka, I.; Chiona, M. Evaluation of Cassava processing and utilization at household level in Zambia. Food Secur. 2019, 11, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Alene, D. Economic impacts of cassava research and extension in Malawi and Zambia. J. Dev. Agric. Econ. 2013, 5, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Haggblade, S.; Nyembe, M. Commercial Dynamics in Zambia’s Cassava Value Chain: Cassava Transformation in Southern Africa (CATISA); Food Security Research Project: Lusaka, Zambia, 2007. [Google Scholar]
- Barratt, N.; Chitundu, D.; Dover, O.; Elsinga, J.; Eriksson, S.; Guma, L.; Haggblade, M.; Haggblade, S.; Henn, T.O.; Locke, F.R.; et al. Cassava as drought insurance: Food security implications of cassava trials in central Zambia. Agrekon 2006, 45, 106–123. [Google Scholar] [CrossRef] [Green Version]
- Chitundu, M.; Droppelmann, K.; Haggblade, S. A Value Chain Task Force Approach for Managing Privatepublic Partnerships: Zambia’s Task Force on Acceleration of Cassava Utilisation; Working Paper No. 21; Food Security Research Project: Lusaka, Zambia, 2006; p. 32. Available online: https://ageconsearch.umn.edu/record/54480/ (accessed on 22 January 2021).
- Pypers, P.; Bimponda, W.; Lodi-Lama, J.P.; Lele, B.; Mulumba, R.; Kachaka, C.; Boeckx, P.; Merckx, R.; Vanlauwe, B. Combining mineral fertilizer and green manure for increased, profitable cassava production. Agron. J. 2012, 104, 178–187. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Prospects of photosynthetic research for increasing agricultural productivity, with emphasis on the tropical c4 amaranthus and the cassava c3-c4 crops. Photosynthetica 2016, 54, 161–184. [Google Scholar] [CrossRef]
- Ezui, K.S.; Franke, A.C.; Mando, A.; Ahiabor, B.D.K.; Tetteh, F.M.; Sogbedji, J. Fertiliser requirements for balanced nutrition of cassava across eight locations in west africa. Field Crop. Res. 2015, 185, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.A.C. Cassava botany and physiology. Cassava Biol. Prod. Util. 2002, 1, 67–89. [Google Scholar] [CrossRef]
- Byju, G.; Suja, G. Mineral nutrition of cassava. Adv. Agron. 2020, 159, 169–235. [Google Scholar] [CrossRef]
- Pellet, D.M.; El-Sharkawy, M.A. Cassava varietal response to fertilization: Growth dynamics and implications for cropping sustainability. Exp. Agric. 1997, 33, 353–365. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, Sustainable agriculture and food security. A review. Agron. Sustain. Dev. 2010, 30, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, T.R.; Horie, T. Leaf nitrogen, photosynthesis, and crop radiation use efficiency: A Review. Crop SC 1989, 29, 90–98. [Google Scholar] [CrossRef]
- Luening, R.; Kelliher, F.M.; De Pury, D.G.G.; Schulze, E.-D. Leaf nitrogen, photosynthesis, conductance and transpiration: Scaling from leaves to canopies. Plant. Cell Environ. 1995, 18, 1183–1200. [Google Scholar] [CrossRef]
- Day, A.D.; Ludeke, K.L. Plant Nutrients in Desert Environments; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Howeler, R. Mineral Nutrition and Fertilization of Cassava; Centro Internacional de Agricultura Tropical (CIAT): Cali, CO, USA, 1981. [Google Scholar]
- Martin, M.H.; Marschner, H. The Mineral Nutrition of Higher Plants. J. Ecol. 1988, 76, 1250. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.H.; Watanabe, K.; Takaragawa, H.; Nakabaru, M.; Kawamitsu, Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Cock, J.H.; Franklin, D.; Sandoval, G.; Juri, P. Ideal cassava plant for maximum yield. Crop Sci. 1979, 19, 271–279. [Google Scholar] [CrossRef]
- Lenis, J.I.; Calle, F.; Jaramillo, G.; Perez, J.C.; Ceballos, H.; Cock, J.H. Leaf retention and cassava productivity. Field Crop. Res. 2006, 95, 126–134. [Google Scholar] [CrossRef]
- Veltkamp, H.J. Physiological Causes of Yield Variation in Cassava (Manihot Esculenta Crantz); Agricultural University Wageningen: Wageningen, The Netherlands, 1985. [Google Scholar]
- Keating, B.A.; Evenson, J.; Fukai, S. Environmental effects on growth and development of cassava (Manihot Esculenta Crantz.) i. Crop development. Field. Crop. Res. 1982, 5, 271–281. [Google Scholar] [CrossRef]
- Ezui, K.S.; Franke, A.C.; Ahiabor, B.D.K.; Tetteh, F.M.; Sogbedji, J.; Janssen, B.H.; Mando, A.; Giller, K.E. Understanding cassava yield response to soil and fertilizer nutrient supply in west africa. Plant Soil 2017, 420, 331–347. [Google Scholar] [CrossRef] [Green Version]
- Nhamo, N. Evaluating yield gaps in cassava production systems in Zambia. In Proceedings of the World Congress on Root and Tuber Crops, Nanning, China, 18–22 January 2016. [Google Scholar]
- Chapoto, A.; Chabala, L.; Lungu, O. A long history of low productivity in Zambia: Is it time to do away with blanket recommendations? Zambia Soc. Sci. J. 2016, 6, 6. [Google Scholar]
- Tsuji, T.; Mambo, A.; Phiri, L.K.; Msoni, R.; Sokotela, S.B.; Yerokun, O.A. Studies on nutrient distribution in some Zambian soils with special reference to sulphur using GIS (Geographic Information Systems) II. Evaluation of plant-available sulphur and its distribution in major Zambian soils. Soil Sci. Plant Nutr. 2005, 51, 943–952. [Google Scholar] [CrossRef]
- Fukuda, W.M.G.; Guevara, C.L.; Kawuki, R.; Ferguson, M.E. Selected Morphological and Agronomic Descriptors for the Characterization of Cassava; IITA: Ibadan, Nigeria, 2010; Volume 19. [Google Scholar]
- Manrique, L.A.; Kinry, J.R.; Hodges, T.; Axness, D.S. Dry matter production and radiation interception of potato. Crop Sci. 1991, 31, 1044. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Project for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.r-project.org/index.html (accessed on 22 January 2021).
- Zhang, L.; Hu, Z.; Fan, J.; Zhou, D.; Tang, F. A meta-analysis of the canopy light extinction coefficient in terrestrial ecosystems. Front. Earth Sci. 2014, 8, 599–609. [Google Scholar] [CrossRef]
- Biratu, G.K. Effect of Chicken Manure application on cassava biomass and root yields in two agro-ecologies of Zambia. Agriculture 2018, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Biratu, G.K.; Elias, E.; Ntawuruhunga, P.; Sileshi, G.W. Cassava response to the integrated use of manure and NPK fertilizer in Zambia. Heliyon 2018, 4, e00759. [Google Scholar] [CrossRef] [Green Version]
- Pellet, D.; El-Sharkawy, M.A. Cassava Varietal response to phosphorus fertilization. ii. phosphorus uptake and use efficiency. Field Crop. Res. 1993, 35, 13–20. [Google Scholar] [CrossRef]
- Lahai, M.T.; Ekanayake, I.J. Accumulation and distribution of dry matter in relation to root yield of cassava under a fluctuating water table in inland valley ecology. Afr. J. Biotechnol. 2009, 8, 4895–4905. [Google Scholar]
- Howeler, R.H.; Cadavid, L.F. Accumulation and distribution of dry matter and nutrients during a 12-month growth cycle of cassava. Field Crop. Res. 1983, 7, 123–139. [Google Scholar] [CrossRef]
- Alves, A.A.; Setter, T. response of cassava to water deficit: Leaf area growth and abscisic acid. Crop Sci. 2000, 40, 131–137. [Google Scholar] [CrossRef]
- Cock, J.H.; El-Sharkawy, M.A. The Physiological Response of Cassava to Stress In Proceedings of the VIIth Symposium of the International Society for Tropical Root Crops, Gosier, Guadeloupe, French, 1–6 July 1985.
- Karim, K.Y.; Ifie, B.; Dzidzienyo, D.; Danquah, E.Y.; Blay, E.T.; Whyte, J.B.A.; Kulakow, P.; Rabbi, I.; Parkes, E.; Omoigui, L.; et al. Genetic characterization of cassava ( Manihot Esculenta Crantz ) genotypes using agro-morphological and single nucleotide polymorphism markers. Physiol. Mol. Biol. Plants 2020, 26, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Kawano, K.; Daza, P.; Amaya, A.; Rios, M.; Goncalves, W.M.F. T Evaluation of cassava germplasm for productivity. Crop Sci. 1978, 18, 8–11. [Google Scholar] [CrossRef]
- El-sharkawy, M.A.; Tafur, S.M.D. Eco-Physiological Research for Breeding Improved Cassava Cultivars in improved cassava cultivars in favourable and stressful environments in tropical/subtropical biosystems. Environ. Res. J. 2014, 6, 144–211. [Google Scholar]
- El-Sharkawy, M.A.; Cock, J.H.; Lynam, J.K.; del Pilar Hernàndez, A.; Cadavid, L.F.L. Relationships between Biomass, Root-Yield and Single-Leaf Photosynthesis in Field-Grown Cassava. Field Crop. Res. 1990, 25, 183–201. [Google Scholar] [CrossRef]
- Onasanya, O.O.; Hauser, S.; Necpalova, M.; Salako, F.K.; Kreye, C.; Tariku, M.; Six, J.; Pypers, P. On-Farm Assessment of Cassava Root Yield Response to Tillage, Plant Density, Weed Control and Fertilizer Application in Southwestern Nigeria. Field Crop. Res. 2021, 262, 108038. [Google Scholar] [CrossRef]
- Manrique, L.A. Response of cassava to liming on a strongly acid ultisol of Panama. Commun. Soil Sci. Plant Anal. 1987, 18, 115–130. [Google Scholar] [CrossRef]
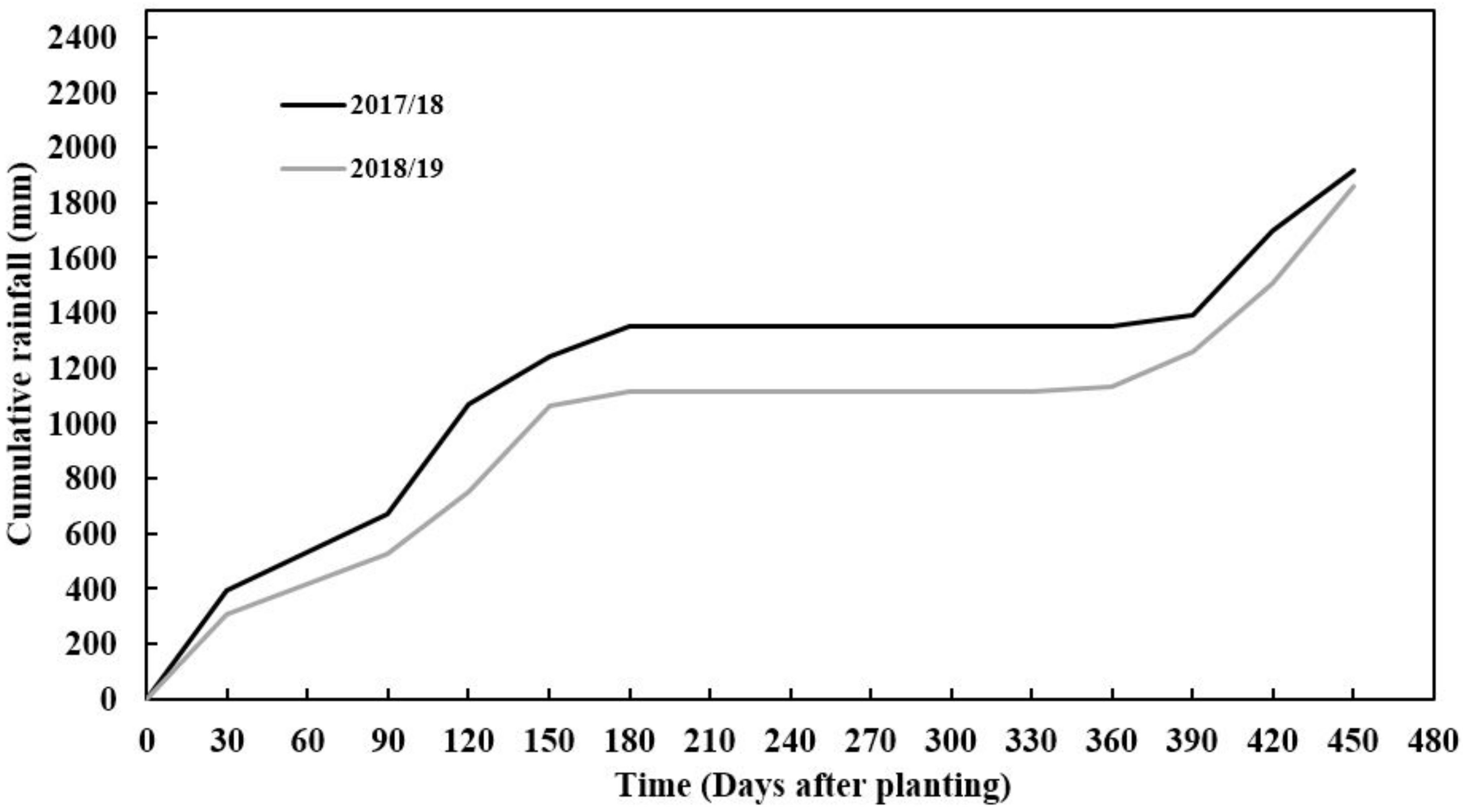
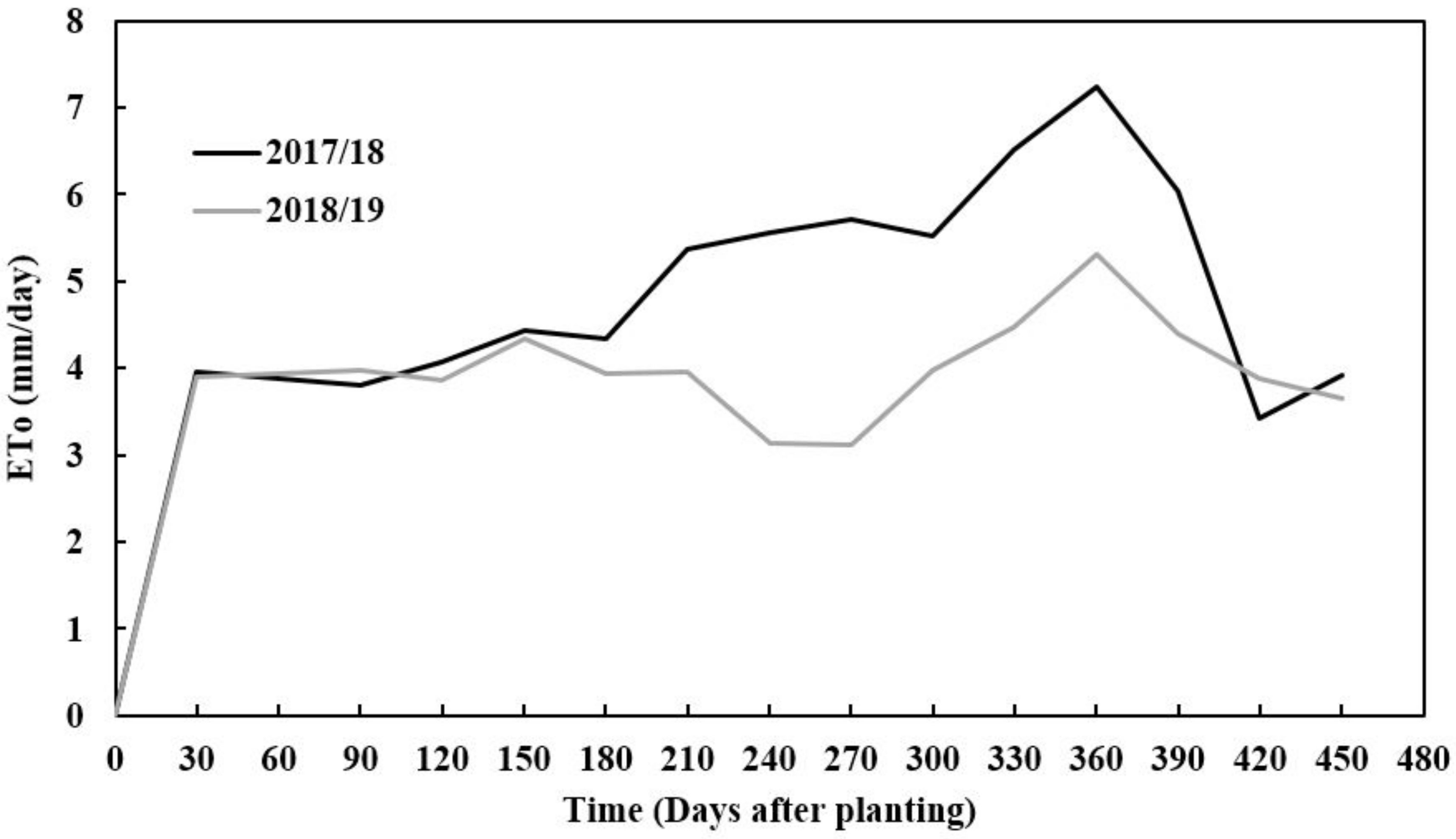



| Parameter | Unit | Mean 2018 | Mean 2019 | Cassava Suitability Production Levels |
|---|---|---|---|---|
| pH (CaCl2) | - | 4.47 | 4.49 | 4.5–7.0 |
| Total N | % | 0.22 | 0.24 | 0.20–0.50 |
| Org C | % | 0.23 | 0.29 | 2.0–4.0 |
| Exchangeable P | (cmol kg−1) | 2.6 | 2.7 | 10–14 |
| Exchangeable K | (cmol kg−1) | 0.08 | 0.08 | 0.15–2.5 |
| Exchangeable Ca | (cmol kg−1) | 0.87 | 0.95 | 1.0–5.0 |
| Exchangeable Mg | (cmol kg−1) | 0.17 | 0.21 | 0.4–1.0 |
| Exchangeable Na | (cmol kg−1) | 0.004 | 0.002 | |
| Micronutrients | ||||
| Zn | (mg kg−1) | 0.61 | 0.58 | 0.5–1.0 |
| Cu | (mg kg−1) | 5.12 | 5.19 | 0.1–0.3 |
| Mn | (mg kg−1) | 50 | 49 | 5–10 |
| Fe | (mg kg−1) | 72 | 69 | 1–10 |
| Particle size (%) | ||||
| Sand | 74 | 73 | ||
| Clay | 4.3 | 4.2 | ||
| Silt | 20.7 | 20.4 | ||
| Textural class | sandy clay loam | sandy clay loam |
| Trait | Fertility | Variety | Year | Fertility:Variety | Fertility:Year | Variety:Year | Fertility:Variety:Year |
|---|---|---|---|---|---|---|---|
| Seasonal LAI | *** | *** | ns | *** | ** | *** | ns |
| Stem yield | *** | *** | *** | ns | * | *** | ns |
| Fresh root yield | ** | *** | *** | ns | *** | ns | ** |
| Root yield | *** | *** | *** | ns | * | ns | ns |
| Fresh biomass | *** | *** | *** | ns | *** | * | * |
| Dry biomass | *** | *** | *** | ns | *** | ns | ns |
| Harvest index | ** | *** | ** | ns | ns | * | ns |
| Tuber number | *** | *** | ns | ** | ** | ns | ns |
| Root diameter | *** | *** | ns | ** | ns | ns | ns |
| Plant height | *** | *** | ns | ** | *** | ** | ns |
| SPAD | ** | *** | * | * | *** | ns | ns |
| Treatments | Traits | ||||
|---|---|---|---|---|---|
| Fertilization Regimes | Variety | Fresh Root Yield (gm−2) | Dry Root Yield (gm−2) | Fresh Biomass (gm−2) | Dry Biomass (gm−2) |
| Control | V1 | 1891ab | 823abc | 3044abcd | 1294bcd |
| V2 | 1658ab | 710ab | 2766abc | 1187abc | |
| V3 | 1370a | 603a | 2398a | 1044a | |
| Lime | V1 | 2072abc | 818abc | 3257abcd | 1309bcd |
| V2 | 1874ab | 710ab | 2996abcd | 1206abc | |
| V3 | 1564ab | 608a | 2684ab | 1082ab | |
| Fertilizer only | V1 | 2834cd | 1096de | 4340e | 1698fg |
| V2 | 2242abcd | 921bcd | 3628cde | 1509def | |
| V3 | 2363bcd | 826bc | 3669de | 1340cde | |
| Fertilizer + lime | V1 | 3012d | 1162e | 4504e | 1768g |
| V2 | 2401bcd | 952cde | 3844de | 1558efg | |
| V3 | 1975abc | 787abc | 3363bcd | 1328cde | |
| SE(Fertilizer) | 101.6 ** | 22.2 *** | 97.7 *** | 25.2 *** | |
| SE(Variety) | 73.1 *** | 19.3 *** | 74.3 *** | 19.9 *** | |
| SE(Fertilizer × Variety) | 156.8 ns | 38.6 ns | 155.8 ns | 41.13 ns | |
| Traits | Year | Leaf Yield | Stem Yield | Root Yield | Biomass | HI | Tuber Number | Root Diameter | PH | SPAD | MeanLAI | RUE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf yield | 1 | 1 | ||||||||||
| 2 | 1 | |||||||||||
| Stem yield | 1 | 0.664 *** | 1 | |||||||||
| 2 | 0.639 *** | 1 | ||||||||||
| Root yield | 1 | 0.731 *** | 0.449 ** | 1 | ||||||||
| 2 | 0.795 *** | 0.774 *** | 1 | |||||||||
| Biomass | 1 | 0.814 *** | 0.626 *** | 0.976 *** | 1 | |||||||
| 2 | 0.819 *** | 0.831 *** | 0.993 *** | 1 | ||||||||
| HI | 1 | 0.272 *** | −0.217ns | 0.755 *** | 0.599 *** | 1 | ||||||
| 2 | 0.572 *** | 0.499 ** | 0.901 *** | 0.849 *** | 1 | |||||||
| Tuber number | 1 | 0.732 *** | 0.671 *** | 0.7601 ** | 0.831 *** | 0.333 * | 1 | |||||
| 2 | 0.718 *** | 0.694 *** | 0.776 *** | 0.799 *** | 0.589 *** | 1 | ||||||
| Root diameter | 1 | 0.603 *** | 0.595 *** | 0.761 *** | 0.809 *** | 0.389 * | 0.666 *** | 1 | ||||
| 2 | 0.653 *** | 0.758 *** | 0.807 *** | 0.827 *** | 0.648 *** | 0.666 *** | 1 | |||||
| PH | 1 | 0.814 *** | 0.771 *** | 0.662 *** | 0.772 *** | 0.175 ns | 0.743 *** | 0.752 *** | 1 | |||
| 2 | 0.599 *** | 0.556 *** | 0.691 *** | 0.697 *** | 0.543 ** | 0.582 *** | 0.743 *** | 1 | ||||
| SPAD | 1 | 0.820 *** | 0.591 *** | 0.758 *** | 0.815 *** | 0.398 * | 0.788 *** | 0.683 *** | 0.807 *** | 1 | ||
| 2 | 0.668 *** | 0.541 ** | 0.691 *** | 0.703 *** | 0.547 ** | 0.633 *** | 0.715 *** | 0.616 *** | 1 | |||
| MeanLAI | 1 | 0.696 *** | 0.567 *** | 0.870 *** | 0.898 *** | 0.558 *** | 0.896 *** | 0.755 *** | 0.759 *** | 0.826 *** | 1 | |
| 2 | 0.749 *** | 0.807 *** | 0.892 *** | 0.911 *** | 0.728 *** | 0.839 *** | 0.837 *** | 0.648 *** | 0.636 *** | 1 | ||
| RUE | 1 | 0.717 *** | 0.609 *** | 0.884 *** | 0.918 *** | 0.529 ** | 0.862 *** | 0.823 *** | 0.759 *** | 0.772 *** | 0.905 *** | 1 |
| 2 | 0.835 *** | 0.743 *** | 0.898 *** | 0.909 *** | 0.730 *** | 0.838 *** | 0.749 *** | 0.603 *** | 0.882 *** | 0.624 *** | 1 |
| Response Variables | Variables | Regression Coefficient | p-Value | R2 | p-Value for Regression Model |
|---|---|---|---|---|---|
| Root yield | Constant | −568.10 | <0.001 *** | 0.95 | <0.001 |
| Stem Dry Matter | 0.28 | 0.28 ns | |||
| Mean LAI | 458.67 | <0.001 *** | |||
| Tuber Number | −24.47 | 0.04 * | |||
| RUE | 291.69 | 0.02 * | |||
| Biomass | Constant | 1492.02 | <0.001 *** | 0.99 | <0.001 |
| Root yield | 1.55 | <0.001 *** | |||
| SPAD | −3.01 | 0.02 * | |||
| Leaf Matter | −0.81 | 0.06 ns | |||
| HI | −25.17 | <0.001 *** | |||
| Mean LAI | 143.66 | 0.001 ** | |||
| Tuber Number | −4.71 | 0.15 ns | |||
| RUE | 11.66 | 0.72 ns | |||
| Stem yield | Constant | 348.98 | 0.09 ns | 0.99 | <0.001 |
| Root yield | −0.55 | 0.01 * | |||
| SPAD | 0.84 | 0.28 ns | |||
| Leaf Matter | −0.75 | 0.01 * | |||
| HI | −7.06 | 0.05 ns | |||
| Biomass | 0.67 | <0.001 *** | |||
| PH | −0.09 | 0.52 ns | |||
| Root Diameter | −0.61 | 0.10 ns | |||
| Mean LAI | 21.52 | 0.44 ns | |||
| Tuber Number | −5.962 | 0.001 ** | |||
| RUE | 27.53 | 0.11 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwamba, S.; Kaluba, P.; Moualeu-Ngangue, D.; Winter, E.; Chiona, M.; Chishala, B.H.; Munyinda, K.; Stützel, H. Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils. Agronomy 2021, 11, 1757. https://doi.org/10.3390/agronomy11091757
Mwamba S, Kaluba P, Moualeu-Ngangue D, Winter E, Chiona M, Chishala BH, Munyinda K, Stützel H. Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils. Agronomy. 2021; 11(9):1757. https://doi.org/10.3390/agronomy11091757
Chicago/Turabian StyleMwamba, Sydney, Peter Kaluba, Dany Moualeu-Ngangue, Etti Winter, Martin Chiona, Benson H. Chishala, Kalaluka Munyinda, and Hartmut Stützel. 2021. "Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils" Agronomy 11, no. 9: 1757. https://doi.org/10.3390/agronomy11091757
APA StyleMwamba, S., Kaluba, P., Moualeu-Ngangue, D., Winter, E., Chiona, M., Chishala, B. H., Munyinda, K., & Stützel, H. (2021). Physiological and Morphological Responses of Cassava Genotypes to Fertilization Regimes in Chromi-Haplic Acrisols Soils. Agronomy, 11(9), 1757. https://doi.org/10.3390/agronomy11091757







