Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed Rape Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Efficacy Evaluation of Fungicides for Control of Sclerotinia Stem Rot under Field Conditions
2.1.1. Experimental Method
2.1.2. Inoculums Preparation and Plant Inoculation
2.1.3. Fungicide Application
2.1.4. Sclerotinia Disease Assessment
2.1.5. Yield and Seed Quality
2.1.6. Statistical Analysis
2.2. In Vitro Sensitivity Evaluation of S. sclerotiorum Isolates to Fungicides
2.2.1. Fungicide Sensitivity Screening
2.2.2. Effect of Fungicides on Sclerotia Formation
2.2.3. Statistical Analysis
3. Results
3.1. Fungicide Efficacy Evaluation under Field Conditions
3.2. In-Vitro Sensitivity Evaluation of S. sclerotiorum Isolates
3.2.1. Inhibition of Hyphal Growth
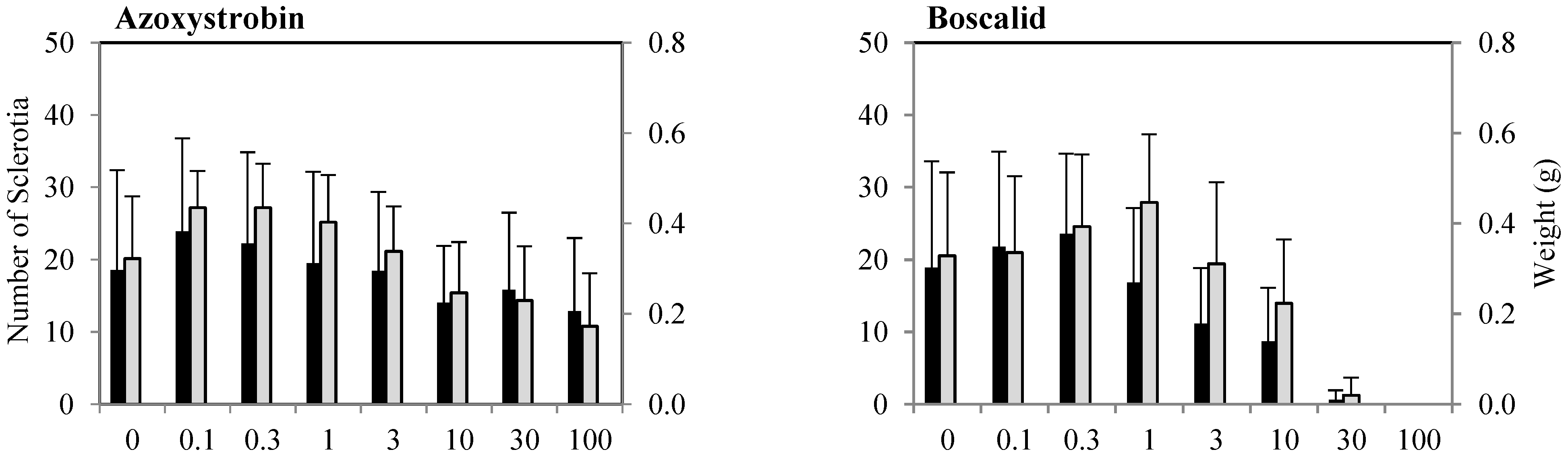
3.2.2. Inhibition of Sclerotia Formation
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Ficke, A.; Grieu, C.; Brurberg, M.B.; Brodal, G. The role of precipitation and petal and leaf infections in Sclerotinia stem rot of spring oilseed Brassica crops in Norway. Eur. J. Plant Pathol. 2018, 152, 885–900. [Google Scholar] [CrossRef]
- Taylor, A.; Coventry, E.; Handy, C.; West, J.S.; Young, C.S.; Clarkson, J.P. Inoculum potential of Sclerotinia sclerotiorum Sclerotia depends on isolate and host plant. Plant Pathol. 2018, 67, 1286–1295. [Google Scholar] [CrossRef] [Green Version]
- Bleiholder, H.; Weber, E.; Lancashire, P.D.; Feller, C.; Buhr, L.; Hess, M.; Wicke, H.; Hack, H.; Meier, U.; Klose, R.; et al. Growth Stages of Mono- and Dicotyledonous Plants, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Braunschweig, Germany, 2011; BBCH Monograph. [Google Scholar]
- FRAC (the Fungicide Resistance Action Committee). FRAC Code List 2021: Fungicide Sorted by Mode of Action 2021. Available online: https://www.frac.info/publications/downloads (accessed on 1 August 2021).
- Avenot, H.F.; Michailides, T.J. Resistance to boscalid fungicide in Alternaria alternata isolates from pistachio in California. Plant. Dis. 2007, 91, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Stammler, G.; Speakman, J. Microtiter method to test the sensitivity of Botrytis cinerea to boscalid. J. Phytopathol. 2006, 154, 508–510. [Google Scholar] [CrossRef]
- Stammler, G.; Benzinger, G.; Speakman, J. A rapid and reliable method for monitoring the sensitivity of Sclerotinia sclerotiorum to boscalid. J. Phytopathol. 2007, 155, 746–748. [Google Scholar] [CrossRef]
- Pan, Y.L.; Zhu, G.M.; Guo, J.; Xiao, T.; Gu, B.C. Sensitivity of Sclerotinia sclerotiorum to boscalid and its correlation with other diverse fungicides. Southwest China J. Agric. Sci. 2012, 25, 507–512. [Google Scholar]
- Liu, S.; Fu, L.; Hai, H.; Jiang, J.; Che, Z.; Tian, Y.; Chen, G. Sensitivity to boscalid in field isolates of Sclerotinia sclerotiorum from rapeseed in Henan Province, China. J. Phytopathol. 2018, 166, 227–232. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, J.; Zhang, Y.; He, S.; Zhu, F. Baseline sensitivity and toxic actions of boscalid against Sclerotinia sclerotiorum. Crop. Prot. 2018, 110, 83–90. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hou, Y.P.; Wang, J.X.; Yang, G.F.; Liang, X.Y.; Zhou, M.G. Activity of a novel strobilurin fungicide benzothiostrobin against Sclerotinia sclerotiorum. Pestic. Biochemist. Physiol. 2014, 115, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Lu, Y.J.; Zhou, M.G.; Wang, X.F. Mutation in β-tubulin of Sclerotinia sclerotiorum conferring resistance to carbendazim in rapeseed field isolate. Chin. J. Oil Crop. Sci. 2003, 25, 56–60. [Google Scholar]
- Liang, H.-J.; Di, Y.-L.; Li, J.-L.; You, H.; Zhu, F.-X. Baseline sensitivity of pyraclostrobin and toxicity of SHAM to Sclerotinia sclerotiorum. Plant. Dis. 2015, 99, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Tóthová, M.; Hudec, K.; Tóth, P. Sensitivity of Sclerotinia sclerotiorum to strobilurin fungicides in Slovakia. Plant Prot. Sci. 2019, 56, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Thaning, C.; Welch, C.J.; Borowicz, J.J.; Hedman, R.; Gerhardson, B. Suppression of Sclerotinia sclerotiorum apothecial formation by the soil bacterium Serratia plymuthica: Identification of a chlorinated macrolide as one of the causal agents. Soil Biol. Biochem. 2001, 33, 1817–1826. [Google Scholar] [CrossRef]
- Whipps, J.M.; Sreenivasaprasad, S.; Muthumeenakshi, S.; Rogers, C.W.; Challen, M.P. Use of Coniothyrium minitans as a biocontrol agent and some molecular aspects of sclerotial mycoparasitism. Eur. J. Plant Pathol. 2008, 121, 323–330. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Knüfer, J. Effects of host plant resistance and fungicide application on phoma stem canker, growth parameters and yield of winter oilseed rape. Crop. Ptotect. 2018, 112, 313–321. [Google Scholar] [CrossRef]
- Hom, N.H.; Backer, H.C.; Möllers, C. Non-destructive analysis of rapeseed quality by NIRS of small seed samples and single seeds. Euphytica 2007, 153, 27–34. [Google Scholar] [CrossRef]
- Carmer, S.G.; Nyquist, W.E.; Walker, W.M. Least significant differences in combined analyses of experiments with two- or three-factor treatment designs. Agron. J. 1989, 81, 665–672. [Google Scholar] [CrossRef]
- Li, J.-L.; Liu, X.-Y.; Di, Y.-L.; Liang, H.-J.; Zhu, F.-X. Baseline sensitivity and control efficacy of DMI fungicide epoxiconazole against Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2015, 141, 237–246. [Google Scholar] [CrossRef]
- Mueller, D.S.; Dorrance, A.E.; Derksen, R.C.; Ozkan, E.; Kurle, J.E.; Grau, C.R.; Gaska, J.M.; Hartman, G.L.; Bradley, C.A.; Pedersen, W.L. Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis. 2002, 86, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.-X.; Chen, Y.; Wang, J.-X.; Yu, W.-Y.; Tang, Z.-H.; Chen, C.-J.; Zhou, M.-G. Activity of carbendazim, dimethachlon, iprodione, procymidone and boscalid against Sclerotinia stem rot in Jiangsu Province of China. Phytoparasitica 2009, 37, 421–429. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.-P.; Chen, C.-J.; Zhou, M.G. Detection of resistance in Sclerotinia sclerotiorum to carbendazim and dimethachlon in Jiangsu Province of China. Aust. Plant Pathol. 2014, 43, 307–312. [Google Scholar] [CrossRef]
- Yang, D.J.; Wang, B.; Wang, J.X.; Chen, Y.; Zhou, M.G. Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and Sclerotinia stem rot of rape. Biol. Control 2009, 51, 61–65. [Google Scholar] [CrossRef]
- Kuang, J.; Hou, Y.-P.; Wang, J.-X.; Zhou, M.-G. Sensitivity of Sclerotinia sclerotiorum to fludioxonil: In vitro determination of baseline sensitivity and resistance risk. Crop. Prot. 2011, 30, 876–882. [Google Scholar] [CrossRef]
- Bradley, C.A.; Lamey, H.A.; Endres, G.J.; Henson, R.A.; Hanson, B.K.; McKay, K.R.; Halvorson, M.; LeGare, D.G.; Porter, P.M. Efficacy of fungicides for control of Sclerotinia stem rot of canola. Plant Dis. 2006, 90, 1129–1134. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.J.; Roberts, D.P.; Jiang, M.L.; Zhang, Y.B. Decreased incidence of disease caused by Sclerotinia sclerotiorum and improved plant vigor of oilseed rape with Bacillus subtilis Tu-100. Appl. Microbiol. Biotechnol. 2005, 68, 802–807. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Qin, H.; Huang, L.; Han, Q. Inhibitory efficacy of endophytic Bacillus subtilis EDR4 against Sclerotinia sclerotiorum on rapeseed. Biol. Cont. 2014, 78, 67–76. [Google Scholar] [CrossRef]
- Matheron, M.E.; Matejka, J.C. In vitro and field comparison of six new fungicides with iprodione and vinclozolin for control of leaf drop of lettuce caused by Sclerotinia sclerotiorum. Plant Dis. 1989, 73, 727–730. [Google Scholar] [CrossRef]
- Smith, F.D.; Phipps, P.M.; Stipes, R.J. Agar plate, soil plate, and field evaluation of fluazinam and other fungicides for control of Sclerotinia minor on peanut. Plant Dis. 1991, 75, 1138–1143. [Google Scholar] [CrossRef]
- Walker, R.; Powell, A.A.; Seddon, B. Bacillus isolates from the spermosphere of peas and dwarf French beans with antifungal activity against Botrytis cinerea and Pythium species. J. Appl. Microbiol. 1998, 84, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Bressollier, P.; Sorokulova, I.B.; Verneuil, B.; Urdaci, M.C. Amicoumacin antibiotic production and genetic diversity of Bacillus subtilis strains isolated from different habitats. Res. Microbiol. 2002, 153, 269–276. [Google Scholar] [CrossRef]
- Wu, Y.C.; Yuan, J.; Raza, W.; Shen, Q.R.; Huang, Q.W. Biocontrol traits and antagonistic potential of Bacillus amyloliquefaciens strain NJZJSB3 against Sclerotinia sclerotiorum, a causal agent of canola stem rot. J. Microbiol. Biotechnol. 2014, 24, 1327–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molaei, H.; Abrinbana, M.; Ghosta, Y. Baseline sensitivities to azoxystrobin and tebuconazole in Sclerotinia sclerotiorum isolates from sunflower in Iran related to sensitivities to carbendazim and iprodione. J. Phytopath. 2020, 168, 353–362. [Google Scholar] [CrossRef]


| Commercial Product | Active Substance (a.s.) | a.s. Content | Application Rate | Mode of Action | Manufacture | Field (F)/In Vitro (I) |
|---|---|---|---|---|---|---|
| Ortiva | Azoxystrobin | 250 g/L | 0.5 kg/ha | QoI | Syngenta Agro GmbH | F and I |
| Cantus | Boscalid | 500 g/kg | 1.0 L/ha | SDHI | BASF SE | F and I |
| Treso | Fludioxonil | 500 g/kg | 0.75 kg/ha | Inhibitor of MAP | Syngenta Agro GmbH | F and I |
| Proline | Prothioconazole | 250 g/L | 0.7 L/ha | DMI | Bayer Crop Science | I |
| Folicur | Tebuconazole | 250 g/L | 1.5 L/ha | DMI | Bayer Crop Science | F and I |
| Custodia | Azoxystrobin + Tebuconazole | 120 gL 200 g/L | 1.0 L/ha | DMI + QoI | ADAMA Deutschland GmbH | F |
| Pictor Active | Boscalid + Pyraclostrobin | 150 g/L 250 g/L | 1.0 L/ha | SDHI + QoI | BASF SE | F |
| Propulse | Fluopyram + Prothioconazole | 125 g/L 125 g/L | 1.0 L/ha | DMI + SDHI | Bayer Crop Science | F |
| Serenade ASO | Bacillus amyloliquefaciens * strain QST 713 | 13.9 g/L (1012 cfu/kg) | 2.0 L/ha | Microbial | Bayer Crop Science | F and I |
| Treatments | DSI ± SD 1,2 (%) | Efficacy of Fungicide 3 (%) | Yield ± SD 1 (dt/ha) | Yield Relative to UTC 4 (%) | TGW ± SD 1 (g) | TGW Relative to UTC 4 (%) | Oil Content ± SD 1 (%) | Oil Relative to UTC 4 (%) | Protein Content ± SD 1 (%) | Protein Relative to UTC 4 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculated-untreated control | 82.5 ± 4.8 a | - | 19.6 ± 4.7 a | 100 | 5.2 ± 0.2 a | 100 | 40.8 ± 1.3 a | 100 | 20.0 ± 1.5 a | 100 |
| Azoxystrobin | 35.3 ± 17.1 c | 57.2 | 34.6 ± 2.1 c | 176.4 | 5.5 ± 0.1 bc | 105.3 | 42.9 ± 0.8 b | 105.3 | 20.1 ± 0.5 a | 100.2 |
| Boscalid | 32.2 ± 5.6 c | 60.9 | 35.8 ± 2.2 cd | 182.3 | 5.6 ± 0.1 c | 107.0 | 42.8 ± 0.5 b | 104.9 | 20.3 ± 0.7 ab | 100.7 |
| Fludioxonil | 10.8 ± 7.1 e | 86.9 | 37.6 ± 2.4 cd | 191.5 | 5.4 ± 0.1 abc | 104.9 | 43.2 ± 0.9 bc | 105.9 | 20.1 ± 0.8 a | 100.1 |
| Tebuconazole | 34.4 ± 7.2 c | 58.3 | 33.8 ± 3.9 bc | 172.0 | 5.3 ± 0.3 ab | 101.5 | 41.7 ± 1.8 a | 102.3 | 20.4 ± 0.9 b | 100.2 |
| Azoxystrobin + Tebuconazole | 25.3 ± 7.3 cd | 69.7 | 38.9 ± 1.2 d | 197.9 | 5.5 ± 0.2 bc | 105.3 | 42.0 ± 0.9 b | 103.1 | 20.2 ± 1.1 a | 100.4 |
| Boscalid + Pyraclostrobin | 12.8 ± 5.2 e | 84.5 | 39.0 ± 1.1 d | 198.7 | 5.5 ± 0.2 bc | 105.3 | 42.7 ± 1.5 b | 104.7 | 20.5 ± 0.9 b | 101.3 |
| Fluopyram + Prothioconazole | 17.5 ± 6.8 d | 78.8 | 37.1 ± 1.6 cd | 189.0 | 5.5 ± 0.1 bc | 105.3 | 43.3 ± 0.7 bc | 105.5 | 20.1 ± 0.6 a | 100.0 |
| B. amyloliquefaciens strain QST 713 | 52.2 ± 7.1 b | 36.7 | 30.5 ± 3.0 b | 155.2 | 5.5 ± 0.1 bc | 105.3 | 41.4 ± 0.6 a | 101.5 | 20.1 ± 0.7 a | 100.1 |
| Variables | Linear Equation | rs | p-Value |
|---|---|---|---|
| Sclerotinia disease severity/Yield | y = 42.8734 − 0.2603x | −0.96 | <0.001 * |
| Sclerotinia disease severity/TGW | y = 5.5531 − 0.0033x | −0.63 | 0.045 * |
| Sclerotinia disease severity/Oil content | y = 43.4102 − 0.0322x | −0.86 | 0.003 * |
| Sclerotinia disease severity/Protein content | y = 20.3088 − 0.0029x | −0.33 | 0.382 ns |
| Fungicides | Concentration (μg a.s. mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 1 | 3 | 10 | 30 | 100 | |
| Azoxystrobin | 9.0 ± 8.3 a | 26.9 ± 18.5 a | 38.0 ± 23.9 b | 50.4 ± 22.7 a | 55.8 ± 25.1 a | 61.2 ± 24.2 a | 66.3 ± 19.2 a |
| Boscalid | 6.6 ± 7.7 a | 23.0 ± 13.1 a | 46.7 ± 10.0 bc | 66.4 ± 7.2 b | 78.8 ± 7.7 ab | 88.7 ± 5.9 b | 91.7 ± 6.3 b |
| Fludioxonil | 25.9 ± 24.8 b | 50.9 ± 35.5 b | 65.1 ± 27.1c | 79.0 ± 18.7 bc | 91.9 ± 8.3 b | 98.4 ± 1.7 b | 99.8 ± 0.5 b |
| Prothioconazole | 14.7 ± 24.2 ab | 19.9 ± 24.4 a | 42.9 ± 29.0 b | 61.9 ± 18.5 ab | 84.5 ± 12.1 b | 91.1 ± 7.3 b | 98.1 ± 3.8 b |
| Tebuconazole | 7.8 ± 17.9 a | 12.3 ± 24.1a | 19.9 ± 31.1 a | 43.3 ± 35.0 a | 72.1 ± 28.2 a | 88.9 ± 16.5 b | 97.3 ± 4.4 b |
| B. amyloliquefaciens | 100 ± 0.0 c | 100 ± 0.0 c | 100 ± 0.0 d | 100 ± 0.0 c | 100 ± 0.0 b | 100 ± 0.0 b | 100 ± 0.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani-Noor, N. Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed Rape Cultivation. Agronomy 2021, 11, 1758. https://doi.org/10.3390/agronomy11091758
Zamani-Noor N. Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed Rape Cultivation. Agronomy. 2021; 11(9):1758. https://doi.org/10.3390/agronomy11091758
Chicago/Turabian StyleZamani-Noor, Nazanin. 2021. "Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed Rape Cultivation" Agronomy 11, no. 9: 1758. https://doi.org/10.3390/agronomy11091758







