Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Chemical Characterization
2.3. Soil Carbon and Nitrogen Pools
2.4. Soil Enzyme Activities
2.5. Soil Enzyme Ratios
2.6. Data Analysis
3. Results
3.1. Soil Chemical Characterization
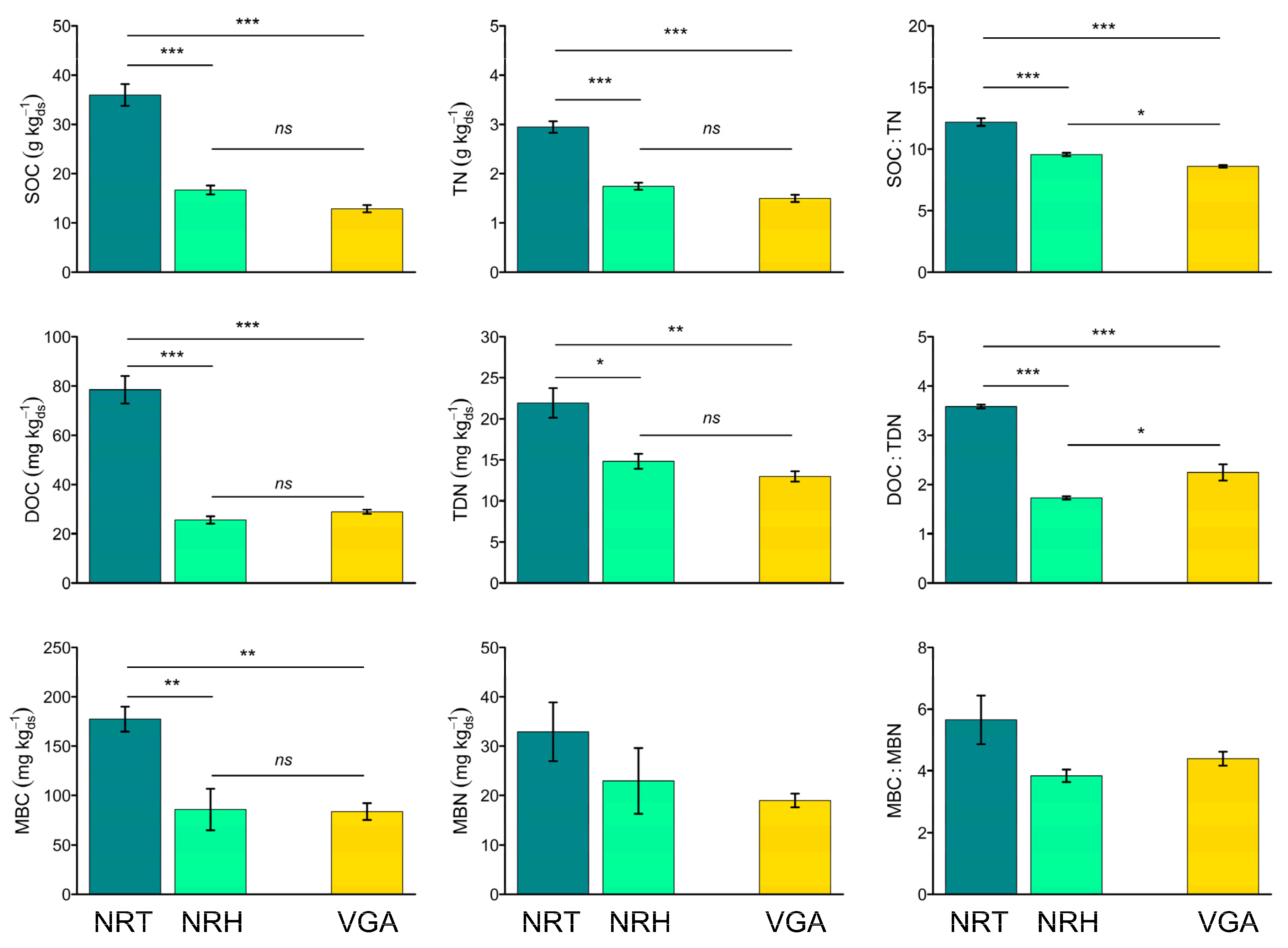
3.2. Soil Carbon and Nitrogen Pools
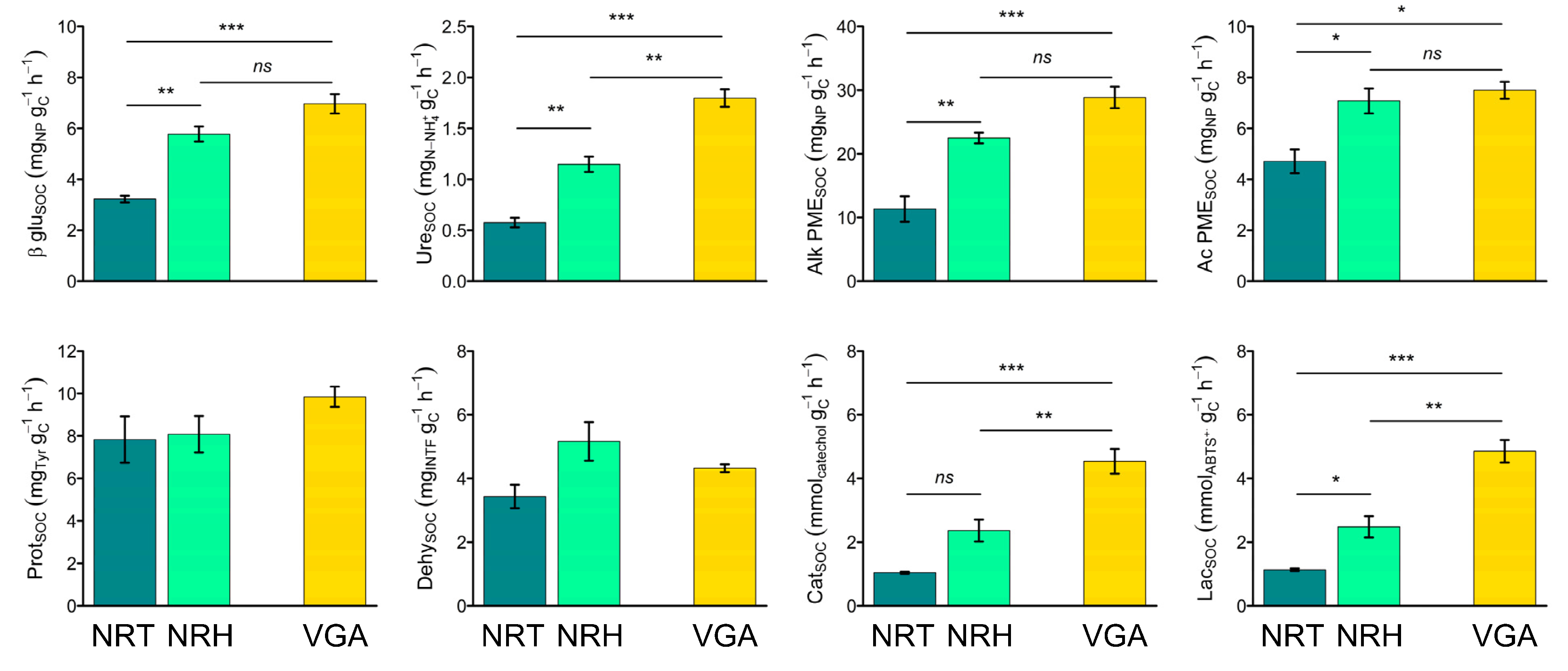
3.3. Soil Enzyme Activities
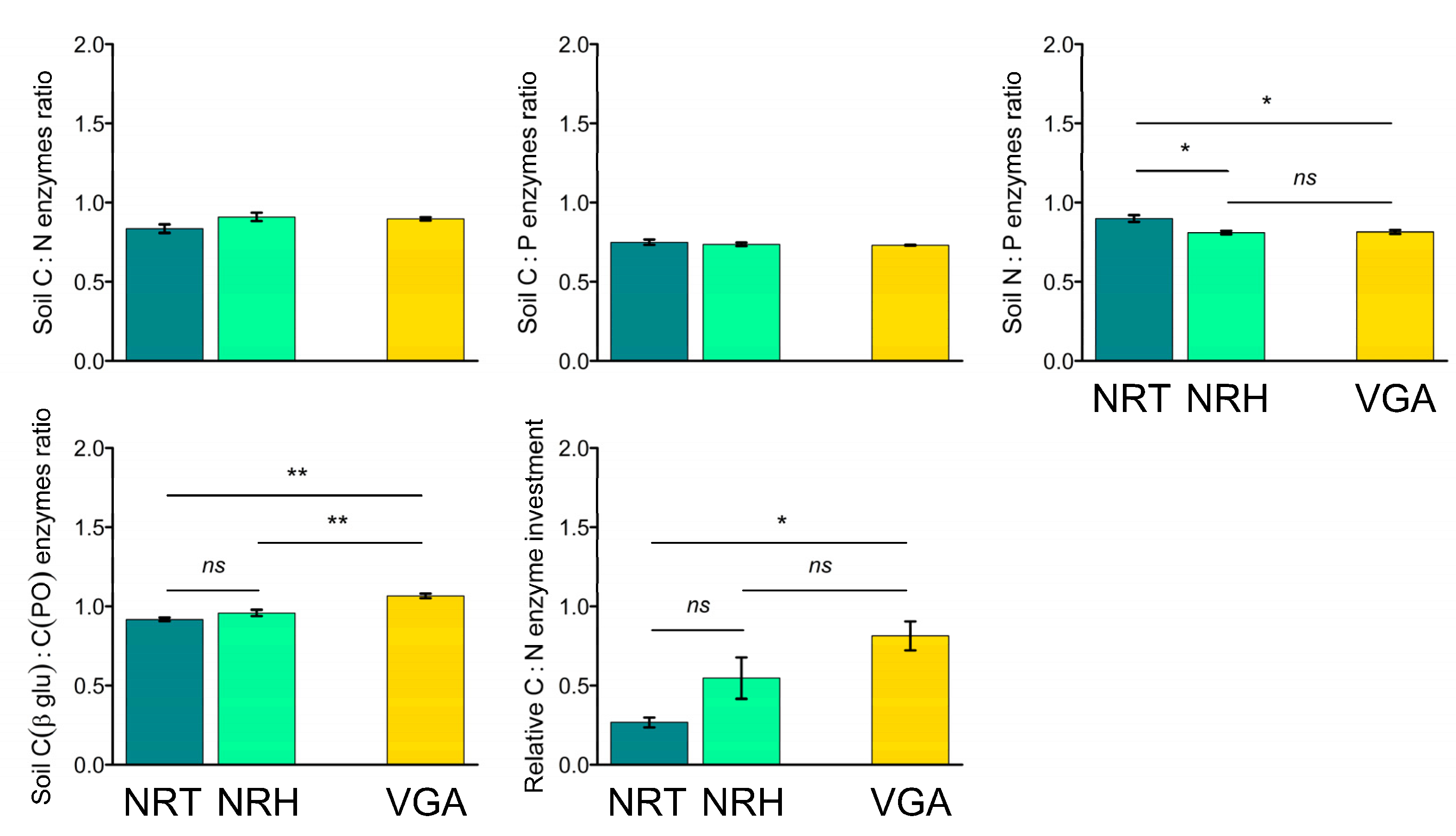
3.4. Soil Eco-Stoichiometric Ratios
4. Discussion
4.1. Influences of Different Natural Recolonization on Soil Quality in the Abandoned Vineyard: Forest vs. Herbaceous Vegetation
4.2. Effects of Natural Recolonization and Conventional Management on Soil Quality in Two Neighboring Vineyards
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, A.N.; Coelho, C.O.A.; De Almeida, A.C.; Figueiredo, A. Soil erosion and hydrological response to land abandonment in a central inland area of Portugal. L. Degrad. Dev. 2010, 21, 260–273. [Google Scholar] [CrossRef]
- Gabarrón-Galeote, M.A.; Trigalet, S.; van Wesemael, B. Effect of land abandonment on soil organic carbon fractions along a Mediterranean precipitation gradient. Geoderma 2015, 249–250, 69–78. [Google Scholar] [CrossRef]
- Perpiña Castillo, C.; Kavalov, B.; Diogo, V.; Jacobs-Crisioni, C.; Batista e Silvia, F.; Lavalle, C. Agricultural land abandonment in the EU within 2015–2030. In JRC Working Papers (No. JRC113718); Joint Research Centre: Ispra, Italy, 2018; pp. 1–7. [Google Scholar]
- Raiesi, F. Land abandonment effect on N mineralization and microbial biomass N in a semi-arid calcareous soil from Iran. J. Arid. Environ. 2012, 76, 80–87. [Google Scholar] [CrossRef]
- Lasanta, T.; Nadal-Romero, E.; Arnáez, J. Managing abandoned farmland to control the impact of re-vegetation on the environment. The state of the art in Europe. Environ. Sci. Policy 2015, 52, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Gabarrón-Galeote, M.A.; Trigalet, S.; van Wesemael, B. Soil organic carbon evolution after land abandonment along a precipitation gradient in southern Spain. Agric. Ecosyst. Environ. 2015, 199, 114–123. [Google Scholar] [CrossRef]
- Bonet, A.; Pausas, J.G. Species richness and cover along a 60-year chronosequence in old-fields of southeastern Spain. Plant Ecol. 2004, 174, 257–270. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, G.Y.; Tang, Z.S.; Shangguan, Z.P. Global patterns of the effects of land-use changes on soil carbon stocks. Glob. Ecol. Conserv. 2016, 5, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Szoboszlay, M.; Dohrmann, A.B.; Poeplau, C.; Don, A.; Tebbe, C.C. Impact of land-use change and soil organic carbon quality on microbial diversity in soils across Europe. FEMS Microbiol. Ecol. 2017, 93, 1–12. [Google Scholar] [CrossRef]
- Baddeley, J.A.; Edwards, A.C.; Watson, C.A. Changes in soil C and N stocks and C:N stoichiometry 21 years after land use change on an arable mineral topsoil. Geoderma 2017, 303, 19–26. [Google Scholar] [CrossRef]
- Atallah, T.; Sitt, K.; El Asmar, E.; Bitar, S.; Ibrahim, L.; Khatib, M.N.; Darwish, T. Effect of abandonment of olive orchards on soil organic carbon sequestration in Mediterranean Lebanon. Soil Res. 2015, 53, 745–752. [Google Scholar] [CrossRef]
- Novara, A.; La Mantia, T.; Rühl, J.; Badalucco, L.; Kuzyakov, Y.; Gristina, L.; Laudicina, V.A. Dynamics of soil organic carbon pools after agricultural abandonment. Geoderma 2014, 235–236, 191–198. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Dang, H.; Ye, C.; Zhang, Q. Soil nitrogen and denitrification potential as affected by land use and stand age following agricultural abandonment in a headwater catchment. Soil Use Manag. 2012, 28, 361–369. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Analysis on soil nutrient characteristics for sustainable land use in Danangou catchment of the Loess Plateau, China. Catena 2003, 54, 17–29. [Google Scholar] [CrossRef]
- Gispert, M.; Emran, M.; Pardini, G.; Doni, S.; Ceccanti, B. The impact of land management and abandonment on soil enzymatic activity, glomalin content and aggregate stability. Geoderma 2013, 202–203, 51–61. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Floch, C.; Capowiez, Y.; Criquet, S. Enzyme activities in apple orchard agroecosystems: How are they affected by management strategy and soil properties. Soil Biol. Biochem. 2009, 41, 61–68. [Google Scholar] [CrossRef]
- Mazzon, M.; Cavani, L.; Margon, A.; Sorrenti, G.; Ciavatta, C.; Marzadori, C. Changes in soil phenol oxidase activities due to long-term application of compost and mineral N in a walnut orchard. Geoderma 2018, 316, 70–77. [Google Scholar] [CrossRef]
- Giacometti, C.; Mazzon, M.; Cavani, L.; Triberti, L.; Baldoni, G.; Ciavatta, C.; Marzadori, C. Rotation and fertilization effects on soil quality and yields in a long term field experiment. Agronomy 2021, 11, 636. [Google Scholar] [CrossRef]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Gallardo-Lancho, J.F. Organic matter properties in cultivated versus set-aside arable soils. Agric. Ecosyst. Environ. 1998, 67, 267–274. [Google Scholar] [CrossRef]
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme activities and microbiological and biochemical processes in soil. In Enzymes in the Environment: Activity, Ecology, and Applications; Burns, R.G., Dick, R.P., Eds.; CRC Press: New York, NY, USA, 2002; pp. 1–33. ISBN 0-8247-0614-5. [Google Scholar]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Trasar-cepeda, C.; Dick, R.P. Soil enzyme activity : A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Balota, E.L.; Kanashiro, M.; Filho, A.C.; Andrade, D.S.; Dick, R.P. Soil Enzyme Activities Under Long-Term Tillage and Crop Rotation Systems in Subtropical Agro-Ecosystems. Braz. J. Microbiol. 2004, 35, 300–306. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Haddix, M.L.; Lee, D.D.; Conant, R.T.; Paul, E.A. A litter-slurry technique elucidates the key role of enzyme production and microbial dynamics in temperature sensitivity of organic matter decomposition. Soil Biol. Biochem. 2012, 47, 18–26. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic activity of loess soil in organic and conventional farming systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: The growth rate hypothesis in reverse. Biogeochemistry 2011, 102, 31–43. [Google Scholar] [CrossRef]
- Cheeke, T.E.; Phillips, R.P.; Brzostek, E.R.; Rosling, A.; Bever, J.D.; Fransson, P. Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol. 2017, 214, 432–442. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Dijkstra, F.A.; Phillips, R.P.; Zhu, B.; Wang, P.; Cheng, W. Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biol. Biochem. 2021, 157, 108246. [Google Scholar] [CrossRef]
- de Santiago-Martín, A.; Vaquero-Perea, C.; Valverde-Asenjo, I.; Quintana Nieto, J.R.; González-Huecas, C.; Lafuente, A.L.; Vázquez de la Cueva, A. Impact of vineyard abandonment and natural recolonization on metal content and availability in Mediterranean soils. Sci. Total Environ. 2016, 551–552, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cavani, L.; Manici, L.M.; Caputo, F.; Peruzzi, E.; Ciavatta, C. Ecological restoration of a copper polluted vineyard: Long-term impact of farmland abandonment on soil bio-chemical properties and microbial communities. J. Environ. Manag. 2016, 182, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R.; Cole, C.V.; Watandbe, F.; Dean, L. Estimation of Available Phosphorus in Soil by Extraction with sodium Bicarbonate. J. Chem. Inf. Model. 1954, 53, 1689–1699. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA micronutrients soil test. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Eiol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Ciavatta, C.; Govi, M.; Antisari, L.V.; Sequi, P. Determination of organic carbon in aqueous extracts of soils and fertilizers. Commun. Soil Sci. Plant Anal. 1991, 22, 795–807. [Google Scholar] [CrossRef]
- Cavani, L.; Ciavatta, C.; Gessa, C. Identification of organic matter from peat, leonardite and lignite fertilisers using humification parameters and electrofocusing. Bioresour. Technol. 2003, 86, 45–52. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphates in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- von Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Perucci, P.; Casucci, C.; Dumontet, S. An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol. Biochem. 2000, 32, 1927–1933. [Google Scholar] [CrossRef]
- Floch, C.; Alarcon-Gutiérrez, E.; Criquet, S. ABTS assay of phenol oxidase activity in soil. J. Microbiol. Methods 2007, 71, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Liu, G.; Li, P.; Xue, S. Ecological stoichiometry of plant-soil-enzyme interactions drives secondary plant succession in the abandoned grasslands of Loess Plateau, China. Catena 2021, 202, 105302. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Green, S.M.; Dungait, J.A.J.; Wen, X.; Quine, T.A. Soil enzyme activity and stoichiometry along a gradient of vegetation restoration at the Karst Critical Zone Observatory in Southwest China. L. Degrad. Dev. 2019, 30, 1916–1927. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lair, G.J.; Gerzabek, M.H.; Haberhauer, G. Sorption of heavy metals on organic and inorganic soil constituents. Environ. Chem. Lett. 2007, 5, 23–27. [Google Scholar] [CrossRef]
- Ungaro, F.; Staffilani, F.; Tarocco, P. Assessing and mapping topsoil organic carbon stock at regional scale: A scorpan kriging approach conditional on soil map delineations and land use. Land Degrad. Dev. 2010, 21, 565–581. [Google Scholar] [CrossRef]
- Francaviglia, R.; Renzi, G.; Ledda, L.; Benedetti, A. Organic carbon pools and soil biological fertility are affected by land use intensity in Mediterranean ecosystems of Sardinia, Italy. Sci. Total Environ. 2017, 599–600, 789–796. [Google Scholar] [CrossRef]
- Romeo, F.; Settineri, G.; Sidari, M.; Mallamaci, C.; Muscolo, A. Responses of soil quality indicators to innovative and traditional thinning in a beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2020, 465, 118106. [Google Scholar] [CrossRef]
- Saha, M.; Das, M.; Sarkar, A. Distinct nature of soil organic carbon pools and indices under nineteen years of rice based crop diversification switched over from uncultivated land in eastern plateau region of India. Soil Tillage Res. 2021, 207, 104856. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Klimkowicz-Pawlas, A.; Smreczak, B. Characterization of organic matter fractions in the top layer of soils under different land uses in Central-Eastern Europe. Soil Use Manag. 2019, 35, 595–606. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L. Mosaic Patterns of thermal stress in the rocky intertidal zone: Implications for Climate Change. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Agnelli, A.; Bol, R.; Trumbore, S.E.; Dixon, L.; Cocco, S.; Corti, G. Carbon and nitrogen in soil and vine roots in harrowed and grass-covered vineyards. Agric. Ecosyst. Environ. 2014, 193, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Abad, J.; Hermoso De Mendoza, I.; Marín, D.; Orcaray, L.; Santesteban, L.G. Cover crops in viticulture. A systematic review (1): Implications on soil characteristics and biodiversity in vineyard. OENO One 2021, 55, 295–312. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A. Enzymes and soil fertility. In Enzymes in Agricultural Sciences; Gianfreda, L., Rao, M., Eds.; OMICS Group: Hyderabad, India, 2014. [Google Scholar]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Mania, E.; Alliani, N.; Giovannozzi, M.; Petrella, F.; Gangemi, L.; Guidoni, S. May vineyard floor management affects indicators of soil quality? Xe Congrès Int. des Terroirs Vitic. 2014, 2, 186–191. [Google Scholar]

| Means ± SE | Pairwise Comparison | |||||
|---|---|---|---|---|---|---|
| NRT | NRH | VGA | NRT vs. NRH | NRT vs. VGA | NRH vs. VGA | |
| pHH2O | 8.04 ± 0.01 | 8.08 ± 0.06 | 8.22 ± 0.02 | ns | * | ns |
| K.exc | 197 ± 17 | 143 ± 32 | 140 ± 6 | ns | ns | ns |
| Mg.exc | 174 ± 13 | 119 ± 5 | 123 ± 2 | ** | * | ns |
| Na.exc | 28.1 ± 3.9 | 22.0 ± 3.3 | 18.3 ± 1.3 | ns | ns | ns |
| Olsen-P | 4.16 ± 0.28 | 2.54 ± 0.34 | 2.12 ± 0.60 | ns | * | ns |
| Means ± SE | Pairwise Comparison | |||||
|---|---|---|---|---|---|---|
| NRT | NRH | VGA | NRT vs. NRH | NRT vs. VGA | NRH vs. VGA | |
| Total | ||||||
| Cu | 594 ± 139 | 114 ± 32 | 99 ± 11 | * | * | ns |
| Mn | 1000 ± 31 | 1060 ± 17 | 1042 ± 22 | ns | ns | ns |
| Zn | 404 ± 96 | 79 ± 21 | 67 ± 7 | * | * | ns |
| Bioavailable | ||||||
| Cu | 134 ± 58 | 22 ± 8 | 16 ± 2 | ** | ** | ns |
| Mn | 22.9 ± 1.5 | 16.8 ± 0.2 | 17.1 ± 0.4 | ** | * | ns |
| Zn | 5.84 ± 1.40 | 1.39 ± 0.18 | 3.65 ± 0.33 | * | ns | ns |
| Means ± SE | Pairwise Comparison | |||||
|---|---|---|---|---|---|---|
| NRT | NRH | VGA | NRT vs. NRH | NRT vs. VGA | NRH vs. VGA | |
| Humified C | 12.2 ± 1.2 | 6.9 ± 1.0 | 4.8 ± 0.6 | * | ** | ns |
| HR | 33.9 ± 1.3 | 40.7 ± 4.3 | 37.3 ± 2.3 | ns | ns | ns |
| DH | 54.3 ± 4.1 | 73.1 ± 1.8 | 59.9 ± 2.4 | * | ns | ns |
| HI | 0.864 ± 0.144 | 0.369 ± 0.034 | 0.674 ± 0.070 | * | ns | ns |
| Means ± SE | Pairwise Comparison | |||||
|---|---|---|---|---|---|---|
| NRT | NRH | VGA | NRT vs. NRH | NRT vs. VGA | NRH vs. VGA | |
| βglu | 116 ±10 | 96 ± 3 | 89 ± 5 | ns | ns | ns |
| Ure | 20.5 ± 0.5 | 19.1 ± 1.6 | 23.1 ± 1.5 | ns | ns | ns |
| Alk PME | 404 ±65 | 376 ± 32 | 370 ± 19 | ns | ns | ns |
| Ac PME | 168 ± 14 | 119 ± 14 | 96 ± 4 | ns | ** | ns |
| Prot | 277 ± 24 | 136 ± 20 | 128 ± 13 | ** | ** | ns |
| Dehy | 123 ± 12 | 87 ± 14 | 56 ± 5 | ns | ** | ns |
| Cat | 37.3 ± 1.5 | 38.8 ± 3.4 | 57.9 ± 1.9 | ns | ** | ** |
| Lac | 40.6 ± 1.1 | 40.8 ± 3.1 | 62.0 ± 1.9 | ns | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciubba, L.; Mazzon, M.; Cavani, L.; Baldi, E.; Toselli, M.; Ciavatta, C.; Marzadori, C. Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy. Agronomy 2021, 11, 1841. https://doi.org/10.3390/agronomy11091841
Sciubba L, Mazzon M, Cavani L, Baldi E, Toselli M, Ciavatta C, Marzadori C. Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy. Agronomy. 2021; 11(9):1841. https://doi.org/10.3390/agronomy11091841
Chicago/Turabian StyleSciubba, Luigi, Martina Mazzon, Luciano Cavani, Elena Baldi, Moreno Toselli, Claudio Ciavatta, and Claudio Marzadori. 2021. "Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy" Agronomy 11, no. 9: 1841. https://doi.org/10.3390/agronomy11091841
APA StyleSciubba, L., Mazzon, M., Cavani, L., Baldi, E., Toselli, M., Ciavatta, C., & Marzadori, C. (2021). Soil Response to Agricultural Land Abandonment: A Case Study of a Vineyard in Northern Italy. Agronomy, 11(9), 1841. https://doi.org/10.3390/agronomy11091841












