Identification of Genetic Loci on Chromosome 4B for Improving the Grain Number per Spike in Pre-Breeding Lines of Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Genotyping
2.3. Population Structure and Phylogenetic Tree Analysis
2.4. Phenotype Evaluation and Correlation Analysis
2.5. Genome-Wide Association Studies (GWAS)
2.6. KASP Markers Development and Validation
2.7. Candidate Gene Analysis
3. Results
3.1. Phenotype Variation and Trait Correlation Analysis
3.2. Estimation of Population Structure
3.3. GWAS Identify Genetic Loci Associated with Spike-Related Traits
3.4. Chromosome 4BS Genomic Region Confers the Grain Number per Spikelet Trait
3.5. Identification of Candidate Genes and Their Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2010, 62, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R. The importance of grain or kernel number in wheat: A reply to Sinclair and Jamieson. Field Crops Res. 2008, 105, 15–21. [Google Scholar] [CrossRef]
- Gao, F.; Ma, D.; Yin, G.; Rasheed, A.; Dong, Y.; Xiao, Y.; Xia, X.; Wu, X.; He, Z. Genetic Progress in Grain Yield and Physiological Traits in Chinese Wheat Cultivars of Southern Yellow and Huai Valley since 1950. Crop Sci. 2017, 57, 760–773. [Google Scholar] [CrossRef]
- Shearman, V.J.; Sylvester-Bradley, R.; Scott, R.K.; Foulkes, M.J. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar] [CrossRef]
- Xiao, S.H. Yield potential improvement in wheat. In Chinese Wheat Improvement and Pedigree Analysis; Zhuang, Q.S., Ed.; Chinese Agriculture Press: Beijing, China, 2003; pp. 497–519. (In Chinese) [Google Scholar]
- Deng, Z.; Cui, Y.; Han, Q.; Fang, W.; Li, J.; Tian, J. Discovery of Consistent QTLs of Wheat Spike-Related Traits under Nitrogen Treatment at Different Development Stages. Front. Plant Sci. 2017, 8, 2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, B.; Sayed-Tabatabaei, B.E.; Saeidi, G.; Kearsey, M.; Suenaga, K. Mapping QTL for grain yield, yield components, and spike features in a doubled haploid population of bread wheat. Genome 2011, 54, 517–527. [Google Scholar] [CrossRef]
- Wang, J.; Liao, X.; Li, Y.; Zhou, R.; Yang, X.; Gao, L.; Jia, J. Fine mapping a domestication-related QTL for spike-related traits in a synthetic wheat. Genome 2010, 53, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, D.; Meng, Z.; Xu, K.; Yan, J.; Xia, X.; Cao, S.; Tian, Y.; He, Z.; Zhang, Y. QTL mapping for grain yield-related traits in bread wheat via SNP-based selective genotyping. Theor. Appl. Genet. 2019, 133, 857–872. [Google Scholar] [CrossRef]
- Deng, M.; Wu, F.; Zhou, W.; Li, J.; Shi, H.; Wang, Z.; Lin, Y.; Yang, X.; Wei, Y.; Zheng, Y.; et al. Mapping of QTL for total spikelet number per spike on chromosome 2D in wheat using a high-density genetic map. Genet. Mol. Biol. 2019, 42, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Zhao, D.; Zhang, C.; Zhang, Z.; Xue, S.; Lin, F.; Kong, Z.; Tian, D.; Luo, Q. Molecular genetic analysis of five spike-related traits in wheat using RIL and immortalized F2 populations. Mol. Genet. Genom. 2006, 277, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ding, P.; Liu, J.; Li, T.; Zou, Y.; Habib, A.; Mu, Y.; Tang, H.; Jiang, Q.; Liu, Y.; et al. Identification and validation of a major and stably expressed QTL for spikelet number per spike in bread wheat. Theor. Appl. Genet. 2019, 132, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Y.; Isham, K.; Zhao, W.; Wheeler, J.; Klassen, N.; Hu, Y.; Bonman, J.M.; Chen, J. QTL identification and KASP marker development for productive tiller and fertile spikelet numbers in two high-yielding hard white spring wheat cultivars. Mol. Breed. 2018, 38, 135. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Conway, B.; Miller, D.; Marshall, D.; Cooper, A.; Murphy, P.; Chao, S.; Brown-Guedira, G.; Costa, J. Quantitative Trait Loci Mapping for Spike Characteristics in Hexaploid Wheat. Plant Genome 2017, 10, plantgenome2016-10. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Xu, Z.; Fan, X.; Zhou, Q.; Cao, J.; Wang, F.; Ji, G.; Yang, L.; Feng, B.; Wang, T. A Genome-Wide Association Study of Wheat Spike Related Traits in China. Front. Plant Sci. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, C.; Wang, D.; Amand, P.S.; Bernardo, A.; Li, W.; He, F.; Li, L.; Wang, L.; Yuan, X.; et al. High-Resolution Genome-Wide Association Study Identifies Genomic Regions and Candidate Genes for Important Agronomic Traits in Wheat. Mol. Plant 2020, 13, 1311–1327. [Google Scholar] [CrossRef]
- Sakuma, S.; Golan, G.; Guo, Z.; Ogawa, T.; Tagiri, A.; Sugimoto, K.; Bernhardt, N.; Brassac, J.; Mascher, M.; Hensel, G.; et al. Unleashing floret fertility in wheat through the mutation of a homeobox gene. Proc. Natl. Acad. Sci. USA 2019, 116, 5182–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzay, S.; Xu, Y.; Zhang, J.; Katz, A.; Pearce, S.; Su, Z.; Fraser, M.; Anderson, J.A.; Brown-Guedira, G.; DeWitt, N.; et al. Identification of a candidate gene for a QTL for spikelet number per spike on wheat chromosome arm 7AL by high-resolution genetic mapping. Theor. Appl. Genet. 2019, 132, 2689–2705. [Google Scholar] [CrossRef] [Green Version]
- Muqaddasi, Q.H.; Brassac, J.; Koppolu, R.; Plieske, J.; Ganal, M.W.; Röder, M.S. TaAPO-A1, an ortholog of rice aberrant panicle organization 1, is associated with total spikelet number per spike in elite European hexaploid winter wheat (Triticum aestivum L.) varieties. Sci. Rep. 2019, 9, 13853. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, H.; Tian, C.; Sajjad, M.; Gao, C.; Tong, Y.; Wang, X.; Jiao, Y. Transcriptome Association Identifies Regulators of Wheat Spike Architecture. Plant Physiol. 2017, 175, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Longin, C.F.H. Phenotypic and genetic analysis of spike and kernel characteristics in wheat reveals long-term genetic trends of grain yield components. Theor. Appl. Genet. 2018, 131, 2071–2084. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.-P.; Wang, J.-S.; Yang, X.-M.; Liu, W.-H.; Gao, A.-N.; Li, X.-Q.; Li, L.-H. Inheritance and Availability of High Grain Number Per Spike in Two Wheat Germplasm Lines. J. Integr. Agric. 2012, 11, 1409–1416. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Wang, H.; Li, L.; Wu, J.; Yang, X.; Li, X.; Gao, A. QTL mapping of yield-related traits in the wheat germplasm 3228. Euphytica 2010, 177, 277–292. [Google Scholar] [CrossRef]
- Li, L.H.; Yang, X.M.; Li, X.Q.; Dong, Y.C.; Chen, X.M. Introduction of desirable genes from Agropyron cristatum into common wheat by intergeneric hybridization. Sci. Agric. Sin. 1998, 31, 1–5. [Google Scholar]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Dong, S.-S.; Xu, J.-Y.; He, W.-M.; Yang, T.-L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.; Buckler, E.; Zhang, Z. GAPIT: Genome association and prediction integrated tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Liu, X.; Wang, J.; Li, M.; Wang, Q.; Tian, F.; Su, Z.; Pan, Y.; Liu, D.; Lipka, A.E.; et al. GAPIT Version 2: An Enhanced Integrated Tool for Genomic Association and Prediction. Plant Genome 2016, 9, plantgenome2015-11. [Google Scholar] [CrossRef] [Green Version]
- Faris, J.D.; Fellers, J.P.; Brooks, S.A.; Gill, B.S. A Bacterial Artificial Chromosome Contig Spanning the Major Domestication Locus Q in Wheat and Identification of a Candidate Gene. Genetics 2003, 164, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, M.; Wu, J.; Guo, W.; Chen, Y.; Li, G.; Wang, Y.; Shi, W.; Xia, G.; Fu, D.; et al. WheatOmics: A platform combining multiple omics data to accelerate functional genomics studies in wheat. Mol. Plant 2021, 14, 1965–1968. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.-Y.; Wu, K.; Zhang, J.-P.; Liu, W.-H.; Yang, X.-M.; Li, X.-Q.; Lu, Y.-Q.; Li, L.-H. Novel and favorable genomic regions for spike related traits in a wheat germplasm Pubing 3504 with high grain number per spike under varying environments. J. Integr. Agric. 2017, 16, 2386–2401. [Google Scholar] [CrossRef]
- Guo, Z.; Slafer, G.A.; Schnurbusch, T. Genotypic variation in spike fertility traits and ovary size as determinants of floret and grain survival rate in wheat. J. Exp. Bot. 2016, 67, 4221–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, J.; Hu, Y.-G.; Chen, J. Dwarfing genes Rht4 and Rht-B1b affect plant height and key agronomic traits in common wheat under two water regimes. Field Crops Res. 2017, 204, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Rebetzke, G.; Ellis, M.; Bonnett, D.; Mickelson, B.; Condon, A.; Richards, R. Height reduction and agronomic performance for selected gibberellin-responsive dwarfing genes in bread wheat (Triticum aestivum L.). Field Crops Res. 2012, 126, 87–96. [Google Scholar] [CrossRef]
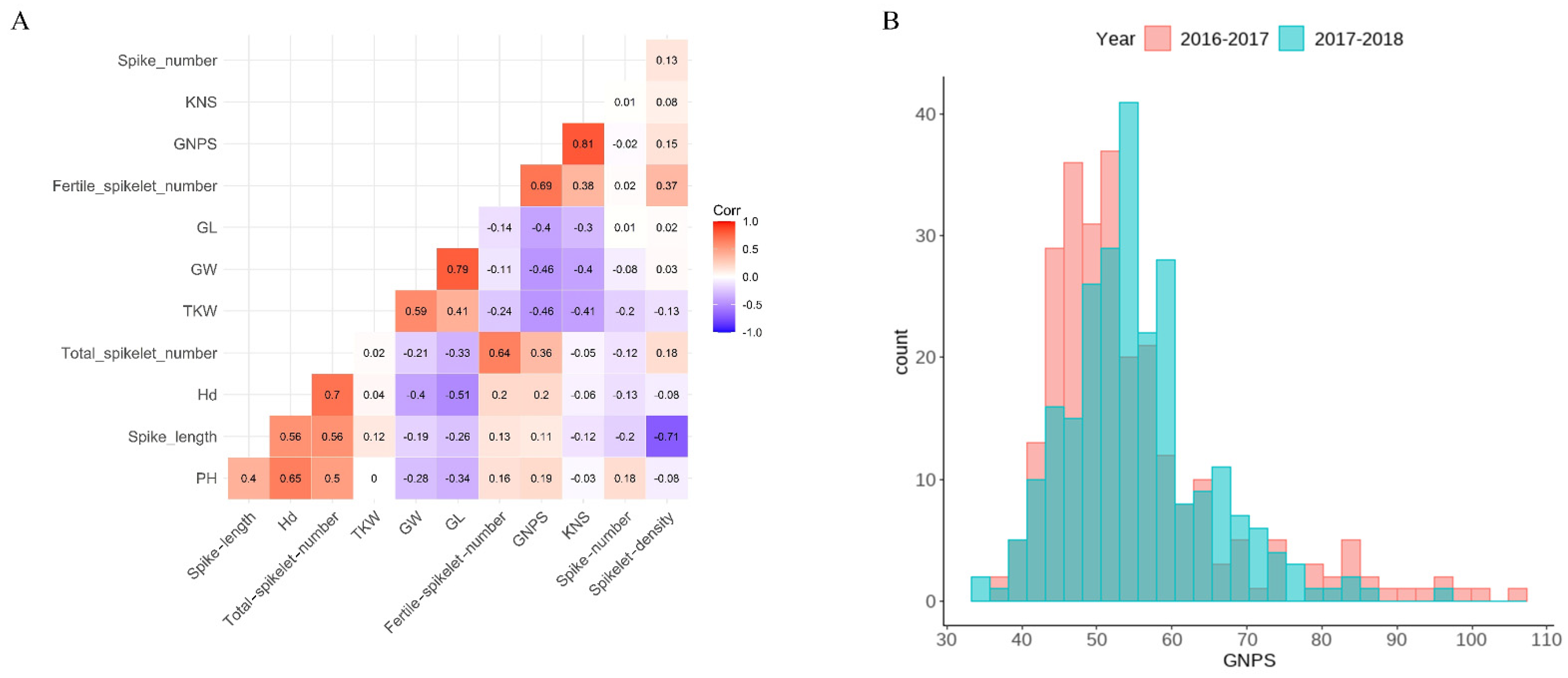
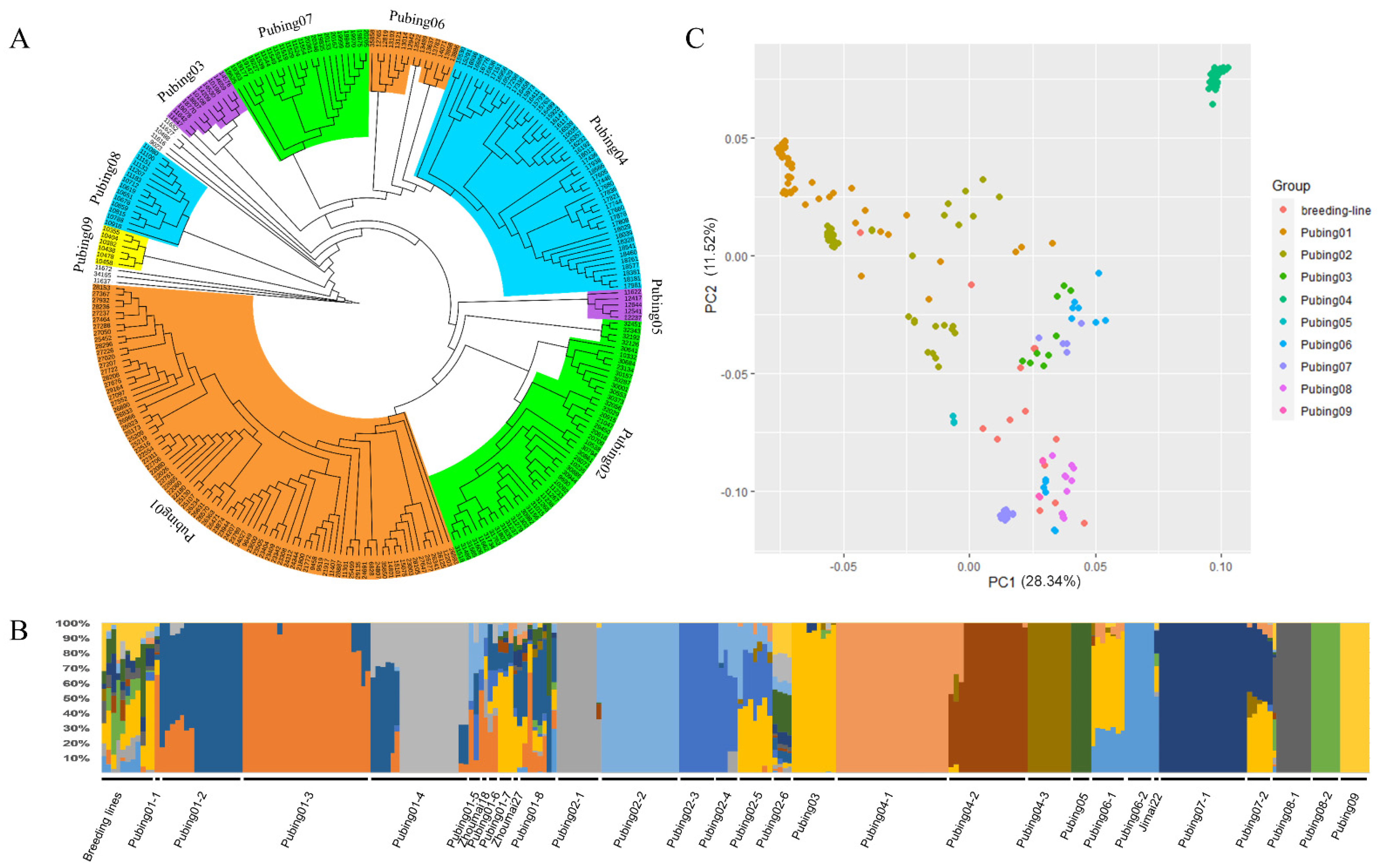
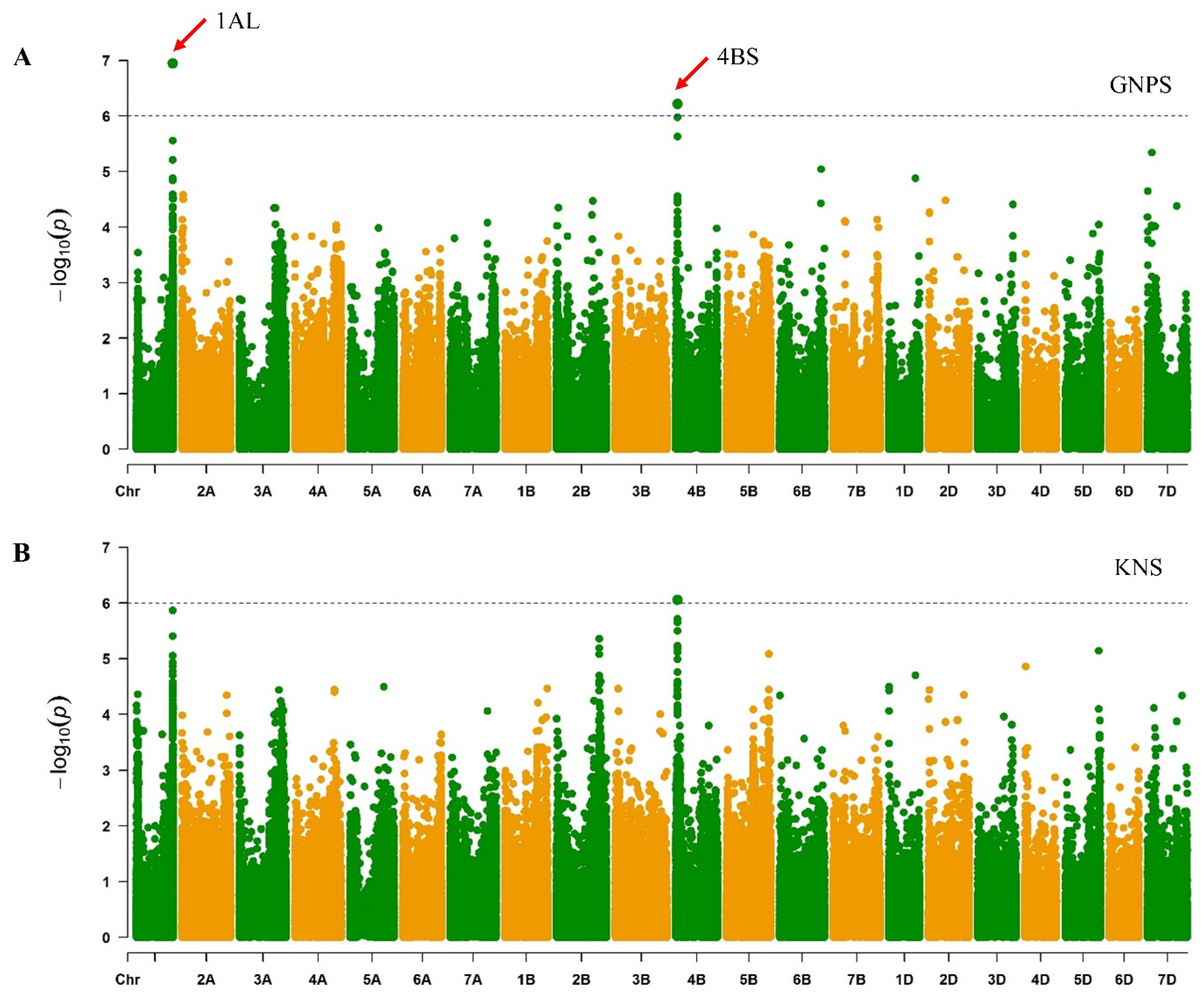
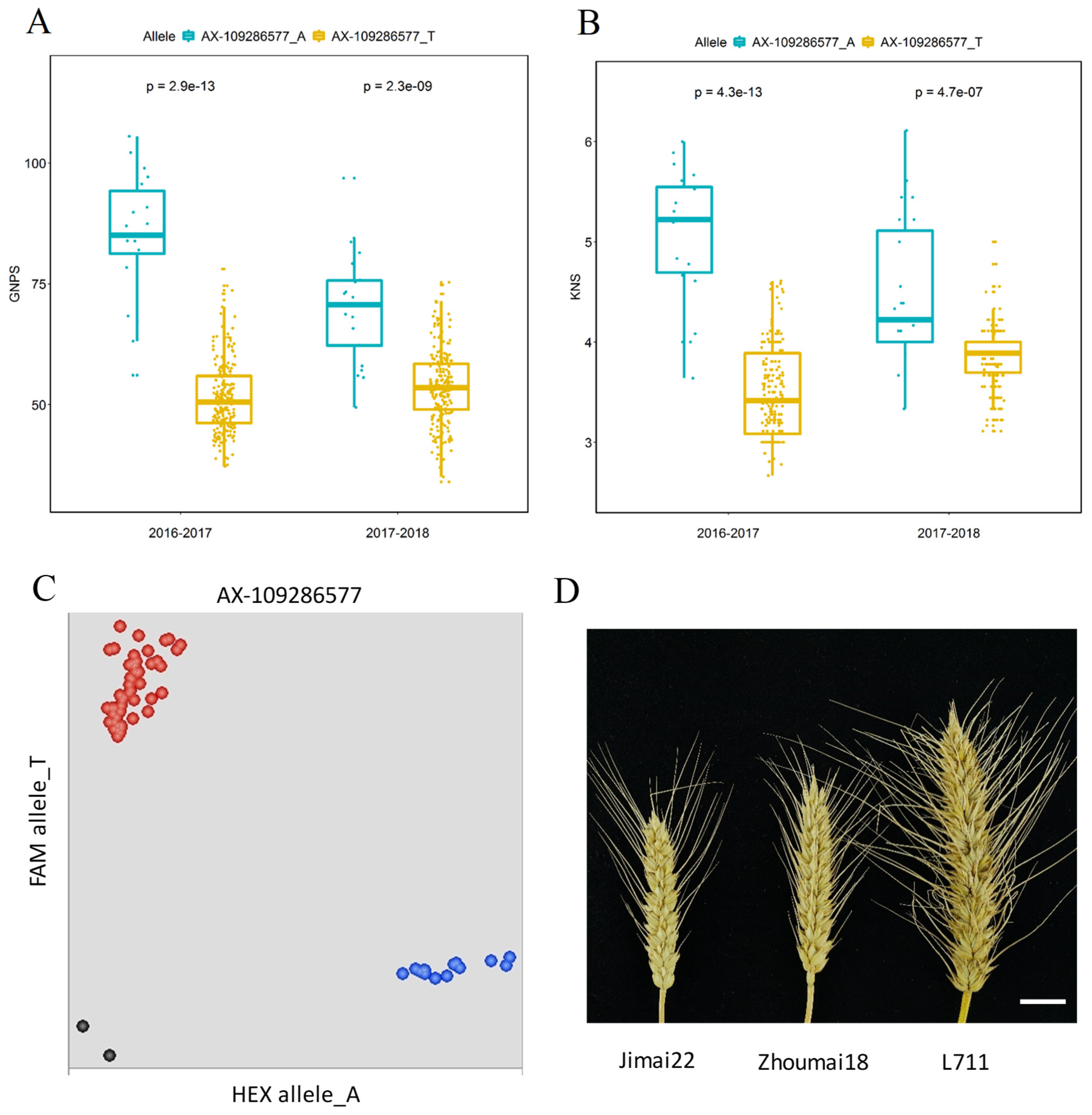
| Trait | 2016–2017 | 2017–2018 | H2 ‡ | ||||
|---|---|---|---|---|---|---|---|
| Variation Range | CV † | Median | Variation Range | CV | Median | ||
| PH(cm) | 65–115 | 9.46% | 83 | 57–93 | 9.34% | 75 | 0.91 |
| Spike_number | 5.9–17.6 | 18.77% | 9.9 | 5.2–17.3 | 15.22% | 10.8 | 0.56 |
| GNPS | 37.2–105.6 | 22.72% | 51.4 | 34.0–96.9 | 17.14% | 54.2 | 0.78 |
| Total_spikelet_number | 16.8–26.4 | 6.75% | 22.3 | 16.5–24.0 | 6.38% | 20.0 | 0.64 |
| Fertile_spikelet_number | 13.6–24.8 | 11.87% | 19.0 | 14.8–23.9 | 7.82% | 19.0 | 0.52 |
| Spike_length(cm) | 7.7–14.5 | 9.73% | 10.7 | 7.1–11.5 | 8.61% | 9.3 | 0.74 |
| Spikelet_density | 16.2–26.7 | 10.27% | 21.0 | 16.8–27.7 | 9.39% | 21.9 | 0.82 |
| KNS | 2.7–6.0 | 17.99% | 3.5 | 3.1–6.1 | 10.67% | 3.9 | 0.68 |
| TKW(g) | 32.9–60.8 | 11.20% | 49.0 | 26.1–68.9 | 14.40% | 47.2 | 0.74 |
| GW(mm) | 2.6–3.9 | 8.19% | 3.5 | 3.0–3.9 | 5.06% | 3.6 | 0.66 |
| GL(mm) | 4.8–7.6 | 7.29% | 6.6 | 5.9–7.9 | 4.05% | 6.9 | 0.65 |
| Hd | 180–188 | 0.99% | 182.0 | 166–179 | 1.24% | 175.0 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yao, Q.; Li, R.; Lu, Y.; Zhou, S.; Han, H.; Liu, W.; Li, X.; Yang, X.; Li, L. Identification of Genetic Loci on Chromosome 4B for Improving the Grain Number per Spike in Pre-Breeding Lines of Wheat. Agronomy 2022, 12, 171. https://doi.org/10.3390/agronomy12010171
Zhang J, Yao Q, Li R, Lu Y, Zhou S, Han H, Liu W, Li X, Yang X, Li L. Identification of Genetic Loci on Chromosome 4B for Improving the Grain Number per Spike in Pre-Breeding Lines of Wheat. Agronomy. 2022; 12(1):171. https://doi.org/10.3390/agronomy12010171
Chicago/Turabian StyleZhang, Jinpeng, Qifu Yao, Ruixin Li, Yuqing Lu, Shenghui Zhou, Haiming Han, Weihua Liu, Xiuquan Li, Xinming Yang, and Lihui Li. 2022. "Identification of Genetic Loci on Chromosome 4B for Improving the Grain Number per Spike in Pre-Breeding Lines of Wheat" Agronomy 12, no. 1: 171. https://doi.org/10.3390/agronomy12010171







