Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Test Setting and Experimental Conditions
2.2.1. Method for Determination of Mass Fraction of Nitrogen and Crude Protein
2.2.2. Method for Determination of the Mass Fraction of Crude Fiber
2.2.3. Method for Determination of Mass Fraction of Crude Fat
2.2.4. Method for Determination of Mass Fraction of Crude Ash
2.2.5. Method of Copper Determination
2.2.6. Method of Manganese Determination
2.2.7. Method of Iron Determination
2.2.8. Method of Zinc Determination
2.2.9. Method of the Mass Fraction of Calcium Determination
2.2.10. Method of the Mass Fraction of Phosphorus Determination
2.2.11. Method of Moisture Content Determination
2.2.12. Method of Vitamin C Determination
2.2.13. Method of the Carotene Determination
2.3. Statistical Processing
3. Results
4. Discussion
5. Conclusions
- The research results prove the usefulness of nettle cultivation. By choosing the right variety, satisfactory productivity, i.e., nutrient, mineral, and vitamin content, is possible to obtain.
- The research results obtained in our study show the advantages of the modified nettle cultivars over the Avicenna cultivar.
- Avicenna cultivar and new cultivars of stinging nettle UD 32/06 and UD 12/16, obtained from the source material collected in the Cretaceous south of the Central Russian Upland, by their nutritional properties, can become the basis for obtaining phytobiotics for the poultry industry.
- Biological resources of wild forms of U. dioica L. from the European south of Russia are a valuable source material for obtaining varieties with high productivity of aboveground mass and stable seed productivity. They provide a high-quality dry weight yield at the level of 0.613–0.683 kg·m−2 and seed productivity of 8.85–10.00 g·m−2.
- The share of the influence of the genotype of nettle varieties on the quality of the aboveground mass is significantly higher than the influence of the conditions of the year (the accounted strength influence was from 91.1 to 97.1%). This indicates the crucial importance of breeding methods for improving the quality of nettle raw materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rokochinskiy, A.; Frolenkova, N.; Turcheniuk, V.; Volk, P.; Prykhodko, N.; Tykhenko, R.; Openko, I. The variability of natural and climatic conditions in investment projects in the field of nature management. J. Water Land Dev. 2021, 48, 48–54. [Google Scholar] [CrossRef]
- Godlewska, K.; Biesiada, A.; Michalak, I.; Pacyga, P. The Effect of Botanical Extracts Obtained through Ultrasound-Assisted Extraction on White Head Cabbage (Brassica Oleracea L. Var. Capitata L.) Seedlings Grown under Controlled Conditions. Sustainability 2020, 12, 1871. [Google Scholar] [CrossRef] [Green Version]
- Jankauskienė, Z.; Gruzdevienė, E. Stinging nettle (Urtica dioica L.)—An alternative fibre plant. In Proceedings of the International Conference: Opportunities and Challenges of National Economic Development, Rezekne, Latvia, 17 April 2008; pp. 175–182. [Google Scholar]
- Bisht, S.; Bhandari, S.; Bisht, N.S. Urtica dioica (L): An undervalued, economically important plant. Agric. Sci. Res. J. 2012, 2, 250–252. [Google Scholar]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; Di Lonardo, S.; Praczyk, M.; Wielgusz, K. The potential of stinging nettle (Urtica dioica L.) as a crop with multiple uses. Ind. Crops Prod. 2014, 68, 42–49. [Google Scholar] [CrossRef]
- Vogl, C.; Hartl, A. Production and processing of organically grown fiber nettle (Urtica dioica L.) and its potential use in the natural textile industry: A review. Am. J. Altern. Agric. 2003, 18, 119–128. [Google Scholar] [CrossRef]
- Codling, E.E.; Rutto, K.L. Stinging nettle (Urtica dioica L.) Growth and mineral uptake from lead-arsenate contaminated orchard soils. J. Plant Nutr. 2014, 37, 393–405. [Google Scholar] [CrossRef]
- Assaf, H.K.; Nafady, A.M.; Allam, A.E.; Hamed, A.N.; Kamel, M.S. Phytochemistry and biological activity of family “Urticaceae”: A review (1957–2019). J. Adv. Biomed. Pharm. Sci. 2019, 3, 150–176. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. 2016. Available online: https://www.efsa.europa.eu (accessed on 30 October 2021).
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B.; Gateau, K.S.H. Carotenoids of Microalgae Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2017, 17, 1140–1172. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products—status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Botanical Pesticides for Eco-Friendly Pest Management. In Pesticides in Crop Production; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K., Singh, S., Prasad, S.M., Chauhan, D.K., Eds.; Wiley: Chichester, UK, 2020; pp. 181–193. [Google Scholar]
- Voytovych, I.; Malovanyy, M.; Zhuk, V.; Mukha, O. Facilities and problems of processing organic wastes by family-type bio-gas plants in Ukraine. J. Water Land Dev. 2020, 45, 185–189. [Google Scholar] [CrossRef]
- Rivera, M.; Wright, E.; Salice, S.; Fabrizio, M. Effect of plant preparations on lettuce yield. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 933, Lisbon, Portugal, 22–27 August 2010; pp. 173–179. [Google Scholar] [CrossRef]
- Peterson, R.; Jensen, P. Effects of Nettle Water on Growth and Mineral Nutrition of Plants. I. Composition and Properties of Nettle Water. Biol. Agric. Hortic. 1985, 2, 303–314. [Google Scholar] [CrossRef]
- Garmendia, A.; Raigón, M.D.; Marques, O.; Ferriol, M.; Royo, J.; Merle, H. Effects of nettle slurry (Urtica dioica L.) used as foliar fertilizer on potato (Solanum tuberosum L.) yield and plant growth. PeerJ 2018, 6, e4729. [Google Scholar] [CrossRef] [PubMed]
- Bozsik, A. Studies on aphicidal efficiency of different stinging nettle extracts. Anz. Schädlingskd. Pfl. Umwelt. 1996, 69, 21–22. [Google Scholar] [CrossRef]
- Dabrowski, Z.T.; Seredynska, U. Characterisation of the Two-Spoted Spider Mite (Tetranychus Urticae Koch, Acari: Tetranychidae), Response to Aqueous Extracts from Selected Plant Species. J. Plant Prot. Res. 2007, 47, 113–124. [Google Scholar]
- Totev, T. Research into growing common nettle for fodder. Rasteviev’d. Nauk. 1964, 1, 95–104. [Google Scholar]
- Rejlová, L.; Chrtek, J.; Trávníček, P.; Lučanová, M.; Vít, P.; Urfus, T. Polyploid evolution: The ultimate way to grasp the nettle. PLoS ONE 2019, 14, e0218389. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.J.; Crompton, C.W.; Woodland, D.W. The biology of canadian weeds: 21. Urtica dioica L. Can. J. Plant Sci. 1977, 57, 491–498. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Rawat, D. Medicinal plants of the family Caryophyllaceae: A review of ethno-medicinal uses and pharmacological properties. Integr. Med. Res. 2015, 4, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, A.A.H.; Otmani, I.S.E.; Derfoufi, S.; Benmoussa, A. Highlights on nutritional and therapeutic value of stinging nettle (Urtica Dioica). Int. J. Pharm. Pharm. Sci. 2015, 7, 8–14. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/8165 (accessed on 17 November 2021).
- Grauso, L.; De Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Moula, N.; Sadoudi, A.; Touazi, L.; Leroy, P.; Geda, F. Effects of stinging nettle (Urtica dioica) powder on laying performance, egg quality, and serum biochemical parameters of Japanese quails. Anim. Nutr. 2019, 5, 410–415. [Google Scholar] [CrossRef]
- Biskupek-Korell, B.; Fischer, H.; Knapwost, C.; Schneider, C.; Wartenberg, S. Breeding of improved clones of Urtica dioica L. with higher fibre contents and qualities. In Proceedings of the NAROSSA®—16th International Conference for Renewable Resources and Plant Biotechnology, Magdeburg, Germany, 7–8 June 2010; Volume 16. [Google Scholar]
- Krjukova, V.; Nekrasova, E. Modern aspects of phitobiotics administration at the poultry. Issues Leg. Regul. Veter-Med. 2020, 4, 107–110. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Trofimov, I.A.; Trofimova, L.S.; Yakovleva, E.P. Fodder’ Production is one of the Basic Growth Factors for Productivity and Agricultural Steadiness. Zemledeliye 2012, 4, 20–22. [Google Scholar]
- Kosolapov, V.M.; Chuikov, V.A.; Khudyakova, H.K.; Kosolapova, V.G. Physico-Chemical Methods of Feed Analysis; Printing House of the Russian Agricultural Academy: Moscow, Russia, 2014; 344p, ISBN 978-5-906592-28-6. Available online: https://www.elibrary.ru/item.asp?id=22447138 (accessed on 30 October 2021).
- Sun, H.; Tang, J.W.; Fang, C.L.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poult. Sci. 2013, 92, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Samolińska, W.; Grela, E.; Kowalczuk-Vasilev, E.; Kiczorowska, B.; Klebaniuk, R.; Hanczakowska, E. Evaluation of garlic and dandelion supplementation on the growth performance, carcass traits, and fatty acid composition of growing-finishing pigs. Anim. Feed Sci. Technol. 2019, 259, 114316. [Google Scholar] [CrossRef]
- Ndomou, S.C.H.; Djikeng, F.T.; Teboukeu, G.B.; Doungue, H.T.; Foffe, H.A.K.; Tiwo, C.T.; Womeni, H.M. Nutritional value, phytochemical content, and antioxidant activity of three phytobiotic plants from west Cameroon. J. Agric. Food Res. 2021, 3, 100105. [Google Scholar] [CrossRef]
- Rajput, P.; Chaudhary, M.; Sharma, R.A. Phytochemical and pharmacological importance of genus Urtica—A review. Int. J. Pharm. Sci. Res. 2018, 9, 1387–1396. [Google Scholar] [CrossRef]
- Andrianova, E.; Egorov, I. New generation protein supplement in combined feeds for broiler chickens. E3S Web Conf. 2021, 262, 02001. [Google Scholar] [CrossRef]
- Ahmadipour, B.; Khajali, F. Expression of antioxidant genes in broiler chickens fed nettle (Urtica dioica) and its link with pulmonary hypertension. Anim. Nutr. 2019, 5, 264–269. [Google Scholar] [CrossRef]
- Чернявских, В.И. Биoлoгические ресурсы Urtica dioica L.: направления исследoваний и перспективы испoльзoвания. Пoлевoй Журнал Биoлoга 2019, 1, 131–149. [Google Scholar] [CrossRef]
- Dumacheva, E.V.; Cherniavskih, V.I. Biological Potential of Legume Grasses in the Natural Cenoses on Eroded Agricultural Lands of the Central Chernozem Zone. Kormoproyzvodstvo 2014, 4, 8–11. [Google Scholar]
- Cherniavskih, V.I.; Titovsky, A.G.; Sharko, R.A.; Shinkarenko, O.V.; Dumacheva, E.V. Experience of Breeding and Seed Production of Alfalfa and other Grasses In CJSC «Krasnaya Yaruga Grain Company». Dostizheniya Nauk. I Tekhniki APK 2012, 12, 14–17. [Google Scholar]
- Cherniavskih, V.I.; Sidelnikov, N.I.; Dumacheva, E.V.; Borodaeva, Z.A.; Glubsheva, T.N.; Gorbacheva, A.A.; Vorobyova, O.V.; Korolkova, S. Biological Resources Of Natural Forage Grassland Of The Cretaceous South Of The European Russia. EurAsian J. BioSci. 2019, 13, 845–849. [Google Scholar]
- Cherniavskih, V.I.; Dumacheva, E.V.; Sidelnikov, N.I.; Lisetsky, F.N.; Gagieva, L.C. Use of Hissopus Officinalis L. Culture For Phytoamelioration of Carbonate Outcrops of Anthropogenic Origin The South of European Russia. Indian J. Ecol. 2019, 46, 221–226. [Google Scholar]
- Dumacheva, E.V.; Cherniavskih, V.I.; Popov, A.M.; Chasovitin, A.Y. Nettle Urtica Avicenna. Selection Achievement Patent RUS 10668, Application No. 75482, 15 August 2018. [Google Scholar]
- Dumacheva, E.V.; Cherniavskih, V.I.; Prisniy, A.V.; Vorobyova, O.V.; Gorbacheva, A.A.; Glubsheva, T.N.; Grigorenko, S.E. Studies of Biological Resources of Urtica Dioica L. as Initial Material for Breeding. J. Int. Pharm. Res. 2018, 45, 473–476. [Google Scholar]
- State Commission of the Russian Federation on Testing and Protection of Breeding Achievements. Test Guidelines for DUS-testing of Urtica dioica L.; RTG/1142/1; FGBU: Moscow, Russia, 2019; Volume 6, pp. 485–494. [Google Scholar]
- Medvedsky, V.A.; Bazylev, M.V.; Bolshakova, L.P.; Munayar, H.F. Biological Bases of Mineral Nutrition of Agricultural Birds. Scientific Review. Biol. Sci. 2016, 2, 93–108. Available online: https://science-biology.ru/ru/article/view?id=998 (accessed on 16 October 2021).
- Rutto, L.K.; Xu, Y.; Ramírez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkova, K.; Polesny, Z. Ethnobotanical review of wild edible plants used in the Czech Republic. J. Appl. Bot. Food Qual. 2015, 88, 49–67. [Google Scholar] [CrossRef]
- Anishchenko, L.; Potsepai, S.N.; Semenova, Y.G.; Bel’chenko, S.A. Dynamic Rows Of Grass Vegetation Associations of Re-growth Successions And Accumulation of Heavy Metals on The Territories of The Former Rural Settlements. Int. J. Appl. Fundam. Res. 2016, 4, 15. [Google Scholar]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ushanova, V.M.; Lebedeva, O.I.; Relyakh, S.M. Investigation of the influence of growing conditions on the chemical com-position of stinging nettle. Chem. Plant Raw Mater. 2001, 3, 97–104. [Google Scholar]
- Biesiada, A.; Kucharska, A.; Sokół-Łętowska, A.; Kuś, A. Effect of the Age of Plantation and Harvest Term on Chemical Composition and Antioxidant Avctivity of Stinging Nettle (Urtica dioica L.). Ecol. Chem. Eng. 2010, 17, 1061–1067. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BPG8-0060-0014 (accessed on 30 October 2021).
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef]
- Sadlik, S. Production of Nettle (Urtica dioica), Environmental and Economic Valuation in Conventional Farming. Master’s Thesis, Department of Economics and Management Agricultural Economics, University of Helsinki, Helsinki, Finland, 2019. [Google Scholar]
- Jankauskiene, Z.; Gruzdevienė, E. Changes in the productivity of wild and cultivated stinging nettle (Urtica dioica L.) as influenced by the planting density and crop age, Produktyvumo pokyčiai skirtingo sodinimo tankio ir įvairaus amžiaus didžiosios dilgėlės (Urtica dioica L.) pasėlyje. Zemdirb.-Agric. 2015, 102, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2015, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Nencu, I.; Vlase, L.; Istudor, V.; Mircea, T. Preliminary Research Regarding Urtica urens L. and Urtica dioica L. Amino Acids 2015, 63, 710–715. [Google Scholar]
- Radman, S.; Žutic’, I.; Fabek, S.; Žlabur, J.Š.; Benko, B.; Toth, N.; Čoga, L. Influence of nitrogen fertilization on chemical composition of cultivated nettle. Emir. J. Food. Agric. 2015, 27, 889–896. [Google Scholar]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of un-derutilized stinging nette leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef] [PubMed]


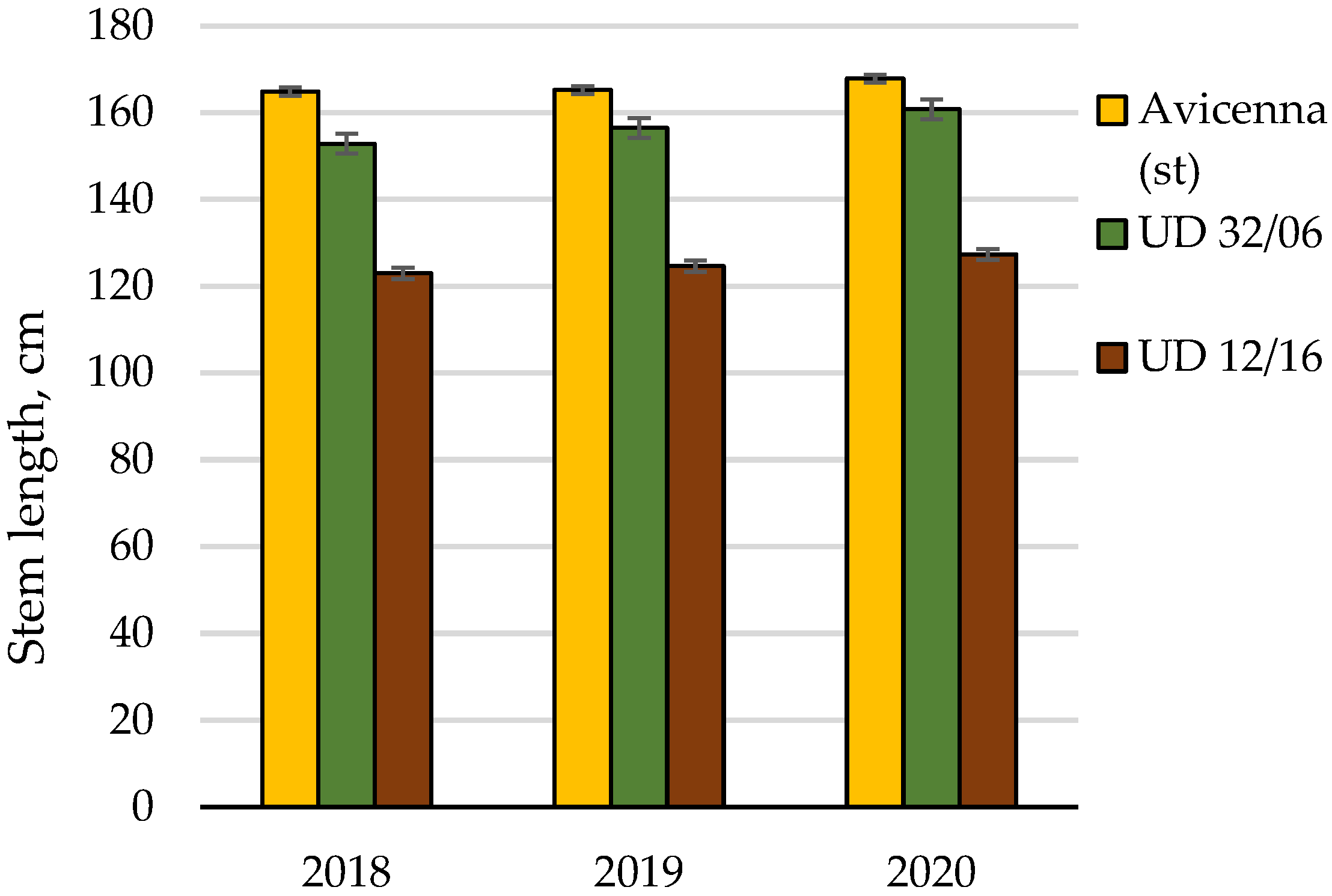

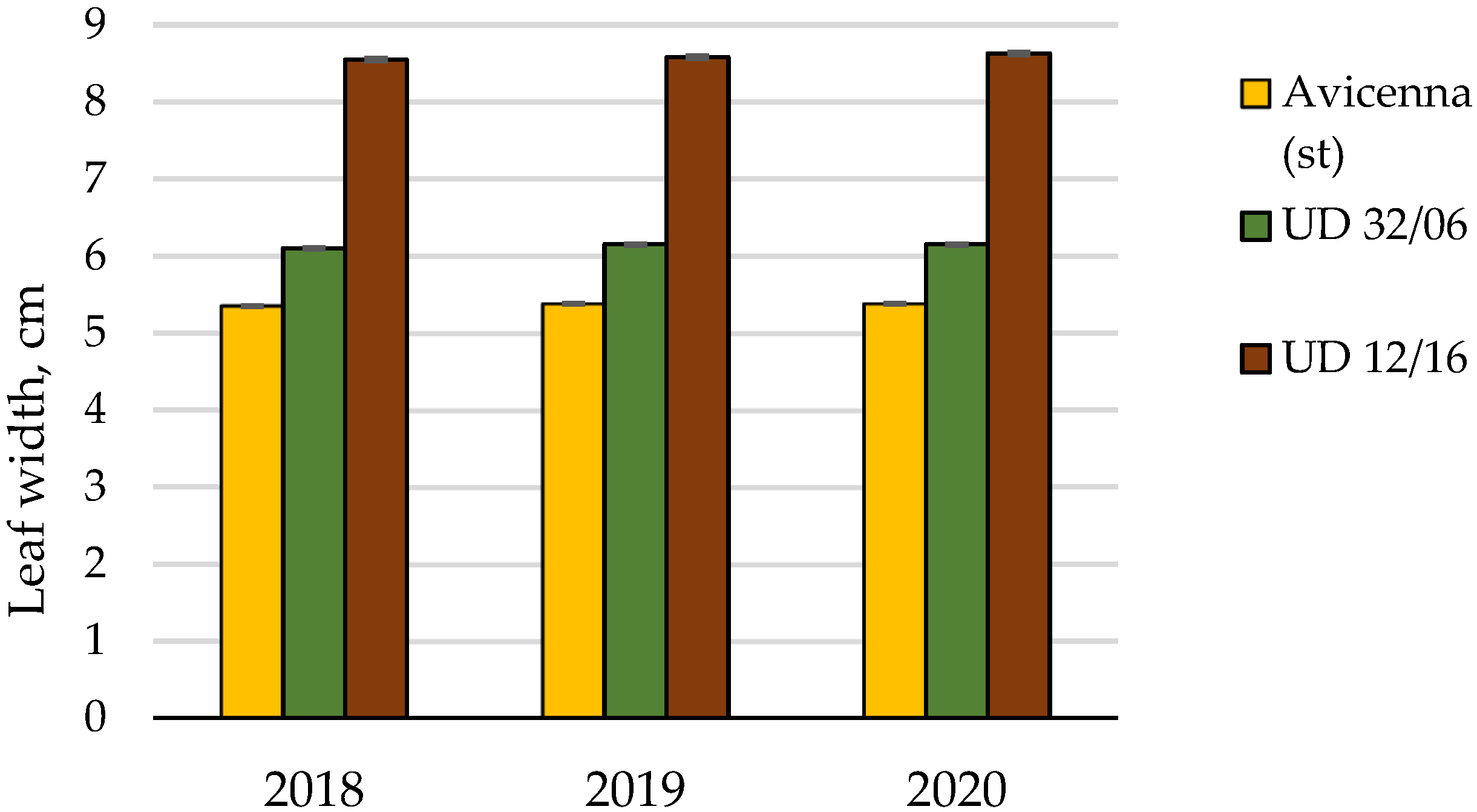

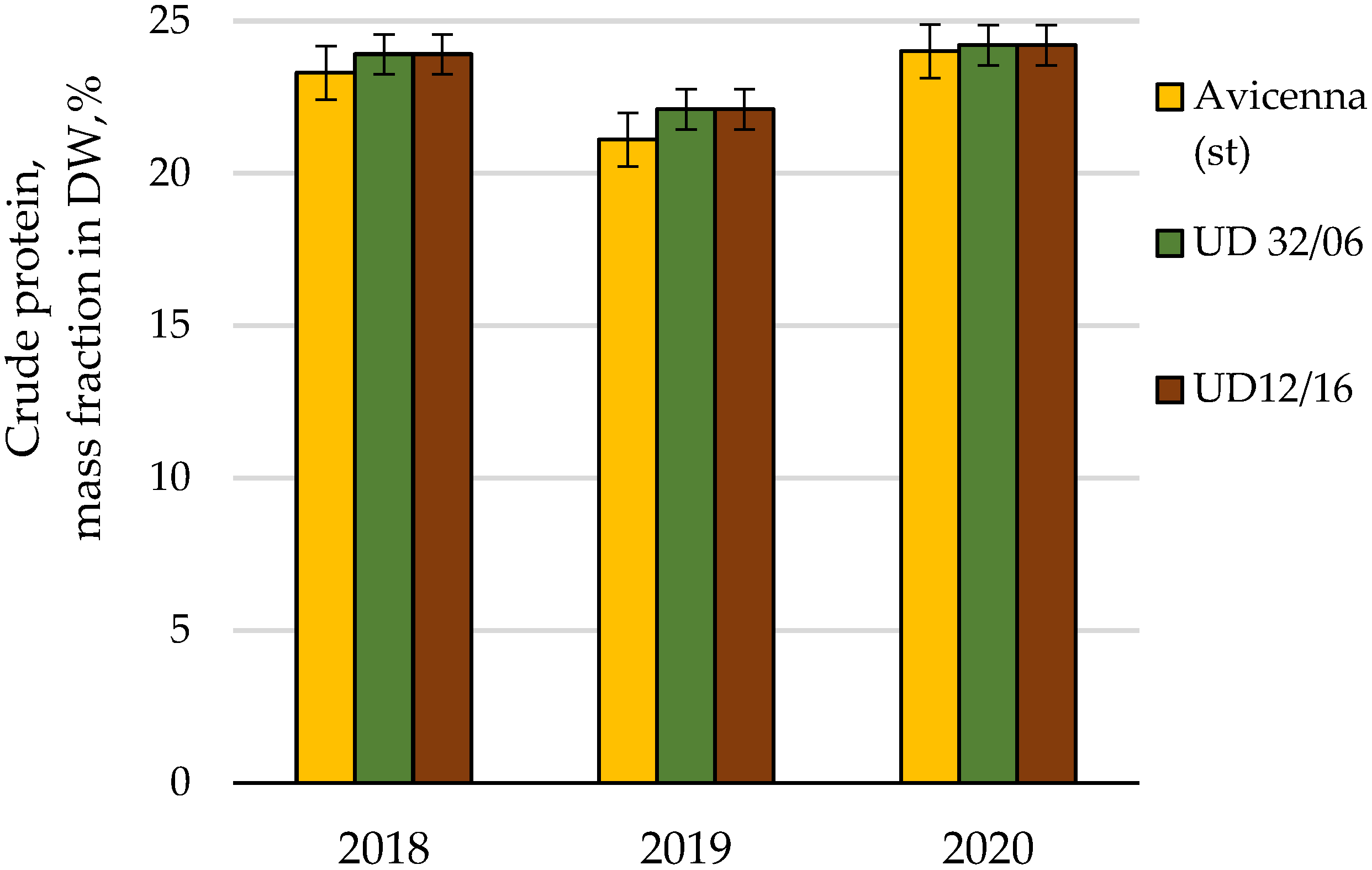
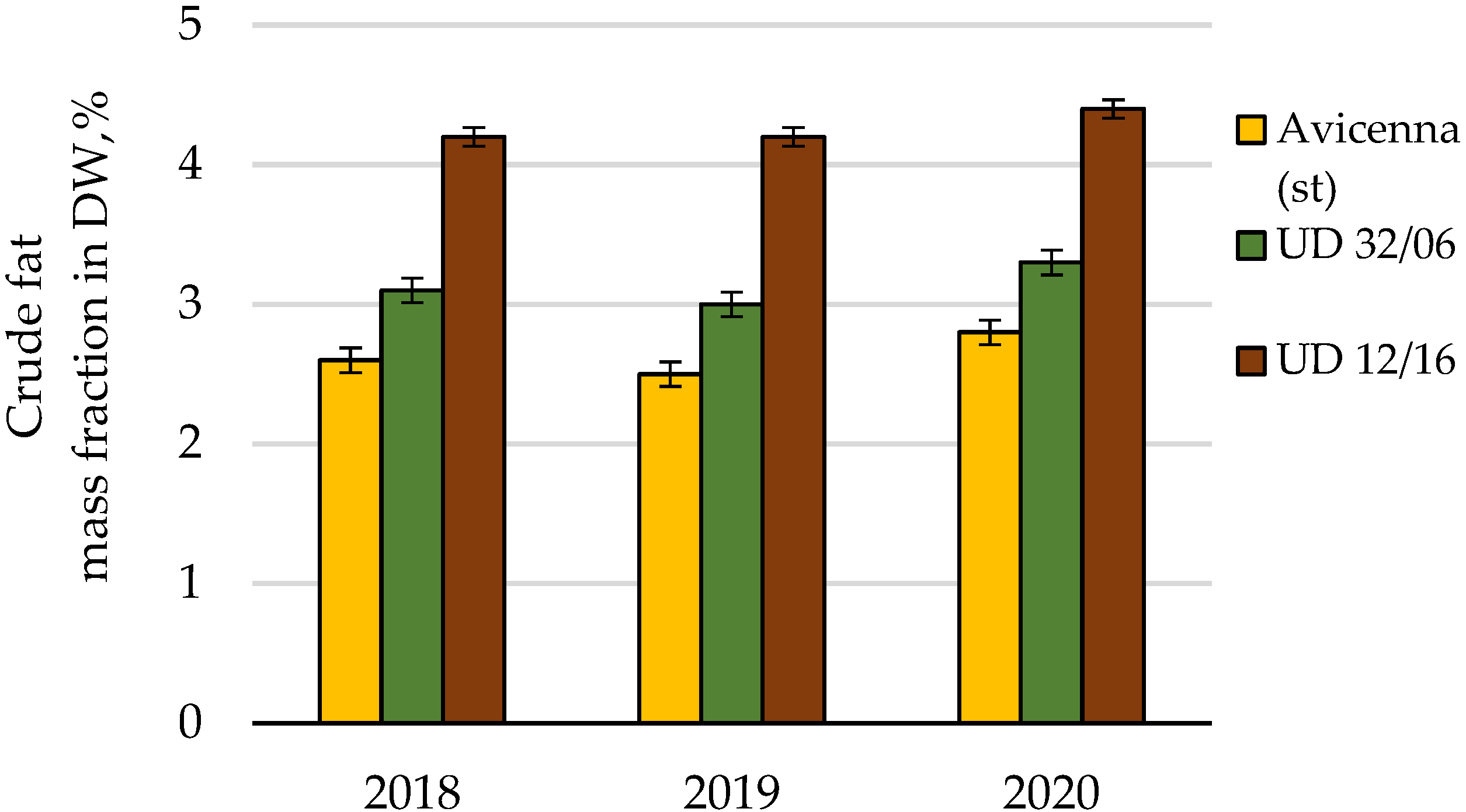
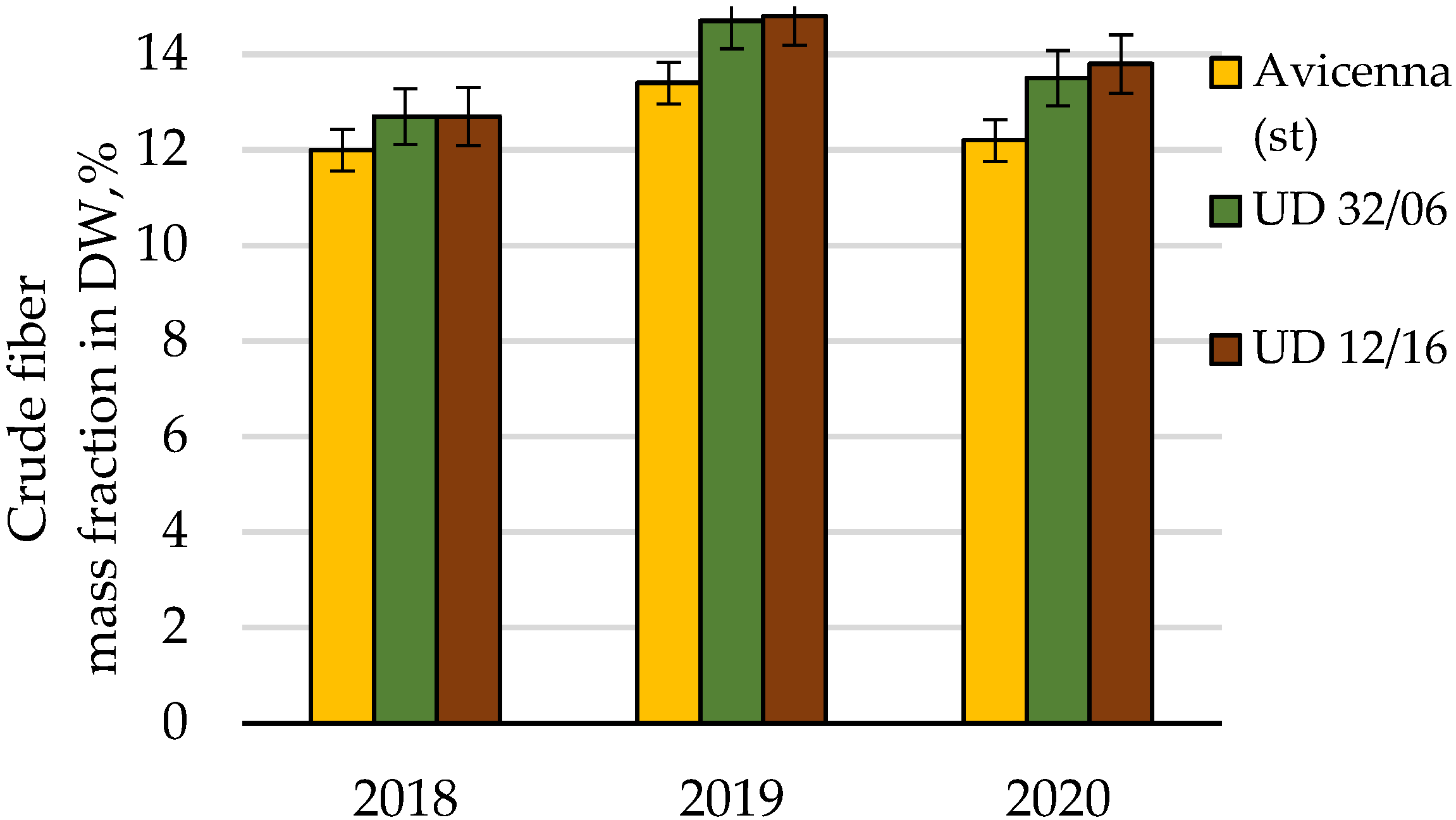
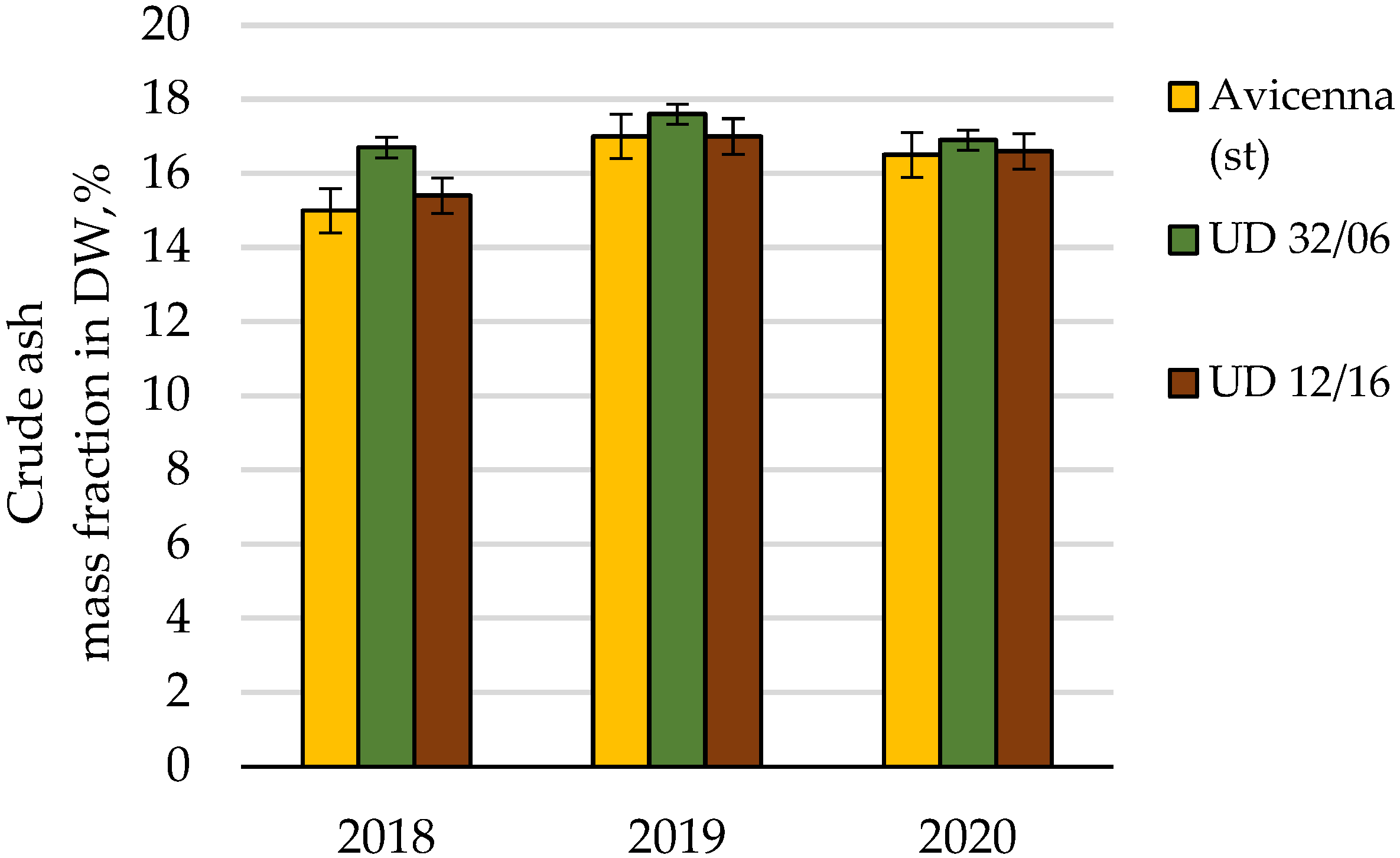



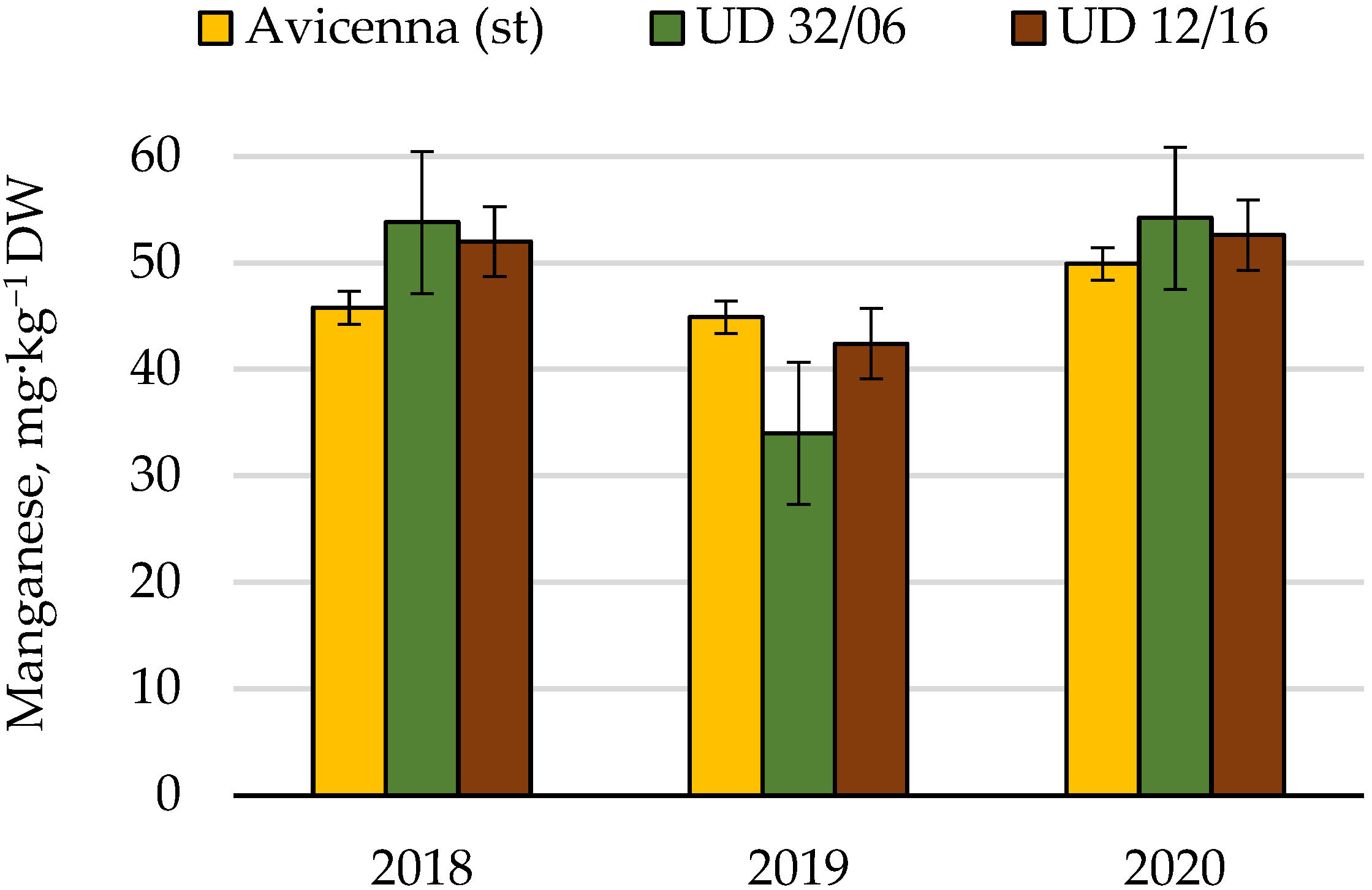

| № | Type/Cultivar | Genealogy | Geographical Co-Ordinates |
|---|---|---|---|
| 1 | Breeding variety Avicenna | Patent № RUS 10668 [43] | |
| 2 | Savage class UD 32/06 | Gully–ravine complex with chalk outcrops Valuisky district, Belgorod region, Russia | 50.287098 N 38.324434 E |
| 3 | Savage class UD 12/06 | Floodplain of the Seversky Donets River, Belgorodsky District, Belgorod Region, Russia | 50.638359 N 36.647003 E |
| Parameter | Description |
|---|---|
| Soil | typical black soil (typical black soil) |
| Humus (according to Tyurin), % | 4.9 |
| pH soil | 6.8 |
| P2O5, mg·kg−1 | 120 |
| K2O, mg·kg−1 | 180 |
| Average annual rainfall, mm | 553 |
| Average annual temperature, °C | +6.3 |
| Frost-free period, months | 8–9 |
| Height above sea level, m | 181 |
| Cultivar | 2018 | 2019 | 2020 |
|---|---|---|---|
| Green mass yield, kg·m−2 | |||
| Avicenna (st) | 2.634 | 2.874 | 2.779 |
| UD 32/06 | 3.072 | 3.400 | 3.411 |
| UD 12/16 | 3.366 | 3.539 | 3.519 |
| 1LSD0.05 | 0.129 | 0.388 | 0.327 |
| Ff | 92.7 | 9.3 | 17.1 |
| F0.05 | 5.1 | 5.1 | 5.1 |
| Dry weight yield, kg·m−2 | |||
| Avicenna (st) | 0.613 | 0.683 | 0.642 |
| UD 32/06 | 0.683 | 0.823 | 0.826 |
| UD 12/16 | 0.642 | 0.867 | 0.852 |
| 1LSD0.05 | 0.044 | 0.115 | 0.106 |
| Ff | 65.1 | 8.0 | 13.5 |
| F0.05 | 5.1 | 5.1 | 5.1 |
| Seed yield, g·m−2 | |||
| Avicenna (st) | 8.85 | 9.45 | 10.00 |
| UD 32/06 | 10.60 | 11.69 | 13.20 |
| UD 12/16 | 10.90 | 12.10 | 14.00 |
| 1LSD0.05 | 0.37 | 0.45 | 0.92 |
| Ff | 99.4 | 113.4 | 61.1 |
| F0.05 | 5.1 | 5.1 | 5.1 |
| Weight of 1000 seeds, g | |||
| Avicenna (st) | 0.186 | 0.199 | 0.210 |
| UD 32/06 | 0.206 | 0.210 | 0.233 |
| UD 12/16 | 0.229 | 0.218 | 0.238 |
| 1LSD0.05 | 0.005 | 0.006 | 0.007 |
| Ff | 185.4 | 25.9 | 50.2 |
| F0.05 | 5.1 | 5.1 | 5.1 |
| Effective Feature | Source of Variation | D | n − 1 | s2 | Ff | Fst0.05 | h2x |
|---|---|---|---|---|---|---|---|
| Green mass yield | Cumulative | 0.944 | 8 | 100.0 | |||
| Conditions of the year | 0.107 | 2 | 11.3 | ||||
| Cultivar | 0.823 | 2 | 0.411 | 117.1 | 6.9 | 87.2 | |
| Random | 0.014 | 4 | 0.004 | 1.5 | |||
| Dry weight yield | Cumulative | 0.085 | 8 | 100.0 | |||
| Conditions of the year | 0.038 | 2 | 44.3 | ||||
| Cultivar | 0.037 | 2 | 0.019 | 7.4 | 6.9 | 43.9 | |
| Random | 0.010 | 4 | 0.002 | 11.8 | |||
| Seed yield | Cumulative | 23.4 | 8 | 100.0 | |||
| Conditions of the year | 7.9 | 2 | 33.8 | ||||
| Cultivar | 14.4 | 2 | 7.2 | 26.8 | 6.9 | 61.6 | |
| Random | 1.1 | 4 | 0.3 | 4.6 | |||
| Weight of 1000 seeds | Cumulative | 0.0023 | 8 | 100.0 | |||
| Conditions of the year | 0.0007 | 2 | 31.8 | ||||
| Cultivar | 0.0014 | 2 | 6.84 × 10−4 | 14.8 | 6.9 | 60.1 | |
| Random | 0.0002 | 4 | 4.6186 × 10−5 | 8.1 |
| Cultivar | 2018 | 2019 | 2020 |
|---|---|---|---|
| Foliage, % | |||
| Avicenna (st) | 35.25 ± 7.38 | 30.45 ± 6.05 | 32.25 ± 4.88 |
| UD 32/06 | 38.63 ± 4.94 | 33.30 ± 5.15 | 35.23 ± 5.73 |
| UD 12/16 | 53.93 ± 2.79 | 40.63 ± 2.83 | 48.90 ± 5.05 |
| The ratio of the length and width of the leaf | |||
| Avicenna (st) | 1.76 ± 0.08 | 1.82 ± 0.09 | 1.98 ± 0.13 |
| UD 32/06 | 1.91 ± 0.12 | 2.15 ± 0.08 | 2.25 ± 0.07 |
| UD 12/16 | 1.60 ± 0.11 | 1.83 ± 0.07 | 1.90 ± 0.05 |
| Productive Feature | Source of Variation | D | n − 1 | s2 | Ff | Fst0.05 | h2x |
|---|---|---|---|---|---|---|---|
| Stem length | Cumulative | 2816.9 | 8 | 100.0 | |||
| Conditions of the year | 39.9 | 2 | 1.4 | ||||
| Cultivar | 2770.3 | 2 | 1385.2 | 826.3 | 6.9 | 98.3 | |
| Random | 6.7 | 4 | 1.7 | 0.2 | |||
| Foliage | Cumulative | 502.3 | 8 | 100.0 | |||
| Conditions of the year | 91.5 | 2 | 18.2 | ||||
| Cultivar | 385.8 | 2 | 192.9 | 30.8 | 6.9 | 76.8 | |
| Random | 25.0 | 4 | 6.3 | 5.0 | |||
| Leaf length | Cumulative | 49.8 | 8 | 100.0 | |||
| Conditions of the year | 6.5 | 2 | 13.0 | ||||
| Cultivar | 42.3 | 2 | 21.2 | 84.6 | 6.9 | 85.0 | |
| Random | 1.0 | 4 | 0.2 | 2.0 | |||
| Leaf width | Cumulative | 16.942 | 8 | 100.00 | |||
| Conditions of the year | 0.004 | 2 | 0.02 | ||||
| Cultivar | 16.937 | 2 | 8.468 | 30548.3 | 6.9 | 99.97 | |
| Random | 0.001 | 4 | 0.0003 | 0.01 | |||
| The ratio of the length and width of the leaf | Cumulative | 0.311 | 8 | 100.0 | |||
| Conditions of the year | 0.126 | 2 | 40.6 | ||||
| Cultivar | 0.173 | 2 | 0.086 | 29.0 | 6.9 | 55.6 | |
| Random | 0.012 | 4 | 0.003 | 3.8 | |||
| Length of female inflorescence | Cumulative | 1665.0 | 8 | 100.0 | |||
| Conditions of the year | 145.0 | 2 | 8.7 | ||||
| Cultivar | 1487.1 | 2 | 743.6 | 90.6 | 6.9 | 89.3 | |
| Random | 32.8 | 4 | 8.2 | 2.0 |
| Productive Feature | Source of Variation | D | n − 1 | s2 | Ff | Fst0.05 | h2x |
|---|---|---|---|---|---|---|---|
| Crude protein | Cumulative | 10.56 | 8 | 100.0 | |||
| Conditions of the year | 9.62 | 2 | 91.1 | ||||
| Cultivar | 0.55 | 2 | 0.27 | 2.8 | 6.9 | 5.2 | |
| Random | 0.39 | 4 | 0.10 | 3.7 | |||
| Crude fat | Cumulative | 4.05 | 8 | 100.0 | |||
| Conditions of the year | 0.11 | 2 | 2.7 | ||||
| Cultivar | 3.93 | 2 | 1.96 | 196.5 | 6.9 | 97.1 | |
| Random | 0.01 | 4 | 0.01 | 0.2 | |||
| Crude fiber | Cumulative | 8.14 | 8 | 100.0 | |||
| Conditions of the year | 5.13 | 2 | 63.0 | ||||
| Cultivar | 2.77 | 2 | 1.38 | 22.5 | 6.9 | 34.0 | |
| Random | 0.25 | 4 | 0.06 | 3.0 | |||
| Vitamin C | Cumulative | 2393.4 | 8 | 100.0 | |||
| Conditions of the year | 144.9 | 2 | 6.1 | ||||
| Cultivar | 1274.8 | 2 | 637.4 | 2.6 | 6.9 | 53.3 | |
| Random | 973.7 | 4 | 243.4 | 40.7 | |||
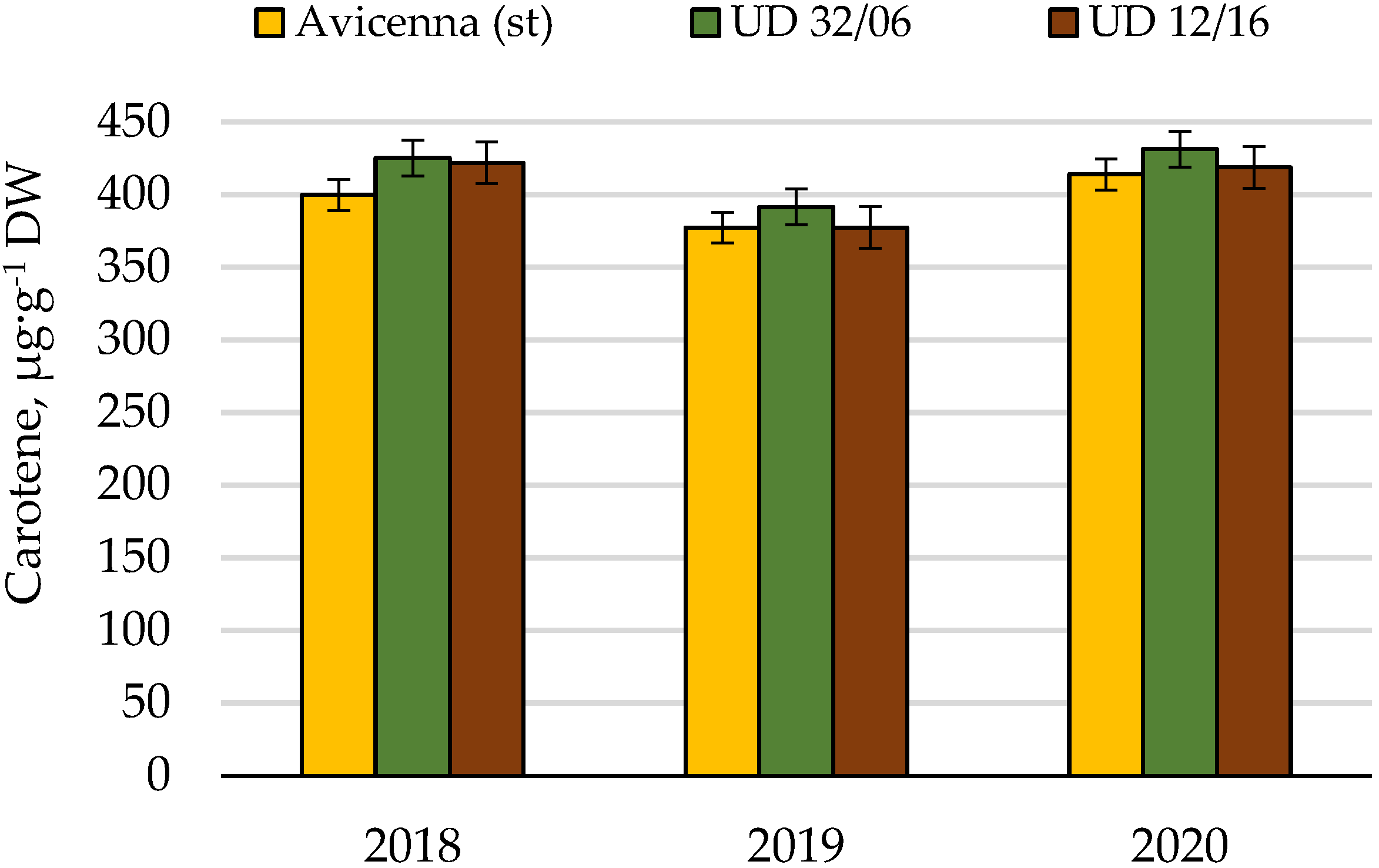
| Carotene | Cumulative | 3385.7 | 8 | 100.0 | |||
| Conditions of the year | 2707.1 | 2 | 80.0 | ||||
| Cultivar | 542.0 | 2 | 271.0 | 7.9 | 6.9 | 16.0 | |
| Random | 136.6 | 4 | 34.1 | 4.0 | |||
| Crude ash | Cumulative | 5.27 | 8 | 100.0 | |||
| Conditions of the year | 3.40 | 2 | 64.5 | ||||
| Cultivar | 1.32 | 2 | 0.66 | 4.8 | 6.9 | 25.1 | |
| Random | 0.55 | 4 | 0.14 | 10.4 | |||
| Calcium | Cumulative | 0.020 | 8 | 100.0 | |||
| Conditions of the year | 0.002 | 2 | 8.5 | ||||
| Cultivar | 0.018 | 2 | 0.009 | 116.4 | 6.9 | 90.0 | |
| Random | 3.1 × 10−4 | 4 | 7.64 × 10−5 | 1.5 | |||
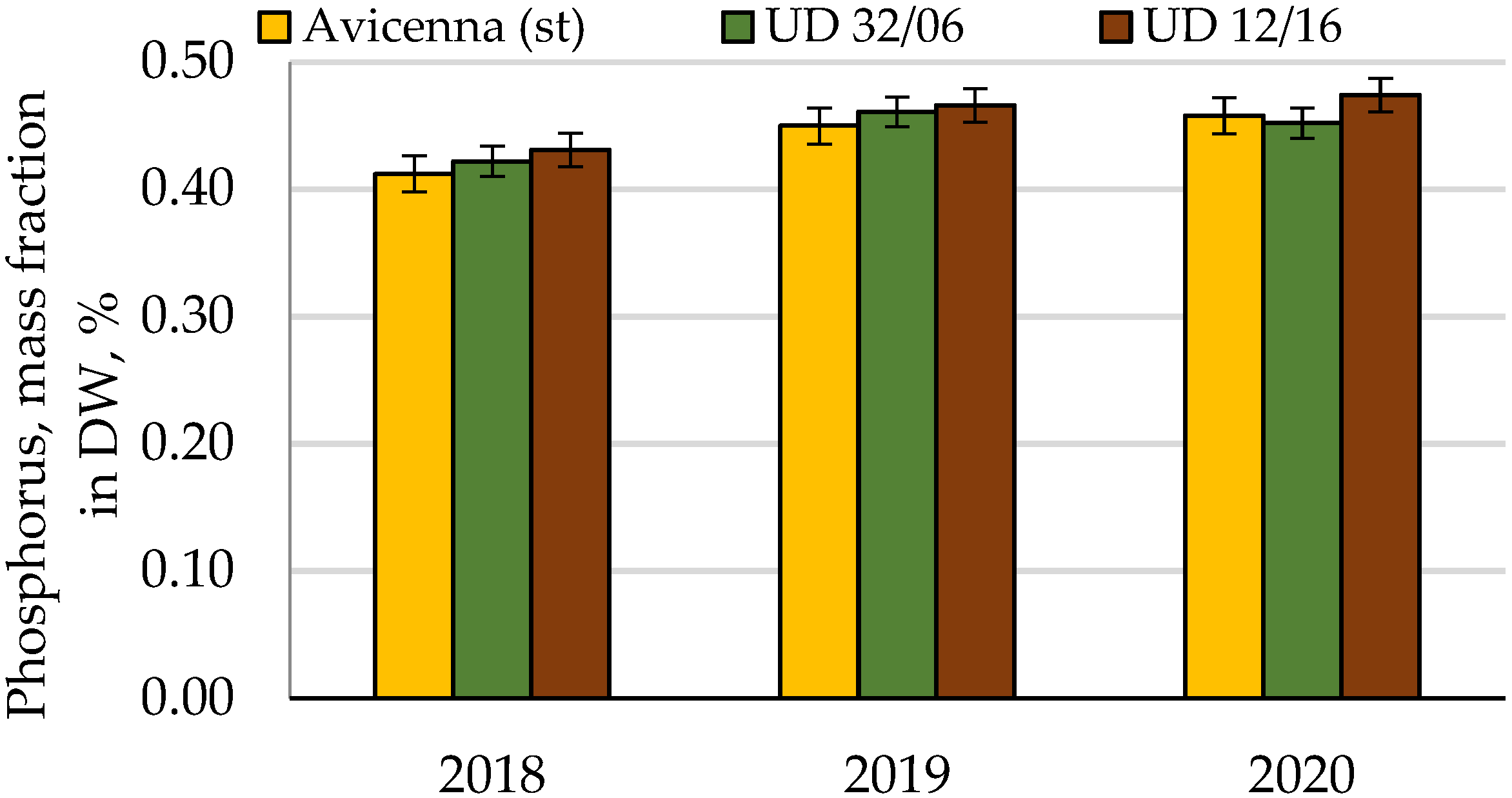
| Phosphorus | Cumulative | 0.004 | 8 | 100.0 | |||
| Conditions of the year | 0.003 | 2 | 83.8 | ||||
| Cultivar | 4.58 × 10−4 | 2 | 2.29 × 10−4 | 7.9 | 6.9 | 12.9 | |
| Random | 1.15 × 10−4 | 4 | 2.88 × 10−5 | 3.3 | |||
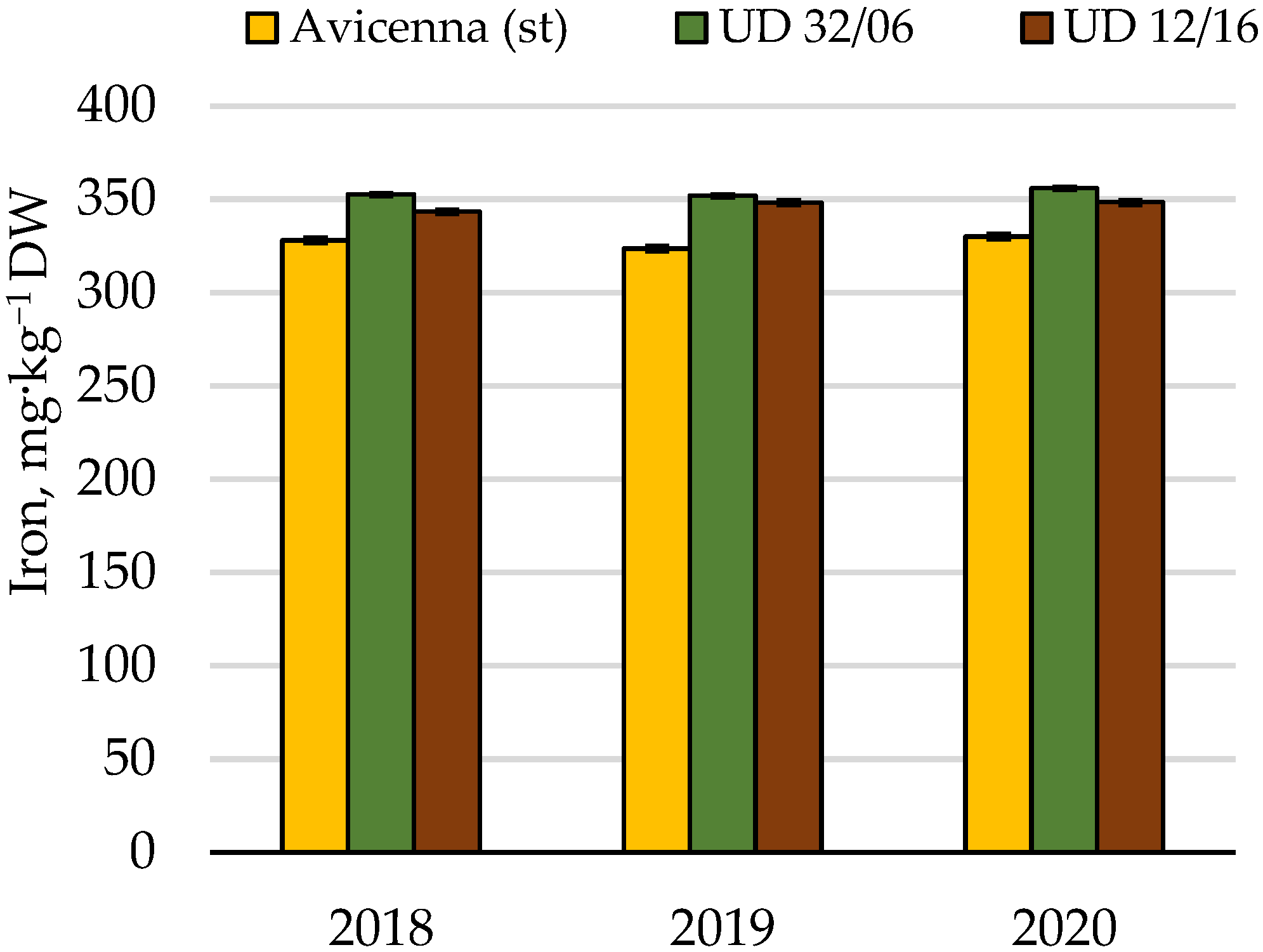
| Iron | Cumulative | 1160.5 | 8 | 100.0 | |||
| Conditions of the year | 23.8 | 2 | 2.1 | ||||
| Cultivar | 1114.3 | 2 | 557.1 | 99.6 | 6.9 | 96.0 | |
| Random | 22.4 | 4 | 5.6 | 1.9 | |||
| Zinc | Cumulative | 135.4 | 8 | 100.0 | |||
| Conditions of the year | 113.1 | 2 | 83.5 | ||||
| Cultivar | 5.7 | 2 | 2.8 | 0.7 | 9.1 | 4.2 | |
| Random | 16.7 | 4 | 4.2 | 12.3 | |||
| Copper | Cumulative | 4.20 | 8 | 100.0 | |||
| Conditions of the year | 2.38 | 2 | 56.8 | ||||
| Cultivar | 0.44 | 2 | 0.22 | 0.6 | 9.1 | 10.5 | |
| Random | 1.37 | 4 | 0.34 | 32.7 | |||
| Manganese | Cumulative | 354.0 | 8 | 100.0 | |||
| Conditions of the year | 244.1 | 2 | 69.0 | ||||
| Cultivar | 7.5 | 2 | 3.8 | 0.14 | 9.1 | 2.1 | |
| Random | 102.3 | 4 | 25.6 | 28.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosolapov, V.M.; Cherniavskih, V.I.; Zarudny, V.A.; Mazur, K.; Konieczna, A.; Tseiko, L.; Dumacheva, E.V.; Dumachev, D.V. Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions. Agronomy 2022, 12, 76. https://doi.org/10.3390/agronomy12010076
Kosolapov VM, Cherniavskih VI, Zarudny VA, Mazur K, Konieczna A, Tseiko L, Dumacheva EV, Dumachev DV. Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions. Agronomy. 2022; 12(1):76. https://doi.org/10.3390/agronomy12010076
Chicago/Turabian StyleKosolapov, Vladimir M., Vladmir I. Cherniavskih, Vladimir A. Zarudny, Kamila Mazur, Anita Konieczna, Leisan Tseiko, Elena V. Dumacheva, and Dmitrij V. Dumachev. 2022. "Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions" Agronomy 12, no. 1: 76. https://doi.org/10.3390/agronomy12010076





