1. Introduction
The use of biofertilizers in Mexico dates back to pre-Columbian times, as mud from lakes (located near what today is Mexico City) loaded with a variety of microorganisms was used to build floating plots (called chinampas) to grow crops [
1]. More recently, Armenta-Bojorquez et al. [
2] indicated that the state of Sinaloa (a highly productive, high input agricultural state in northwestern Mexico) widely adopted the use of biofertilizers for N fixation on legume crops around the 1970s and 1980s. Currently the irrigated intensive production systems in Sinaloa, for the most part, relay on synthetic fertilizers. In contrast, the state of Chiapas, which has fewer input intensive systems, and mostly rainfed agriculture, in southern Mexico, seems to be one of the most enthusiastic and successful states for testing biofertilizers [
3,
4]. Today, with the goal of improving productivity and reducing the costs of production while minimizing environmental impact, the Mexican government promotes the use of biofertilizers across the whole country. They see biofertilizers as a way to cut down the use of synthetic fertilizers; regardless of obvious agroecological and input use differences across a highly diverse country (
https://www.gob.mx/agricultura%7Cregionlagunera/articulos/sagarpa-entrego-2-9-toneladas-de-biofertilizantes-para-el-mejoramiento-del-suelo (accessed on 24 December 2021)). Thus, given the present wave of interest, farmers in Mexico are looking for alternative management practices that could help them reduce their fertilization costs hence, making their activity more profitable. Among these management options, the optimization of mineral fertilizer rates, timing, and methods of application are of great importance [
5,
6], as well as fertilizer sources such as organic fertilizers [
7,
8] and biofertilizers [
9,
10].
The term biofertilizer was defined by Vessey [
11] as “a substance which contains living microorganisms which, when applied to seed, plant surfaces, or soil, colonizes the rhizosphere or the interior of the plant and promotes growth by increasing the supply or availability of primary nutrients to the host plant”. Biofertilizers can contribute to soil fertility through N fixation, phosphorus solubilization, and an extended root system through association with vesicular-arbuscular mycorrhizal fungi in the soil (AMF), which can improve soil exploration for increased water and nutrient uptake; principally for P uptake. This in turn can result in a better tolerance to biotic and abiotic stress, protection against pathogens, and a general increase in plant fitness [
12,
13]. This technology more commonly involves seed inoculation with bacteria of the genera
Azospirillum spp. [
14,
15],
Bacillus spp. [
16,
17], and
Pseudomonas spp. [
18,
19]; as well as mycorrhizas like
Glomus spp. [
20,
21]. However, there are other microorganisms also used as biofertilizers. Itelima et al. [
22] have made a detailed list of biofertilizers, distinguishing among those used for nitrogen fixation, phosphate solubilization, phosphate mobilizers, biofertilizers for micronutrients, and plant growth promoting Rhizobacteria. Commercial biofertilizers products can have a specific microorganism or a consortium of several of these microorganisms as granular or liquid presentations to be applied (inoculated) to seeds, soil, or plant tissues [
23,
24,
25].
The use of biofertilizers emerges as a potential sustainable alternative for improving maize and wheat cropping systems [
26,
27]. A growing interest in Mexico is arising based on the expectation that using biofertilizers may partially substitute the use of synthetic fertilizers [
28,
29,
30,
31,
32]. It has been suggested that biofertilizers can save up to 50% of synthetic fertilizers [
4,
33]. Positive effects from different microorganisms have been reported for laboratory and greenhouse experiments [
16,
17,
34,
35,
36,
37,
38]. However, the potential of biofertilizers for decreasing synthetic fertilizer rates and or increasing the yield of cereals under field conditions in Mexico has been limited and unclear. The quality of biofertilizer products has been questioned due to several factors.
Herrmann & Lesueur [
39] indicated that “…many of the products that are currently available worldwide are often of very poor quality, resulting in the loss of confidence from farmers. The formulation of an inoculant is a crucial multistep process that should result in one or several strains of microorganisms included in a suitable carrier, providing a safe environment to protect them from the often harsh conditions during storage and ensuring survival and establishment after introduction into soils. One of the key issues in formulation development and production is the quality control of the products at each stage of the process.” Furthermore, Husen et al. [
40] found a series of inconsistencies in a study about biofertilizers quality in Indonesia and concluded that there is an urgent need to improve the current quality standard system of biofertilizers. In a study of the effect of biofertilizers on bean (
Vigna sp.) production in Pakistan, Zahir et al. [
41] concluded that “research for the development of inoculum for different advanced genotypes should be continued and more emphasis should be deployed to develop biofertilizers with efficient strains to use them under different climate and soil conditions”. A classic paper addressing the inconsistency and variability of biofertilizers in Mexican agriculture was published by Fuentes-Ramírez and Caballero-Mellado [
42]. More recently, other studies conducted in Mexico have recognized having inconsistent results with the use of biofertilizers [
28,
29,
30]. Even more recently, in 2019, Rodriguez-Ramos et al. [
43] in a multi-location trial reported a lack of response of biofertilizers on wheat (
Triticum aestivum L.) and barley (
Hordeum vulgare L.) in the Mexican state of Guanajuato. In contrast, there is one study conducted in Chiapas, Mexico [
3] that reported definitive advantages of applying biofertilizers together with synthetic fertilizers to increase maize yields. The objective of the present study was to evaluate different commercially available biofertilizers in combination with synthetic fertilizer on the yields of maize and wheat in contrasting climatic and input use conditions in Mexico.
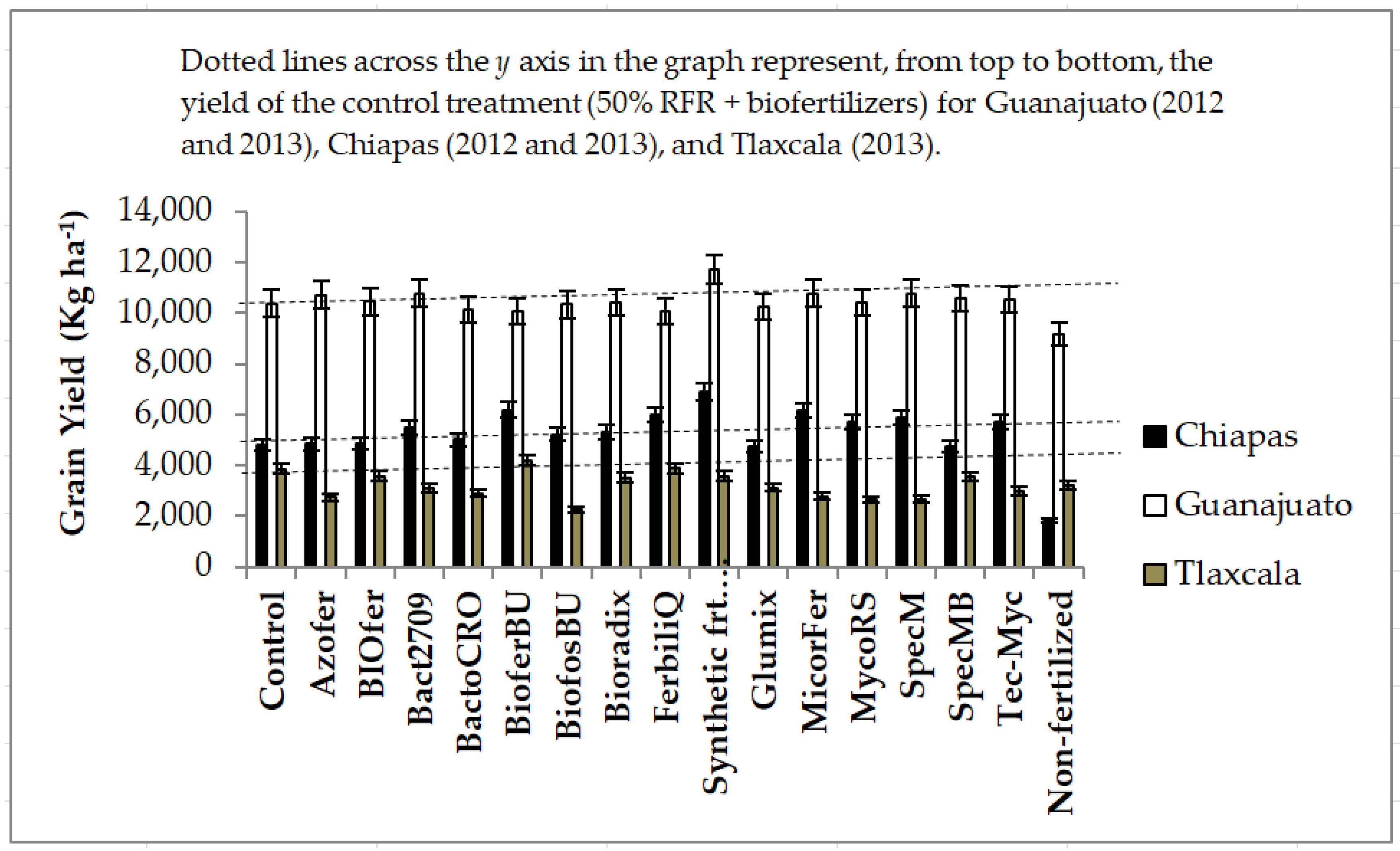
4. Discussion
It was found that there are significant benefits of the biofertilizer MicorrizaFer in Sonora and Chiapas (AMF); locations with low P soils with maize. Given the extent of low P tropical soils in maize production systems in Mexico, this is a very relevant finding. Other than that, in general, no response to biofertilizers was observed in the rest of the locations through the years in maize. The yield response in Chiapas in this study coincides with that reported also in Chiapas by Martínez-Reyes et al. [
3], who found a positive response of maize yield to a combination of synthetic fertilizer plus biofertilizers. It is worthwhile to notice that both the Chiapas and Tlaxcala sites had moderately low soil P availability. The benefits of AMF symbiosis in maize in low P soils have been well documented [
45,
46]. In contrast, the different response of wheat to AMF under low-moderate P has also been documented. Since wheat has a more extensive root system and root exudates, it is less dependent on AMF for P uptake and the response is generally less; while, maize is a crop that is highly dependent on AMF for P uptake due to root architecture [
47]. A generalized lack of wheat yield response to biofertilizers in the present study coincides with another series of experiments conducted in Guanajuato, Mexico by Rodríguez-Ramos et al. [
43] with an inconsistent response of biofertilizers in wheat. Across all experiments, this study concluded that the addition of biofertilizers had an inconsistent response on yield and that, in general, did not compensate cereal yields due to reductions on the recommended synthetic fertilizer rates.
In experiments conducted under irrigation in Guanajuato and Sonora, excluding the one with maize in Sonora, which positively responded to biofertilizers (experiment that demonstrated being nitrogen deficient), it is suggested that the lack of yield response to biofertilizers could be partly be explained by the relatively high soil fertility. In Tlaxcala, there was no difference between the 50% and 100% RFR. There was no additional yield response from the 100% RFR or biofertilizers; indicating plant nutrition limitations were covered by the 50% rate under water limiting rainfed conditions. In Guanajuato, on the other hand, the unfertilized yield was over 9 t ha
−1; indicating high residual soil fertility. High soil fertility could be the result of the residual effect of nutrients caused by the continuous application of considerable high fertilizer rates through the years that are typically employed in these intensive production systems. Sonora ranks first in wheat production in Mexico and Bajío (Guanajuato) ranks second or third every year. Thus, these states are highly productive and use high level of inputs for crop production. Even though in the present study the full recommended fertilizer rates were cut by half, soil fertility still remained relatively high, as observed by the small or almost null gap between the full RFR and the non-fertilized treatments recorded for maize in Guanajuato and wheat in Sonora (
Table 8 and
Table 9). Thus, in these two locations, where relatively high fertility may occur, biofertilizers may not have been as effective in high fertility environments as in low fertility environments like in Chiapas or Sonora, in the case of maize, where the gap between the full RFR and the non-fertilized treatment was a 383% and 280% difference, respectively.
In a classic paper, Fuentes-Ramirez and Caballero-Mellado [
42], reported results from an extensive campaign where biofertilizers were tested in Mexico. They reported that “When nitrogen fertilizers were not applied to traditional and modern maize cultivars, the inoculation with Azospirillum exerted beneficial effects in 95 and 93% of the sites evaluated during 1999 and 2000, respectively. However, when fertilizers were applied in levels higher than 110 kg N/ha, the positive responses on the maize yield were observed only in 55 and 50% of the sites evaluated in 1999 and 2000, respectively”. Banayo et al. [
48] supported the hypothesis of the inconsistency of biofertilizers performance in the Philippines due to relatively high fertility levels in rice production system. They concluded that “…the trends in our results seem to indicate that biofertilizers might be most helpful in rainfed environments with limited inorganic fertilizer input”. At the 50% RFR, the nitrogen fertilizer rates in Guanajuato and Sonora were 100 and 125 kg N ha
−1, respectively, on top of the modest residual fertility levels (
Table 2) (modest residual fertility if 35 kg N are required to produce 1000 kg of wheat, in environments where mean yields are around 6500 kg ha
−1). Even these slightly high fertility levels could have inhibited the response of microorganisms contained in the applied biofertilizers. In further support of this hypothesis about high soil fertility in Guanajuato and Sonora cancelling the benefits of biofertilizers to crop yields, Fukami et al. [
23] observed that the efficiency of
Azospirillum spp. to support crop yields depended on N rates. High N rates would decrease the ability of
Azospirillum spp. to promote positive responses on crop yields, due to a decreased activity of the enzyme nitrogenase, while at low N rates its ability to stimulate a positive response is increased. These results are in general agreement with those reported by Ramírez-Ramos et al. [
43], where, with the exception of the results observed in Villagrán, there was no effect of the biofertilizers on wheat or barley (
Hordeum vulgare) yields in Guanajuato. The present study supports the hypothesis of high soil fertility as yields of maize without fertilizers were high, as compared to the 100% RFR (
Table 8). In addition, relatively high soil supplies of available phosphorus (P) may have, as well as N, inhibited the response to mycorrhizas-based biofertilizers. Davaran et al. [
20] found a negative interaction between
Glomus spp. and P fertilization at levels higher than 50% the locally recommended rate (equivalent to 50 kg P
2O
5 ha
−1), while the mean P
2O
5 applied fertilizer rate in non-responsive sites in the present study was 54 kg P
2O
5 ha
−1 on top of the P in soil residual reserves (
Table 2). Jensen and Jacobson [
47] provided additional evidence about vesicular-arbuscular mycorrhizas being inhibited by high P levels in soil and
vice versa.
On the other hand, a lack of an adequate water supply in rainfed experiments, except for Chiapas where annual precipitation exceeds 1000 mm, could have been the main reason for the lack of response of maize to biofertilizers. Glazova [
48] reported that the efficiency of bio-fertilization directly depended on soil moisture levels, being the optimum at a level as high as 60% soil moisture. In addition, Alahdadi et al. [
49] reported a significant water deficit stress × cultivar × biofertilizer interaction on soybeans (
Glycine max L.). They suggested that by increasing the severity of water deficit stress, the primary root length decreased. This could be the result of a disruption in photosynthesis because of the shortage of soil moisture and decreasing transport of photosynthates to the plant during the growth period.
Cassán et al. [
25] point to a number of possible reasons for restricting the response of biofertilizers in different crops. Crop plant root factors such as surface area, root hair abundance and length, growth rate, response to soil conditions, and exudations determine the relative dependency on AMF for nutrient uptake [
45,
46]. Other causes include complex interactions between microorganisms in biofertilizers and plants; strong stressful crop growing conditions; unsound methods of inoculation; a lack of replicability of experimental conditions; and the interaction of native soil biota with inoculants, i.e., studies under isolated controlled conditions often produce different results under field uncontrolled conditions. Other suggested reasons for the lack of response to biofertilizers include possibly a poor quality of biofertilizer standards [
29]. In the present study, for example, Azofer + Microrriza Fer (
Glomus spp. based) out yielded the control in Sonora and Chiapas; both maize and low available P sites, but the same biofertilizer, sold by the same company, did not show a yield response in any other part of the environment, which may suggest variability in quality of products from different manufacturers, although there are other several factors, inherent to individual biofertilizer users that may damage the product such as prolonged exposure time of biofertilizer products or biofertilizer treated seed to direct sunlight in the field (among a number of other particular practices and environmental conditions. Chávez-Díaz et al. [
1] underlined that there are several factor interacting that need to be considered in order to take advantage of biofertilizers (
Figure 2).
The use of biofertilizers is no doubt, less harmless to the environment than synthetic fertilizers, which is of key importance in today’s world. However, the need of producing food for ≈ 8 billion people (as today, 14 December 2021) is equally or more important and requires increasing the productivity of crops, but crop productivity is unavoidably linked to substantial input use. It is a physics rule. The key for intensive production systems is to find ways to be more sustainable. This objective is possible without sacrificing crop yields. Minimum tillage systems, the use of tools to reasonable apply minerals, such the GreenSeeker® technology, which is capable of estimating the N needs of crops based on actual yield potential, among many other technologies are sound tools that are designed to make modern agriculture productive but friendlier to the environment, to farmer’s income, and to society in general. The downside of biofertilizers is their lack of consistency across products, locations, and years. In addition, the interactions among these factors are complex to understand and apply at the field level, as biological processes are very dynamic in space and time for the microorganisms and for the plant’s environments.
5. Conclusions
The results of these experiments show that only in Chiapas and Sonora (maize) was there was a significant increase in yield with some biofertilizers in combination with inorganic fertilizers. Therefore, one of the main conclusions of this study is that biofertilizers only work in some places and in the places where biofertilizers show a response, only some of them work. These results suggest that we should be cautious before widely recommending the use of biofertilizers across Mexico since their positive response on yields seem to be more of an exception rather than the rule.
From the results observed in the present multiple-locations (with highly contrasting agroecologic characteristics and input use levels), multiple-year study, it is suggested that the lack of response to some environments may be related to the level of precipitation, organic matter content, and residual soil fertility. Further research is needed to test biofertilizers in environments representative of smaller farmers, with even lower input investment and surely more responsiveness to fertilizer applications, than those represented in the current research, since, Tlaxcala and Chiapas experiments were developed under medium input, while Sonora and Guanajuato were high input production systems. Another lesson the experiments left us is, in future research, to include another control treatment with, perhaps, 25% of the locally RFR, since the 50% RFR often yielded the same as the full RFR, indicating that in these locations we did not lower the fertility level to achieve a more accurate evaluation of the efficacy of biofertilizers.
While we found evidence that there can be benefit from some products in low-moderate soil fertility conditions, there were by far more products that provided no benefit and resulted in yield loss. Only four of the 15 biofertilizer products produced a yield response and only one in more than one location (MicorrizaFer). While the benefits of a biofertilizer can be significant under a certain condition with the right product, there is a greater chance of a farmer using a product with no benefit. This shows the need for well-designed field trails in experimental platforms to test products before recommending to farmers to avoid risk of yield loss. Farmers need clear guidance on the use of biofertilizers, what products are recommended, and how much synthetic fertilizer rates can be reduced. Further research on this is required to fine tune recommendations to maximize yields and economic benefits for farmers.