Anti-Browning and Oxidative Enzyme Activity of Rice Bran Extract Treatment on Freshly Cut ‘Fuji’ Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Treatments
2.2. Color Analyses
2.3. Weight Loss
2.4. Total Soluble Solid Content and Titratable Acidity
2.5. Total Phenolic Content
2.6. Total Protein and Oxidative Enzyme Activity Assays
2.7. Microbial Counts
2.8. Sensory Test
2.9. Statistical Analysis
3. Results and Discussion
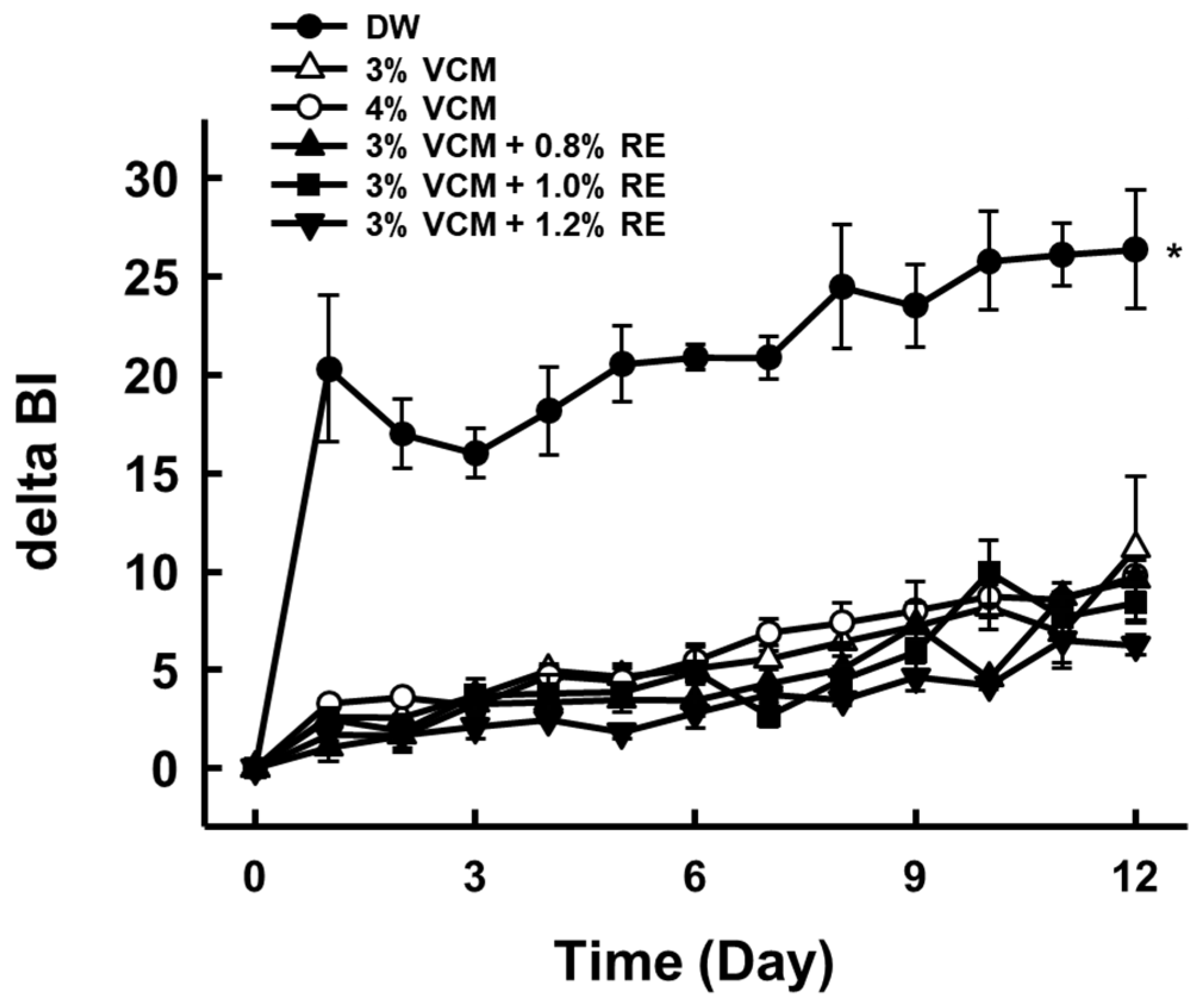
3.1. Effects of Anti-Browning Reagent Mixture on Browning Index
3.2. Effects of Anti-Browning Reagents on the Weight and Appearance of Apples
3.3. Effects of Anti-Browning Treatments on Total Polyphenol Content and Phenol Oxidase Enzyme Activity
3.4. Effects of Anti-Browning Reagent on Microbial Growth in Apples
3.5. Sensory Evaluation with VCM and RE Combination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Jiang, Y.; Li, W.; Tang, Y.; Yun, J. Effects of ascorbic acid and high oxygen modified atmosphere packaging during storage of fresh-cut eggplants. Food Sci. Technol. Int. 2014, 20, 99–108. [Google Scholar] [CrossRef]
- Sinha, N.K. Apples and pears: Production, physicochemical and nutritional quality, and major products. In Handbook of Fruits and Fruit Processing; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 365–383. [Google Scholar]
- Shrestha, L.; Kulig, B.; Moscetti, R.; Massantini, R.; Pawelzik, E.; Hensel, O.; Sturm, B. Optimisation of physical and chemical treatments to control browning development and enzymatic activity on fresh-cut apple slices. Foods 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA film incorporated with ZnO nanoparticle on the quality attributes of fresh-cut apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality control of fresh-cut apples after coating application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Li, W.; Zhen, C.; Wang, K.; Qin, Z.; Gao, H. Transcriptomic analysis of the effects of γ-aminobutyric acid treatment on browning and induced disease resistance in fresh-cut apples. Postharvest Biol. Technol. 2021, 181, 111686. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Herceg, K.; Levaj, B. Influence of antibrowning solutions, air exposure, and ultrasound on color changes in fresh-cut apples during storage. J. Food Process. Preserv. 2017, 41, e13288. [Google Scholar] [CrossRef]
- Pizzocaro, F.; Torreggiani, D.; Gilardi, G. Inhibition of apple polyphenoloxidase (PPO) by ascorbic acid, citric acid and sodium chloride. J. Food Process. Preserv. 1993, 17, 21–30. [Google Scholar] [CrossRef]
- Fan, X.; Sokorai, K.; Phillips, J. Development of antibrowning and antimicrobial formulations to minimize Listeria monocytogenes contamination and inhibit browning of fresh-cut “Granny Smith” apples. Postharvest Biol. Technol. 2018, 143, 43–49. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants-recent progress. Phytochemistry 1986, 26, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, X. Characterisation of polyphenol oxidase and peroxidase and the role in browning of loquat fruit. Czech J. Food Sci. 2015, 33, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Serra, S.; Anthony, B.; Boscolo Sesillo, F.; Masia, A.; Musacchi, S. Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Katiyo, W.; Yang, R.; Zhao, W.; Hua, X.; Gasmalla, M.A.A. Optimization of combined pulsed electric fields and mild temperature processing conditions for red apple juice polyphenol oxidase and peroxidase inactivation. Adv. J. Food Sci. Technol. 2014, 6, 638–646. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Effect of chitosan-ascorbic acid coatings on the refrigerated storage stability of fresh-cut apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef]
- Arab, F.; Alemzadeh, I.; Maghsoudi, V. Determination of antioxidant component and activity of rice bran extract. Sci. Iran. 2011, 18, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Mahunu, G.K.; Zhang, H.; Yang, Q.; Zhang, X.; Li, D.; Zhou, Y. Improving the biocontrol efficacy of Pichia caribbica with phytic acid against postharvest blue mold and natural decay in apples. Biol. Control 2016, 92, 172–180. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Romani, V.P.; Hernández, C.P.; Martins, V.G. Pink pepper phenolic compounds incorporation in starch/protein blends and its potential to inhibit apple browning. Food Packag. Shelf Life 2018, 15, 151–158. [Google Scholar] [CrossRef]
- Sukhonthara, S.; Kaewka, K.; Theerakulkait, C. Inhibitory effect of rice bran extracts and its phenolic compounds on polyphenol oxidase activity and browning in potato and apple puree. Food Chem. 2016, 190, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Suttirak, W.; Manurakchinakorn, S. Potential application of ascorbic acid, citric acid and oxalic acid for browning inhibition in fresh-cut fruits and vegetables. Walailak J. Sci. Technol. WJST 2010, 7, 5–14. [Google Scholar]
- Liu, X.; Ren, J.; Zhu, Y.; Han, W.; Xuan, H.; Ge, L. The preservation effect of ascorbic acid and calcium chloride modified chitosan coating on fresh-cut apples at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Olivas, G.; Mattinson, D.; Barbosa-Cánovas, G. Alginate coatings for preservation of minimally processed ‘Gala’apples. Postharvest Biol. Technol. 2007, 45, 89–96. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria x ananassa Duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Kim, A.N.; Lee, K.Y.; Kim, H.J.; Chun, J.; Kerr, W.L.; Choi, S.G. Effect of grinding at modified atmosphere or vacuum on browning, antioxidant capacities, and oxidative enzyme activities of apple. J. Food Sci. 2018, 83, 84–92. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.-J.; Meng, X.-H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef]
- Solval, K.M.; Xiang, B.; Lee, Y.S. Combined effects of calcium ascorbate treatment and modified atmosphere packaging to improve quality retention of fresh-cut cantaloupes. J. Appl. Packag. Res. 2019, 11, 5. [Google Scholar]
- Adhikary, T.; Gill, P.S.; Jawandha, S.K.; Bhardwaj, R.D.; Anurag, R.K. Browning and quality management of pear fruit by salicylic acid treatment during low temperature storage. J. Sci. Food Agric. 2021, 101, 853–862. [Google Scholar] [CrossRef]
- Singh, S. Method for Preserving Foodstuff European Patent EP1301084B1, 15 June 2001.
- Chowhan, Z. pH-solubility profiles of organic carboxylic acids and their salts. J. Pharm. Sci. 1978, 67, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Rovito, M.A.; De Bonis, M.V.; Ruocco, G. COLDwave TM processing: Cold jet impingement to control bio-substrate drying by microwave and preserve its quality. Heat Mass Transf. 2019, 55, 953–963. [Google Scholar] [CrossRef]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying characteristics and quality attributes of apple slices dried by a non-thermal ultrasonic contact drying method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef]
- Buta, J.G.; Moline, H.E.; Spaulding, D.W.; Wang, C.Y. Extending storage life of fresh-cut apples using natural products and their derivatives. J. Agric. Food Chem. 1999, 47, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Hu, W.; He, Y.; Jiang, A.; Zhang, R. Effect of citric acid combined with UV-C on the quality of fresh-cut apples. Postharvest Biol. Technol. 2016, 111, 126–131. [Google Scholar] [CrossRef]
- Olivas, G.; Barbosa-Cánovas, G. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. Nutr. 2005, 45, 657–670. [Google Scholar] [CrossRef]
- Nelson, L.; Cox, M.M.; Freeman, W. Lehninger Principles of Biochemistry. Third; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Villamil-Galindo, E.; Van de Velde, F.; Piagentini, A.M. Extracts from strawberry by-products rich in phenolic compounds reduce the activity of apple polyphenol oxidase. LWT 2020, 133, 110097. [Google Scholar] [CrossRef]
- Du, Y.; Dou, S.; Wu, S. Efficacy of phytic acid as an inhibitor of enzymatic and non-enzymatic browning in apple juice. Food Chem. 2012, 135, 580–582. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.; Nicolas, J. Phenolic composition and browning susceptibility of various apple cultivars at maturity. J. Food Sci. 1992, 57, 958–962. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Guardo, M.D.; Tadiello, A.; Lorenz, G.; Masuero, D.; Vrhovsek, U.; Velasco, R.; Costa, F.; Farneti, B.; Costa, G. Multidisciplinary approach provides novel insight about fruit flesh browning physiology in apple (Malus × domestica Borkh.). In Proceedings of the III International Symposium on Molecular Markers in Horticulture 1100, Trento, Italy, 25–27 September 2013; pp. 161–166. [Google Scholar]
- Lu, S.; Luo, Y.; Feng, H. Inhibition of apple polyphenol oxidase activity by sodium chlorite. J. Agric. Food Chem. 2006, 54, 3693–3696. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yao, Y.-X.; Heng, Z.; Du, Y.-P.; Feng, C.; Shu-Wei, W. Polyphenolic compound and the degree of browning in processing apple varieties. Agric. Sci. China 2007, 6, 607–612. [Google Scholar]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Myrvik, Q.N.; Volk, W.A. Comparative study of the antibacterial properties of ascorbic acid and reductogenic compounds. J. Bacteriol. 1954, 68, 622. [Google Scholar] [CrossRef] [Green Version]
- Selim, S.A.; El Alfy, S.M.; Abdel Aziz, M.; Mashait, M.S.; Warrad, M.F. Evolution of bactericidal activity of selected food additives against food borne microbial pathogens. Biosci. Biotechnol. Res. Asia 2012, 9, 7–17. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Y.; Dang, H.; Tang, Y.; Zhang, B. Antibacterial effects of phytic acid against foodborne pathogens and investigation of its mode of action. J. Food Prot. 2019, 82, 826–833. [Google Scholar] [CrossRef]
- Oatway, L.; Vasanthan, T.; Helm, J.H. Phytic acid. Food Rev. Int. 2001, 17, 419–431. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of aloe vera gel-based edible coating with natural anti-browning and anti-oxidant additives to improve post-harvest quality of fresh-cut ‘fuji’ apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef] [Green Version]






| Anti-Browning Agent | pH |
|---|---|
| Apple juice | 4.30 ± 0.01 |
| DW | 4.97 ± 0.02 * |
| 100% RE | 0.10 ± 0.02 * |
| 3% VCM | 5.32 ± 0.01 * |
| 4% VCM | 5.28 ± 0.04 * |
| 3% VCM + 0.8% RE | 4.37 ± 0.03 * |
| 3% VCM + 1.0% RE | 4.30 ± 0.02 |
| 3% VCM + 1.2% RE | 4.23 ± 0.01 * |
| Group | Storage Period (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| pH | DW | 4.30 ± 0.01 | 4.26 ± 0.07 | 4.25 ± 0.05 | 4.26 ± 0.06 | 4.32 ± 0.01 |
| 4% VCM | 4.30 ± 0.03 | 4.32 ± 0.07 | 4.27 ± 0.06 | 4.30 ± 0.03 | 4.32 ± 0.06 | |
| VR | 4.34 ± 0.06 | 4.31 ± 0.05 | 4.24 ± 0.09 | 4.32 ± 0.03 | 4.30 ± 0.03 | |
| Brix | DW | 12.70 ± 0.65 | 13.20 ± 0.20 | 13.50 ± 0.40 | 12.67 ± 0.40 | 12.13 ± 0.25 |
| 4% VCM | 13.23 ± 0.29 | 13.36 ± 0.67 | 13.30 ± 0.40 | 13.37 ± 0.35 | 13.17 ± 0.49 | |
| VR | 13.60 ± 0.27 | 13.37 ± 0.31 | 13.23 ± 0.45 | 13.70 ± 0.30 | 13.80 ± 0.75 | |
| Acidity | DW | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.01 |
| 4% VCM | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.26 ± 0.01 | |
| VR | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.28 ± 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Baek, S.M.; Jeong, I.; Heo, W.; Hwang, K.-A.; Han, B.K.; Kim, Y.J. Anti-Browning and Oxidative Enzyme Activity of Rice Bran Extract Treatment on Freshly Cut ‘Fuji’ Apple. Agronomy 2022, 12, 86. https://doi.org/10.3390/agronomy12010086
Lee SH, Baek SM, Jeong I, Heo W, Hwang K-A, Han BK, Kim YJ. Anti-Browning and Oxidative Enzyme Activity of Rice Bran Extract Treatment on Freshly Cut ‘Fuji’ Apple. Agronomy. 2022; 12(1):86. https://doi.org/10.3390/agronomy12010086
Chicago/Turabian StyleLee, Sang Hoon, Soo Min Baek, Inhye Jeong, Wan Heo, Kyung-A Hwang, Bok Kyung Han, and Young Jun Kim. 2022. "Anti-Browning and Oxidative Enzyme Activity of Rice Bran Extract Treatment on Freshly Cut ‘Fuji’ Apple" Agronomy 12, no. 1: 86. https://doi.org/10.3390/agronomy12010086
APA StyleLee, S. H., Baek, S. M., Jeong, I., Heo, W., Hwang, K.-A., Han, B. K., & Kim, Y. J. (2022). Anti-Browning and Oxidative Enzyme Activity of Rice Bran Extract Treatment on Freshly Cut ‘Fuji’ Apple. Agronomy, 12(1), 86. https://doi.org/10.3390/agronomy12010086





