Foliar Spraying of Mannose Alleviates Cadmium Stress by Changing the Subcellular Distribution and Chemical Forms of Cadmium in Wheat Root
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Hydroponic Culture, and Soil Properties
2.2. Experimental Design
2.3. Wheat Seedlings Biomass Measurements and Root Scaning
2.4. Measurements of Chlorophyll Content and Gas Exchange Parameters
2.5. Separation and Subcellular Fractions of Cd in Roots
2.6. Cell Wall Extraction, Fractionation, and Its Components Measurement
2.7. The Chemical Forms of Cd in Wheat Root
2.8. Sample Collection
2.9. Statistical Analysis
3. Results
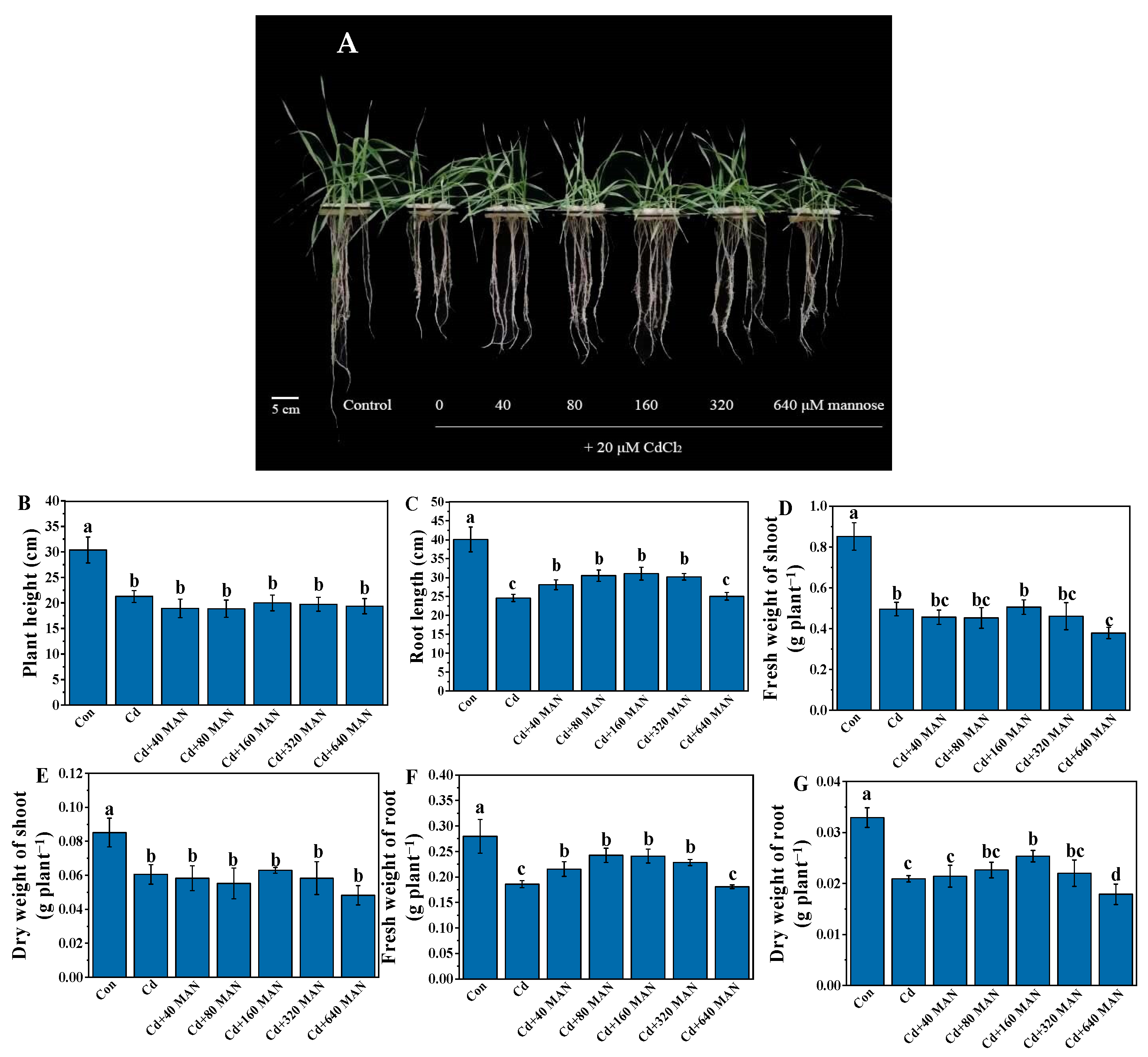
3.1. Effects of MAN on Biomass and Growth of Wheat Seedlings under Cd Stress
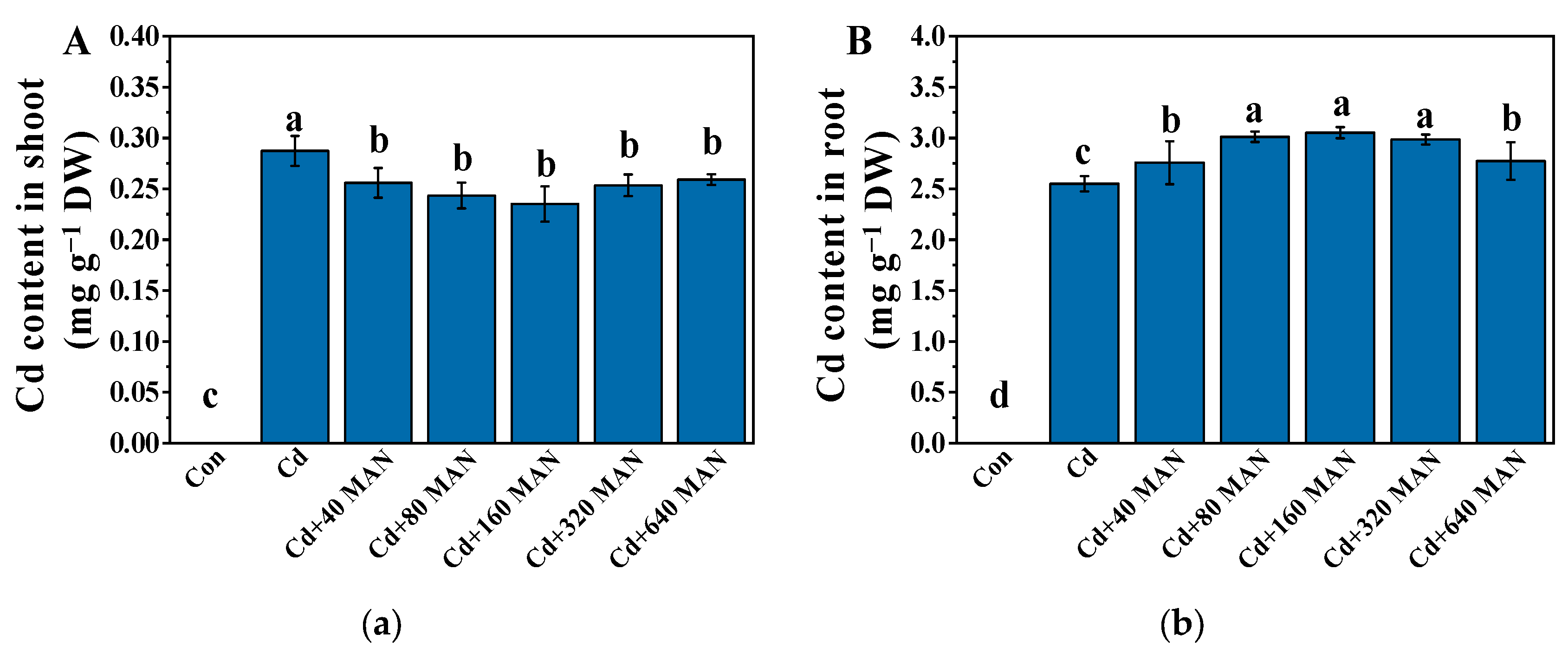
3.2. Effects of MAN on Cd Uptake by Wheat
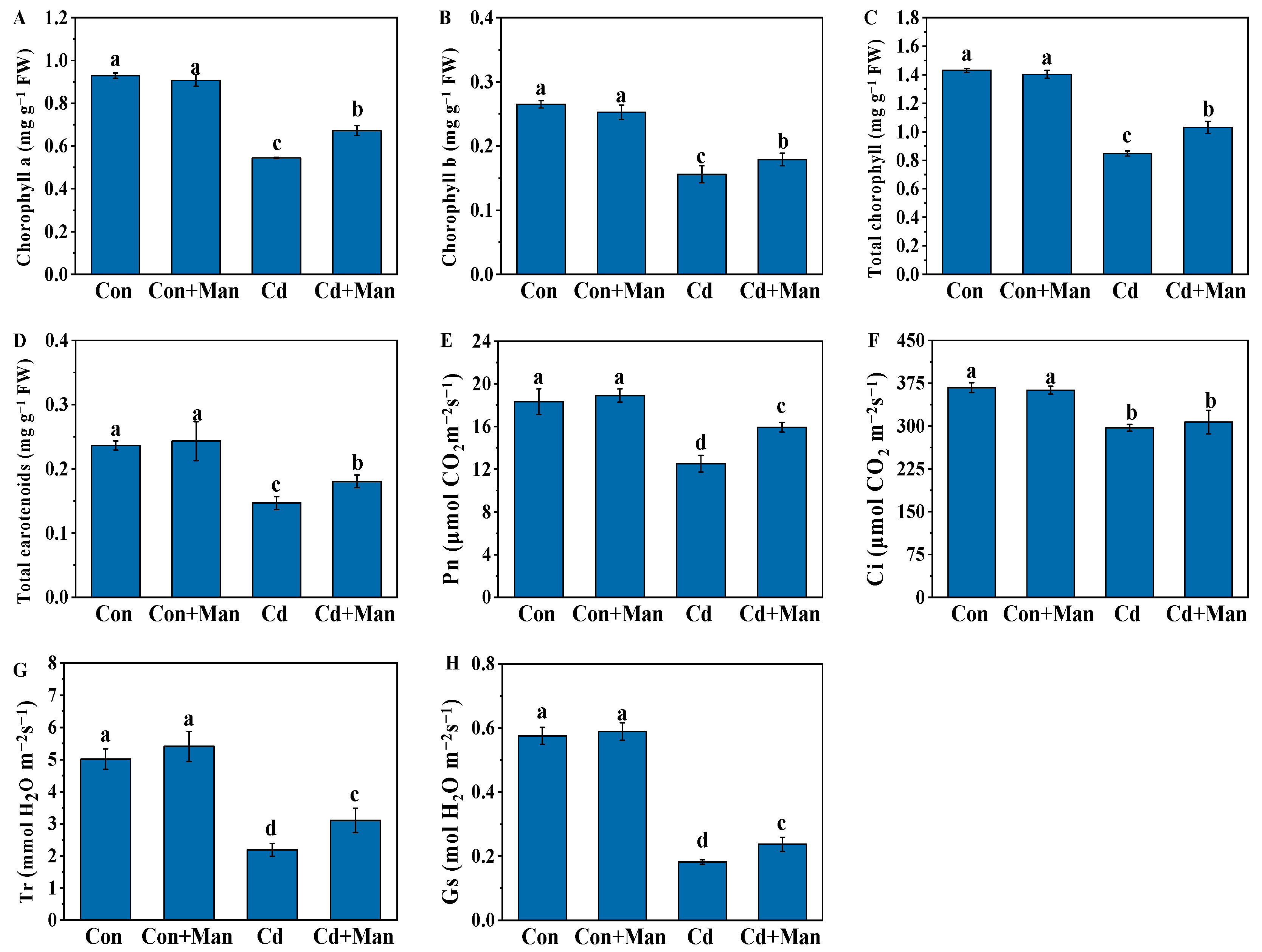
3.3. Effects of MAN on Photosynthetic Pigments and Gas Exchange Parameters
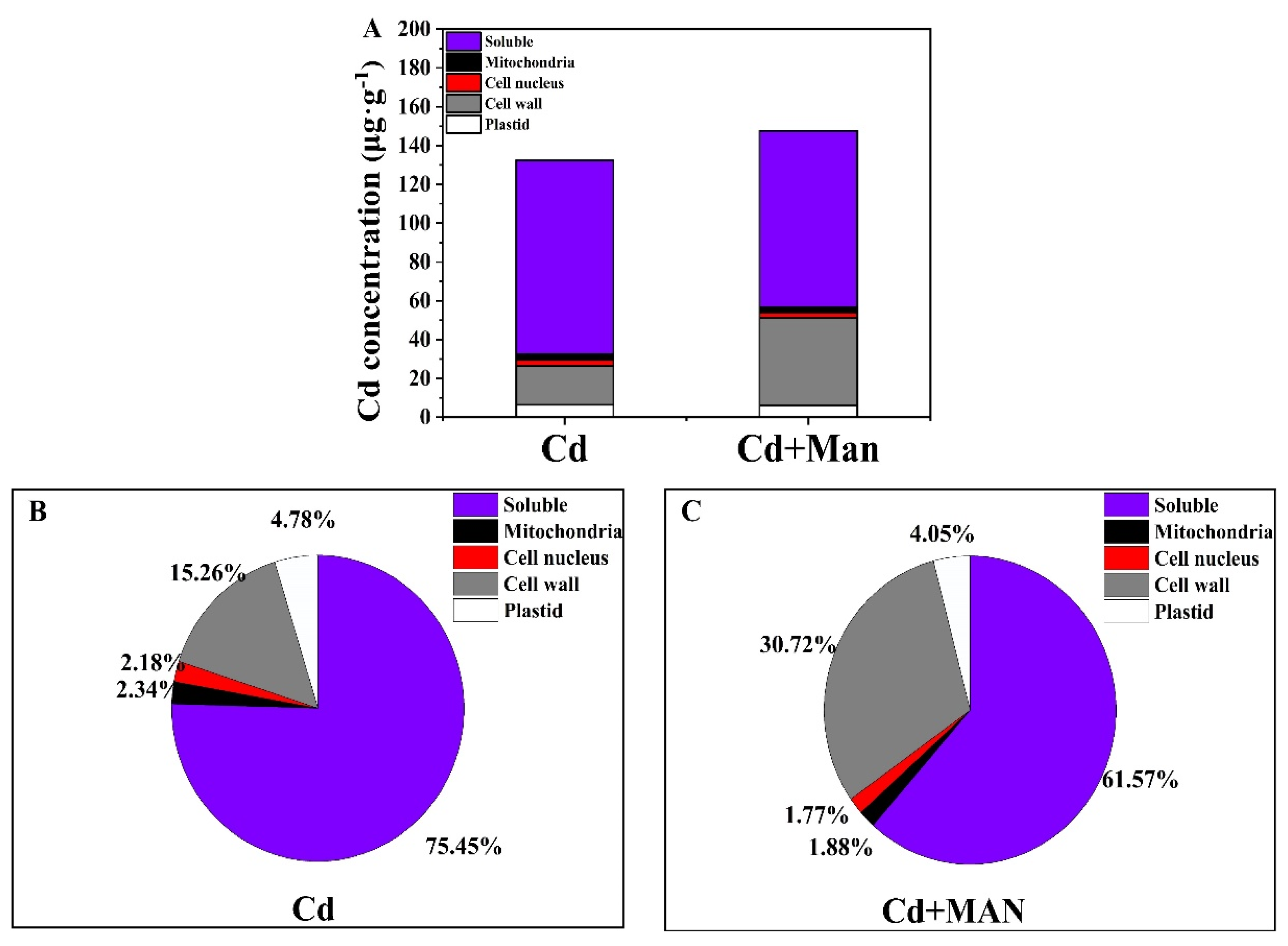
3.4. Effects of MAN on the Cd Subcellular Distribution of Wheat Roots
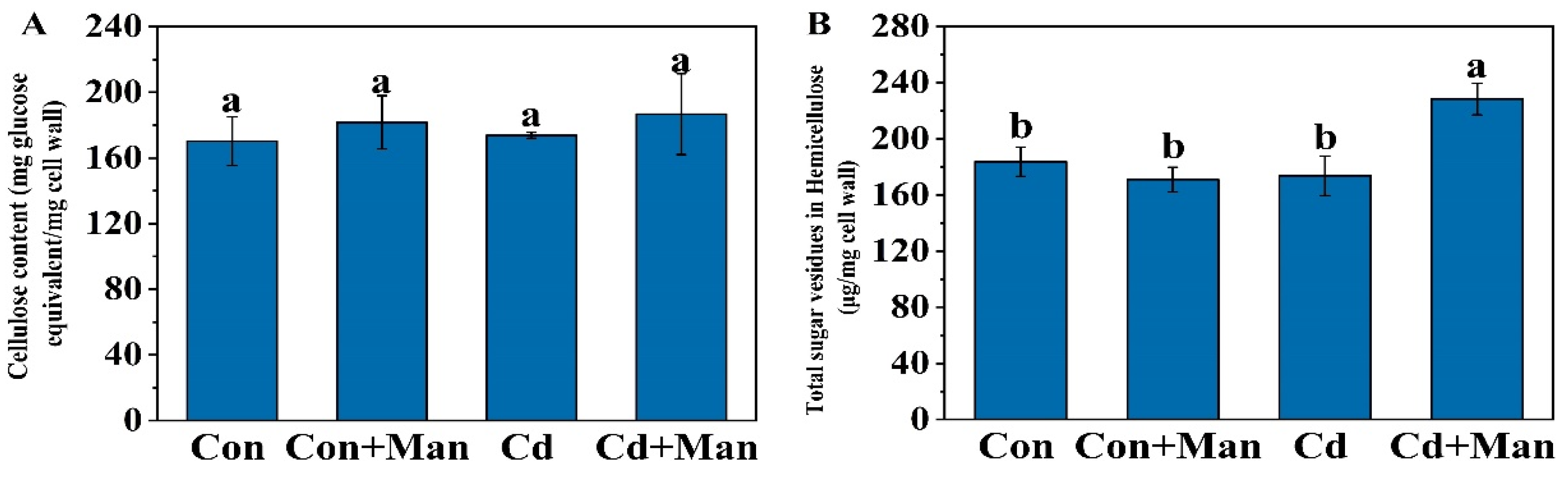
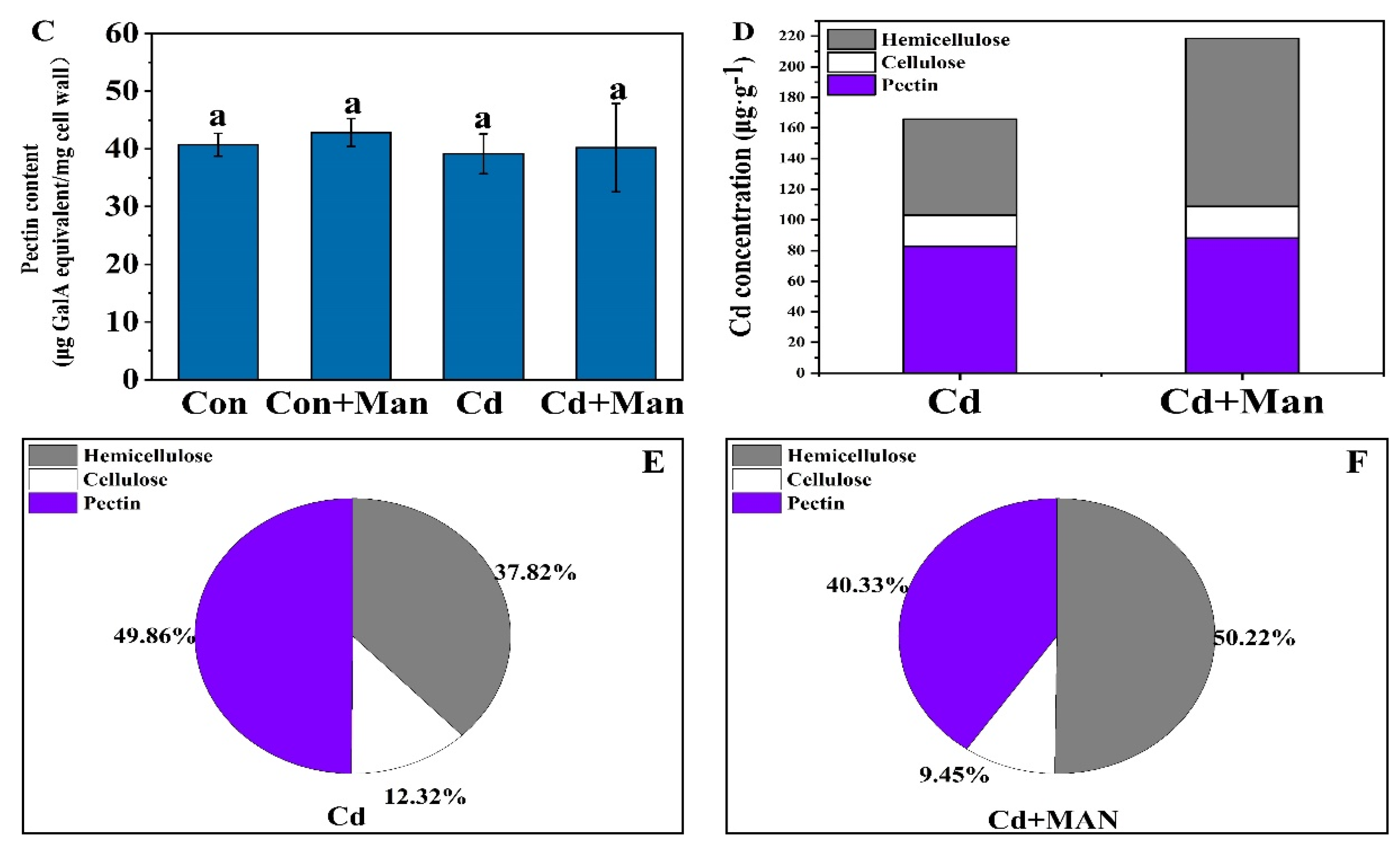
3.5. Effects of MAN on the Cell Wall Composition and Cd Content in Wheat Roots
3.6. Effects of MAN on the Chemical Forms of Cd in Wheat Roots
3.7. Effect of MAN on TF and BCF Values of Cd in Wheat
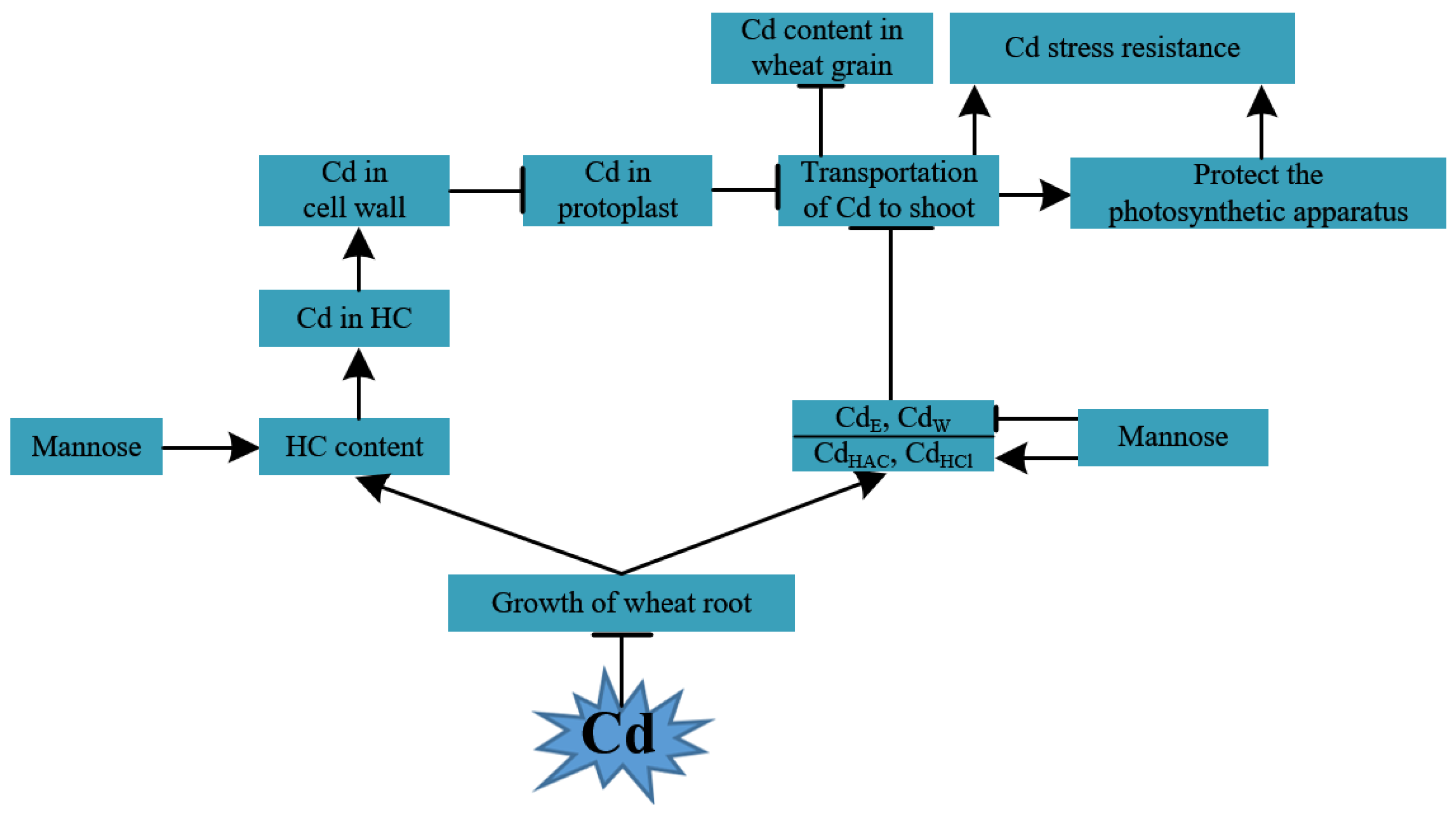
4. Discussion
4.1. MAN Alleviated Cd Stress in Wheat
4.2. MAN Can Enhance Cd Accumulation in the Cell Wall
4.3. MAN Enhanced the Cd-Binding Capacity of Hemicellulose
4.4. MAN Alters the Chemical Forms of Cd
4.5. MAN Inhibits Cd Transport into the Above-Ground Parts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbudin, N.R.; Kamal, N.A. Establishment of material flow analysis (MFA) for heavy metals in a wastewater system. Ain Shams Eng. J. 2021, 12, 1407–1418. [Google Scholar] [CrossRef]
- Rui, W.; Shuang, W.; Kan, W.; Shiqiao, H.; Ruijie, W.; Bo, L.; Min, L.; Liang, L.; Dawei, Z.; Xinpeng, D. Estimation and Spatial Analysis of Heavy Metals in Metal Tailing Pond Based on Improved PLS With Multiple Factors. IEEE Access 2021, 9, 64880–64894. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussiere, S.; Robert, T.; Cornu, J.Y. Cadmium allocation to grains in durum wheat exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2019, 184, 109592. [Google Scholar] [CrossRef]
- Rashid, I.; Murtaza, G.; Dar, A.A.; Wang, Z. The influence of humic and fulvic acids on Cd bioavailability to wheat cultivars grown on sewage irrigated Cd-contaminated soils. Ecotoxicol. Environ. Saf. 2020, 205, 111347. [Google Scholar] [CrossRef]
- Jiang, X.; Dai, J.; Zhang, X.; Wu, H.; Tong, J.; Shi, J.; Fang, W. Enhanced Cd efflux capacity and physiological stress resistance: The beneficial modulations of Metarhizium robertsii on plants under cadmium stress. J. Hazard. Mater. 2022, 437, 129429. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z. Recent Advances in Minimizing Cadmium Accumulation in Wheat. Toxics 2022, 10, 187. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Xu, K.; Wang, J. Cadmium in Cereal Crops: Uptake and Transport Mechanisms and Minimizing Strategies. J. Agric. Food. Chem. 2022, 70, 5961–5974. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, X.; Pan, N.; Shen, Z.; Chen, Y.; Lu, W. Effects of mannose on wheat(Triticum aestivum L.) growth, cadmium transport and oxidative stress under cadmium stress. J. Nanjing Agricult. Univ. 2021, 44, 468–476. [Google Scholar]
- Khan, I.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.S.; Izzet, W.; Alhammad, B.A.; Ali, E.F.; Roy, R.; et al. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12303. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.A.; Hayat, M.T.; Yang, C.; Xu, L.; Zhou, W.J. 5-Aminolevulinic Acid Ameliorates the Growth, Photosynthetic Gas Exchange Capacity, and Ultrastructural Changes Under Cadmium Stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef]
- Naeem, A.; Saifullah; Rehman, M.Z.; Akhtar, T.; Ok, Y.S.; Rengel, Z. Genetic Variation in Cadmium Accumulation and Tolerance among Wheat Cultivars at the Seedling Stage. Commun. Soil Sci. Plant Anal. 2016, 47, 554–562. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Wu, J.; Li, R.; Lu, Y.; Bai, Z. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J. Environ. Manage. 2021, 295, 113081. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- Ishikawa, N.; Ishioka, G.; Yanaka, M.; Takata, K.; Murakami, M. Effects of Ammonium Chloride Fertilizer and its Application Stage on Cadmium Concentrations in Wheat (Triticum aestivum L.) Grain. Plant Prod. Sci. 2015, 18, 137–145. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T. Novel Field Data on Phytoextraction: Pre-Cultivation With Salix Reduces Cadmium in Wheat Grains. Int. J. Phytorem. 2015, 17, 917–924. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Asghar, H.N.; Saleem, M.; Khan, M.Y.; Zahir, Z.A. Synergistic Effect of Rhizobia and Biochar on Growth and Physiology of Maize. Agron. J. 2015, 107, 2327–2334. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, J.; Zhang, Y.; Bi, Z.; Wei, H. Microwave pretreatment can enhance tolerance of wheat seedlings to CdCl2 stress. Ecotoxicol. Environ. Saf. 2011, 74, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Gondor, O.K.; Szalai, G.; Kovacs, V.; Janda, T.; Pal, M. Impact of UV-B on drought- or cadmium-induced changes in the fatty acid composition of membrane lipid fractions in wheat. Ecotoxicol. Environ. Saf. 2014, 108, 129–134. [Google Scholar] [CrossRef]
- Herold, A.; Lewis, D.H. Mannose and green plants—Occurrence, physiology and metabolism, and use as a tool to study role of orthophosphate. New Phytol. 1977, 79, 1–40. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, N.; Malik, S.A. Mannose-Induced Modulations in Antioxidants, Protease Activity, Lipid Peroxidation, and Total Phenolics in Etiolated Wheat Leaves. J. Plant Growth Regul. 2009, 28, 58–65. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in Cd detoxification for the production of low Cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Chu, S.; Hayat, K.; Chi, Y.; Zhi, Y.; Zhang, D.; Zhou, P. Influence of Cd toxicity on subcellular distribution, chemical forms, and physiological responses of cell wall components towards short-term Cd stress in Solanum nigrum. Environ. Sci. Pollut. Res. 2021, 28, 13955–13969. [Google Scholar] [CrossRef]
- Li, H.-Y.; Sun, S.-N.; Zhou, X.; Peng, F.; Sun, R.-C. Structural characterization of hemicelluloses and topochemical changes in Eucalyptus cell wall during alkali ethanol treatment. Carbohydr. Polym. 2015, 123, 17–26. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Chauhan, P.K. Optimization of eco-friendly amendments as sustainable asset for salt-tolerant plant growth-promoting bacteria mediated maize (Zea Mays L.) plant growth, Na uptake reduction and saline soil restoration. Environ. Res. 2022, 211, 113081. [Google Scholar] [CrossRef]
- Knudson, L.L.; Tibbitts, T.W.; Edwards, G.E. Measurement of ozone injury by determination of leaf chlorophyll concentration. Plant Physiol. 1977, 60, 606–608. [Google Scholar] [CrossRef]
- Duan, B.; Ma, Y.; Jiang, M.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015, 75, 33–44. [Google Scholar] [CrossRef]
- Zeng, F.; Zhou, W.; Qiu, B.; Ali, S.; Wu, F.; Zhang, G. Subcellular distribution and chemical forms of chromium in rice plants suffering from different levels of chromium toxicity. J. Plant Nutr. Soil Sci. 2011, 174, 249–256. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Jiang, T.; Liu, Y.; Li, G.X.; Zheng, S.J. Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 2012, 236, 989–997. [Google Scholar] [CrossRef]
- Su, Y.; Liu, J.; Lu, Z.; Wang, X.; Zhang, Z.; Shi, G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ. Exp. Bot. 2014, 97, 40–48. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Gu, J.; Bai, X.; Ren, Y.; Fan, T.; Han, Y.; Jiang, L.; Xiao, F.; Liu, Y.; et al. MAN3 gene regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis thaliana. New Phytol. 2015, 205, 570–582. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, N.; Malik, S.A. Effect of D-mannose on antioxidant defense and oxidative processes in etiolated wheat coleoptiles. Acta Physiol. Plant. 2014, 36, 161–167. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Fan, J.; Liu, R.; Sun, R. Single and Joint Toxicity of Sulfamonomethoxine and Cadmium on Three Agricultural Crops. Soil Sediment Contam. 2015, 24, 454–470. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Q.; Cai, Z. Effect of soil HHCB on cadmium accumulation and phytotoxicity in wheat seedlings. Ecotoxicology 2014, 23, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Nie, Z.; Liu, H.; Zhao, P.; Qin, S.; Shi, Z. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-R.; Zhou, S.-L.; Wu, S.-H. Accumulation of Heavy Metals in Different Parts of Wheat Plant from the Yangtze River Delta, China. Int. J. Agric. Biol. 2016, 18, 1242–1248. [Google Scholar] [CrossRef]
- Wang, P.; Yang, B.; Wan, H.; Fang, X.; Yang, C. The differences of cell wall in roots between two contrasting soybean cultivars exposed to cadmium at young seedlings. Environ. Sci. Pollut. Res. 2018, 25, 29705–29714. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, M.; Le Faucheur, S.; Fortin, C.; Campbell, P.G.C. Cadmium detoxification strategies in two phytoplankton species: Metal binding by newly synthesized thiolated peptides and metal sequestration in granules. Aquat. Toxicol. 2009, 92, 65–75. [Google Scholar] [CrossRef]
- Guan, M.Y.; Zhang, H.H.; Pan, W.; Jin, C.W.; Lin, X.Y. Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. Sci. Total Environ. 2018, 627, 663–670. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Wei, T.; Zhou, R.; Muhammad, H.; Hua, L.; Ren, X.; Guo, J.; Ding, Y. Accumulation and fixation of Cd by tomato cell wall pectin under Cd stress. Environ. Exp. Bot. 2019, 167, 103829. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Xiong, J.; An, L.; Lu, H.; Zhu, C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 2009, 230, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Xie, X.; Weiss, D.J.; Liu, J.; Lu, H.; Yan, C. Kandelia obovata (S. L.) Yong tolerance mechanisms to Cadmium: Subcellular distribution, chemical forms and thiol pools. Mar. Pollut. Bull. 2012, 64, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Qin, X.; Zhao, L.; Liang, X.; Sun, Y.; Xu, Y. Soil application of manganese sulfate effectively reduces Cd bioavailability in Cd-contaminated soil and Cd translocation and accumulation in wheat. Sci. Total Environ. 2022, 814, 152765. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-T.; Du, Z.-J.; Busso, C.-A.; Qi, X.-B.; Wu, H.-Q.; Guo, W.; Wu, D.-F. Differences in root surface adsorption, root uptake, subcellular distribution, and chemical forms of Cd between low- and high-Cd-accumulating wheat cultivars. Environ. Sci. Pollut. Res. 2020, 27, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.-P.; Giehl, R.F.H.; Pitann, B.; Muehling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, P.; Liu, H.; Nie, Z.; Zhu, J.; Qin, S.; Li, C. Selenium inhibits cadmium uptake and accumulation in the shoots of winte wheat by altering the transformation of chemical forms of cadmium in soil. Environ. Sci. Pollut. Res. 2022, 29, 8525–8537. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ahmad, M.; Srivastava, A.K.; Abhilash, P.C.; Sharma, B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere 2021, 267, 129216. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Yang, L.; Gong, H.; Wang, L.; Li, H.; Yu, J.; Li, Y.; Deji, Y.; Nima, C.; Zhao, S.; et al. Bioaccumulation characteristics, transfer model of heavy metals in soil-crop system and health assessment in plateau region, China. Ecotoxicol. Environ. Saf. 2022, 241, 113733. [Google Scholar] [CrossRef] [PubMed]
- Soubasakou, G.; Cavoura, O.; Damikouka, I. Phytoremediation of Cadmium-Contaminated Soils: A Review of New Cadmium Hyperaccumulators and Factors Affecting their Efficiency. Bull. Environ. Contam. Toxicol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ke, Y.; Zhou, Q.; Tao, R.; Wang, Y. Derived regional soil-environmental quality criteria of metals based on Anhui soil-crop systems at the regulated level. Sci. Total Environ. 2022, 825, 154060. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; You, Y.; Wang, J.; Chen, X.; Chu, S.; Wang, R.; Zhang, X.; Yin, S.; Zhang, D.; Zhou, P. Two plant growth-promoting bacterial Bacillus strains possess different mechanisms in affecting cadmium uptake and detoxification of Solanum nigrum L. Chemosphere 2022, 305, 135488. [Google Scholar] [CrossRef] [PubMed]
| Soil Properties | Value (Pot) |
|---|---|
| pH | 7.02 |
| Organic matter (g kg−1) | 14.80 |
| Alkali N (mg kg−1) | 36.05 |
| Available P (mg kg−1) | 30.15 |
| Available K (mg kg−1) | 87.12 |
| Total Cd (mg kg−1) | 3.30 |
| EDTA-Cd (mg kg−1) | 1.71 |
| Treatment | Length (cm) | Surface Area (cm2) | Root Volume (cm3) | Average Diameter (mm) | Tips |
|---|---|---|---|---|---|
| Con | 349 ± 5.32 a | 16.2 ± 0.59 a | 0.098 ± 0.0003 a | 0.242 ± 0.008 a | 2423 ± 9.81 a |
| Cd | 165 ± 8.19 c | 10.7 ± 0.84 c | 0.055 ± 0.0061 d | 0.206 ± 0.006 d | 1584 ± 33.4 cd |
| Cd + 40 Man | 167 ± 15.1 c | 11.8 ± 1.30 bc | 0.067 ± 0.0088 cd | 0.225 ± 0.007 bc | 1694 ± 149.7 bcd |
| Cd + 80 Man | 207 ± 16.9 b | 13.7 ± 0.97 ab | 0.072 ± 0.0043 c | 0.211± 0.002 cd | 1553 ± 67.1 c |
| Cd + 160 Man | 207 ± 4.68 b | 15.2 ± 0.41 a | 0.089 ± 0.0052 ab | 0.234 ± 0.002 ab | 1982 ± 50.9 b |
| Cd + 320 Man | 210 ± 20.9 b | 14.5 ± 1.20 ab | 0.080 ± 0.0052 bc | 0.221± 0.004 bcd | 2023 ± 185.7 b |
| Cd + 640 Man | 155 ± 1.47 c | 10.1 ± 0.29 c | 0.052 ± 0.0023 d | 0.207 ± 0.004 d | 1875 ± 210.2 bcd |
| Treatment | CdE (mg kg−1) | CdW (mg kg−1) | CdNaCl (mg kg−1) | CdHAC (mg kg−1) | CdHCl (mg kg−1) | CdR (mg kg−1) |
|---|---|---|---|---|---|---|
| Cd | 543.1 ± 29.5 | 870.9 ± 20.5 | 410.8 ± 21.6 | 329.8 ± 25.2 | 238.6 ± 32.9 | 111.1 ± 13.7 |
| Cd + Man | 471.1 ± 28.6 * | 815.0 ± 19.4 * | 458.4 ± 33.2 | 498.8 ± 22.2 * | 368.2 ± 32.8 * | 140.9 ± 21.2 |
| Treatment | Root Cd Content (mg kg−1) | Stem Cd Content (mg kg−1) | Leaf Cd Content (mg kg−1) | Grain Cd Content (mg kg−1) |
|---|---|---|---|---|
| Con | 8.73 ± 0.46 | 2.76 ± 0.20 | 1.75 ± 0.18 | 0.19 ± 0.02 |
| MAN | 10.3 ± 0.59 * | 2.46 ± 0.07 | 1.57 ± 0.06 | 0.14 ± 0.01 * |
| Treatment | TFroot-stem | TFstem-leaf | TFleaf-grain | BCFroot | BCFleaf | BCFstem | BCFgrain |
|---|---|---|---|---|---|---|---|
| Con | 0.32 ± 0.03 | 0.64 ± 0.06 | 0.11 ± 0.02 | 2.79 ± 0.15 | 0.88 ± 0.06 | 0.56 ± 0.06 | 0.06 ± 0.01 |
| MAN | 0.24 ± 0.02 * | 0.62 ± 0.05 | 0.09 ± 0.01 * | 3.28 ± 0.19 * | 0.79 ± 0.05 | 0.50 ± 0.19 | 0.05 ± 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Cheng, X.; Pan, N.; Huang, W.; Shi, L.; Lu, W. Foliar Spraying of Mannose Alleviates Cadmium Stress by Changing the Subcellular Distribution and Chemical Forms of Cadmium in Wheat Root. Agronomy 2022, 12, 2273. https://doi.org/10.3390/agronomy12102273
Zheng X, Cheng X, Pan N, Huang W, Shi L, Lu W. Foliar Spraying of Mannose Alleviates Cadmium Stress by Changing the Subcellular Distribution and Chemical Forms of Cadmium in Wheat Root. Agronomy. 2022; 12(10):2273. https://doi.org/10.3390/agronomy12102273
Chicago/Turabian StyleZheng, Xiang, Xue Cheng, Ni Pan, Wei Huang, Liang Shi, and Wei Lu. 2022. "Foliar Spraying of Mannose Alleviates Cadmium Stress by Changing the Subcellular Distribution and Chemical Forms of Cadmium in Wheat Root" Agronomy 12, no. 10: 2273. https://doi.org/10.3390/agronomy12102273





