Effects of Ascorbic Acid and/or α-Tocopherol on Agronomic and Physio-Biochemical Traits of Oat (Avena sativa L.) under Drought Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Layout and Treatments
2.2. Post-Experiment Soil Analysis
2.3. Agronomic Analysis
2.4. Physio-Biochemical Features
2.4.1. Leaf Pigment Contents
2.4.2. Soluble Sugar Content (SSC), Glycine Betaine (GB), Total Proline Content (TPC), and Soluble Protein Content (SPC)
2.4.3. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA)
2.4.4. Antioxidant Enzymatic Assays
2.5. Analysis through Statistical Software
3. Results and Discussion
3.1. Effect on Soil Physicochemical Properties
3.2. Agronomic Features
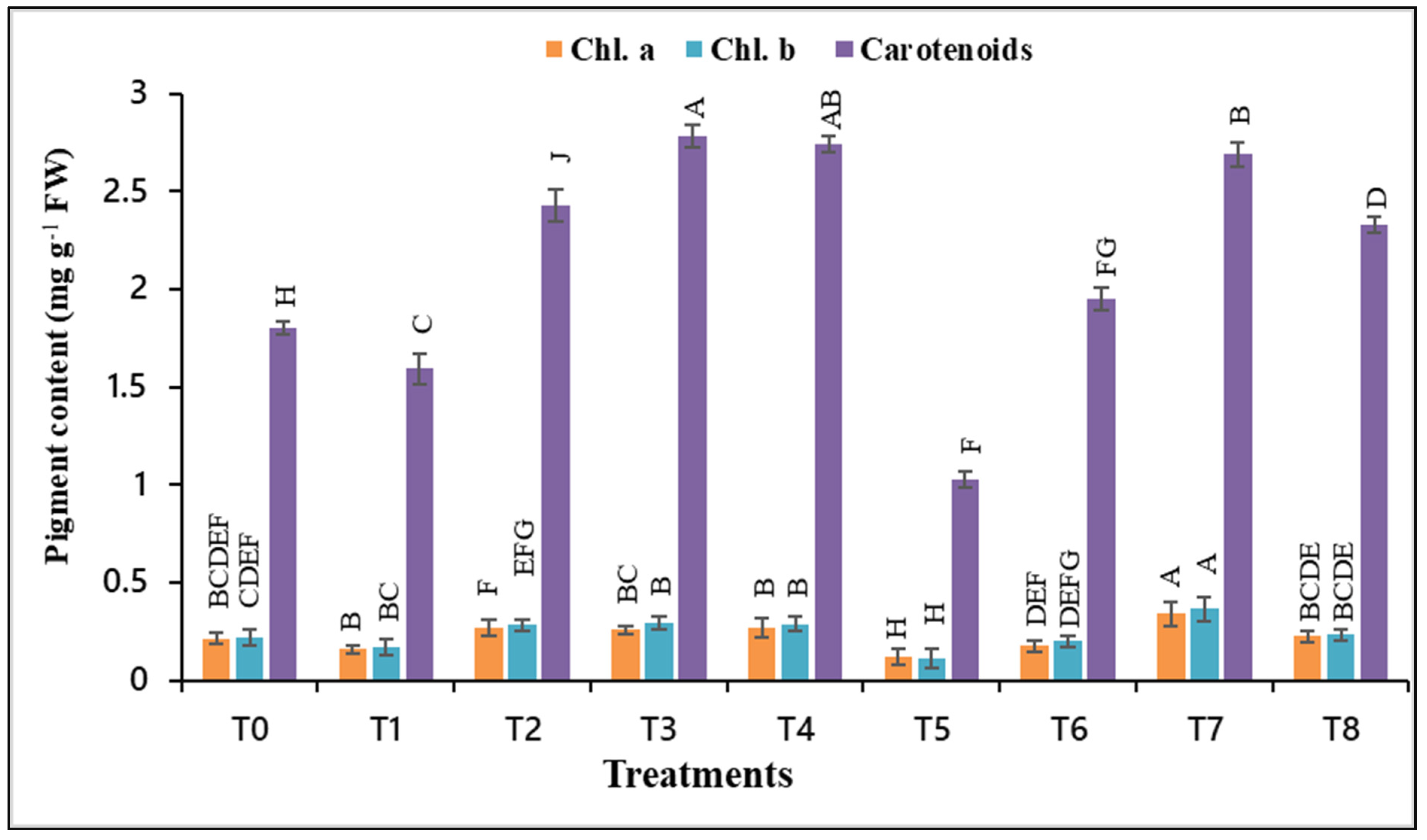
3.3. AsA and/or α-toc Mitigate the Effect of Drought Stress on Photosynthetic Pigments
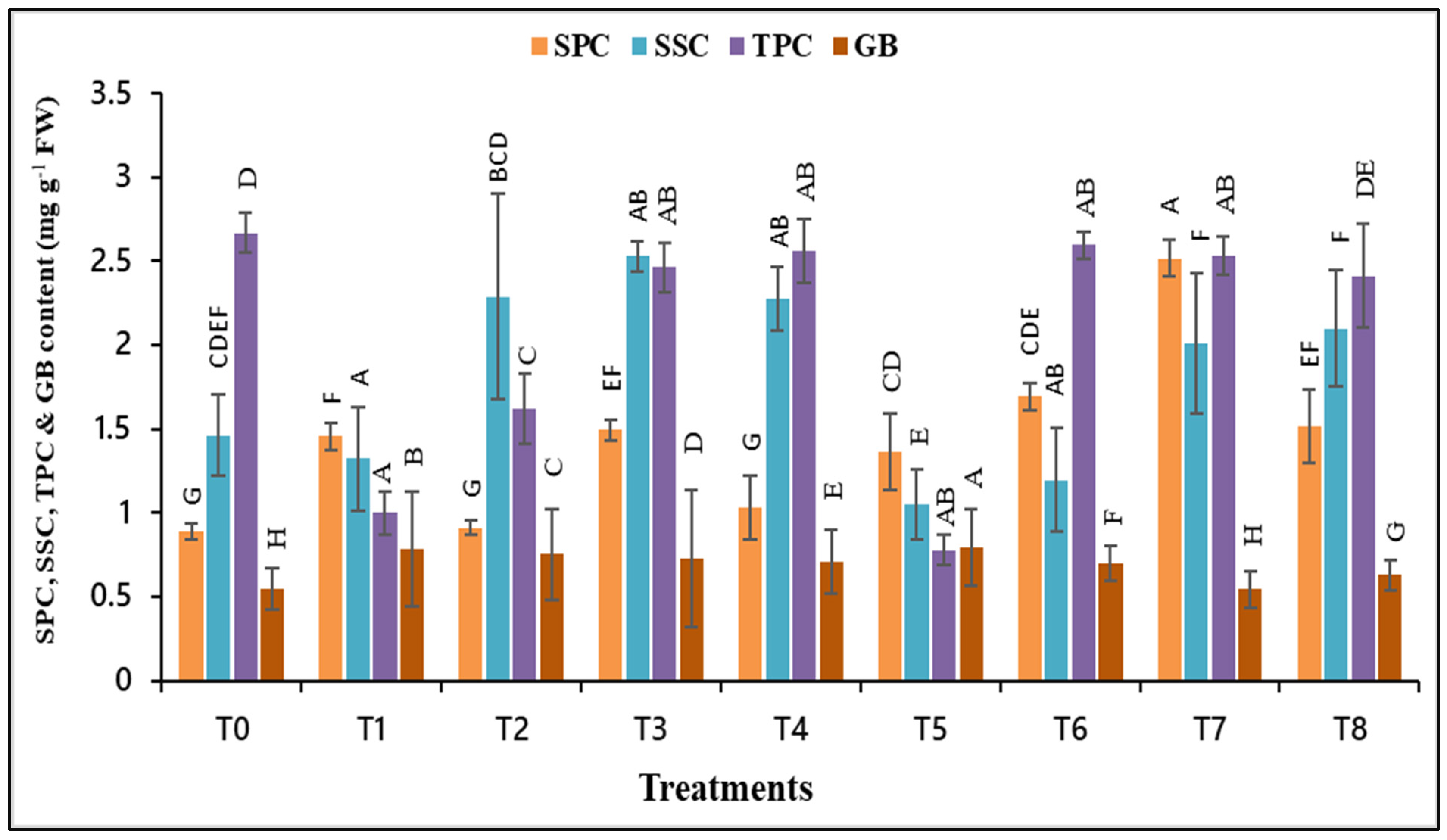
3.4. Impact of AsA and/or α-toc on Soluble Protein, Sugar, Proline, and GB under Drought Stress
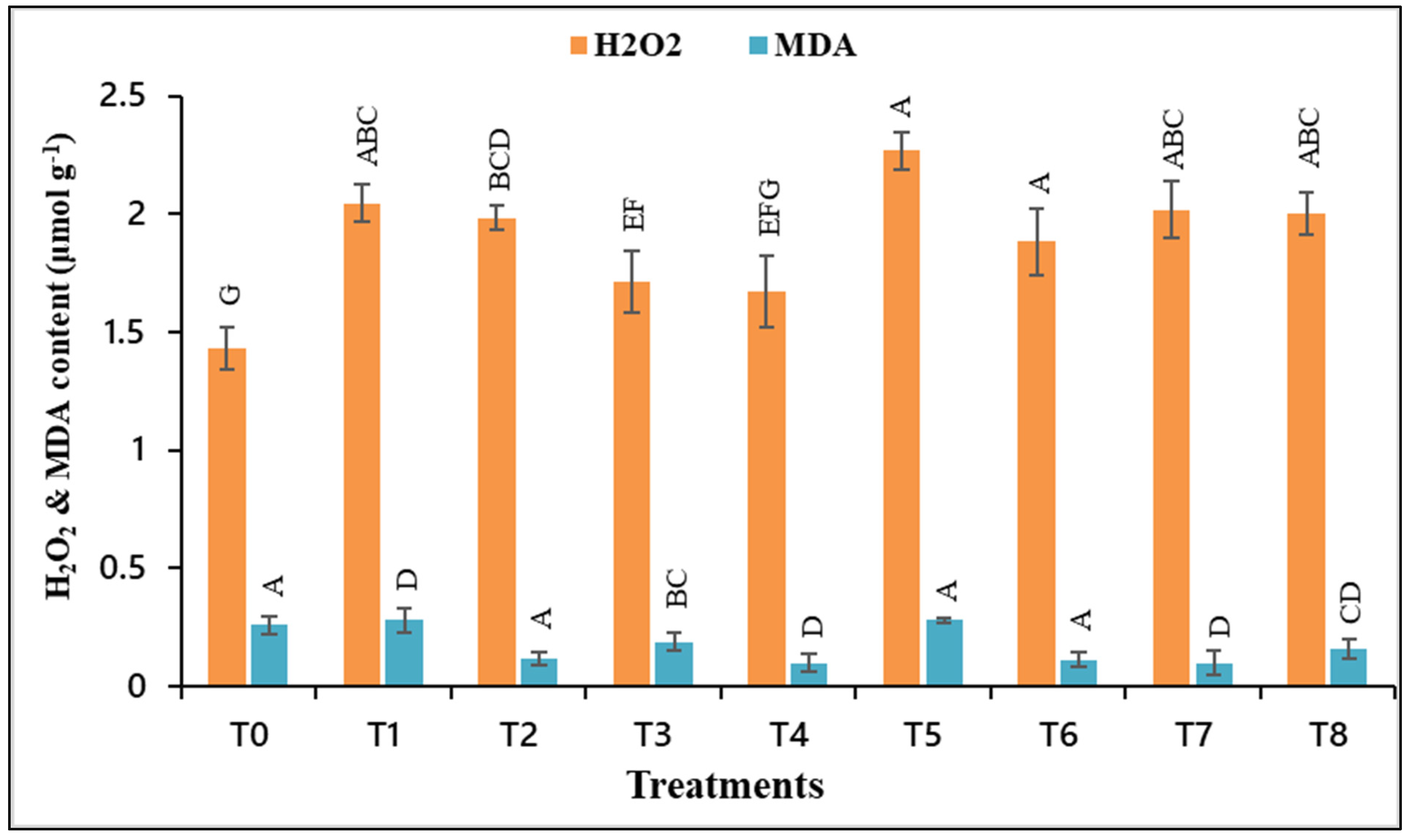
3.5. H2O2 and MDA
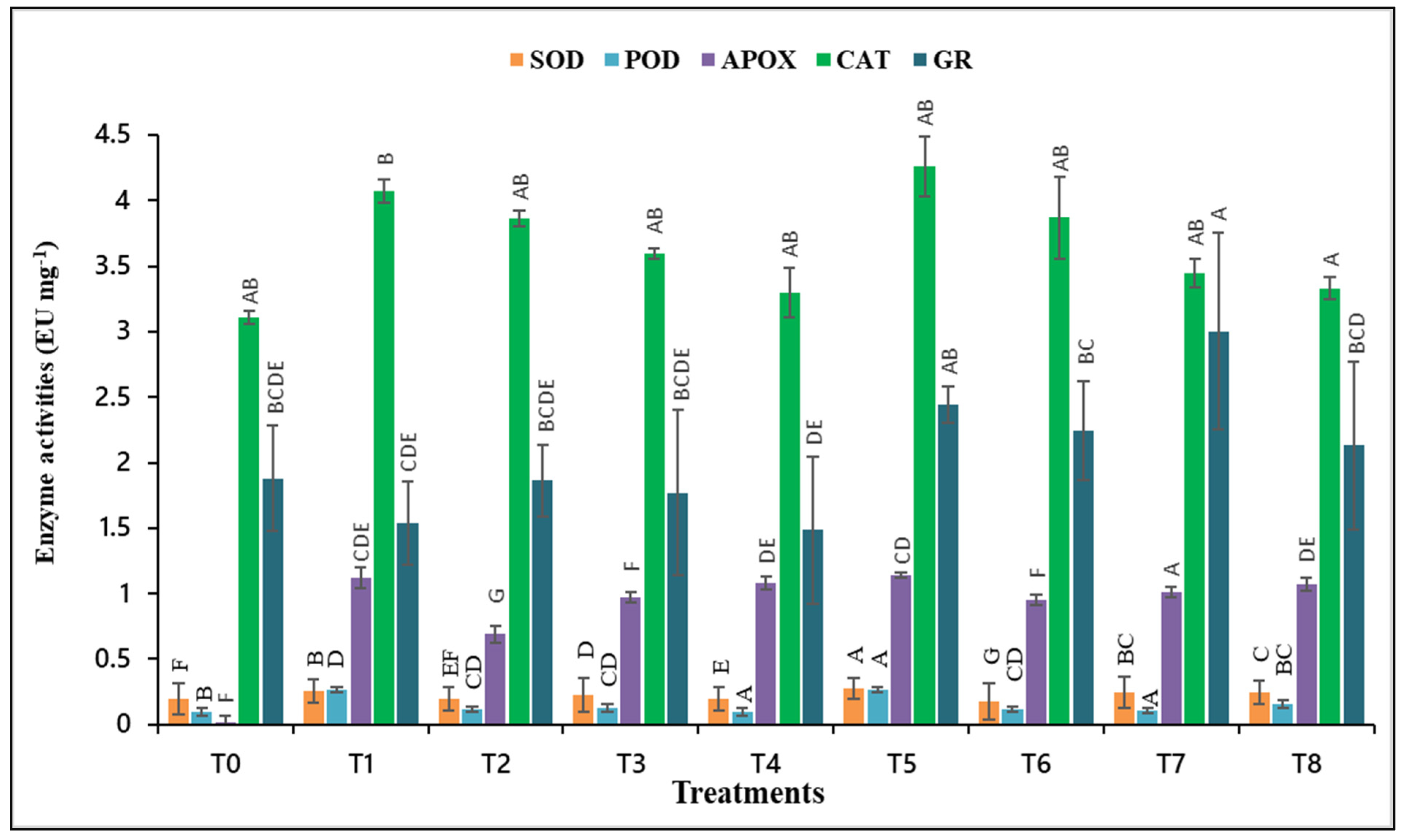
3.6. Antioxidant Enzyme Activities
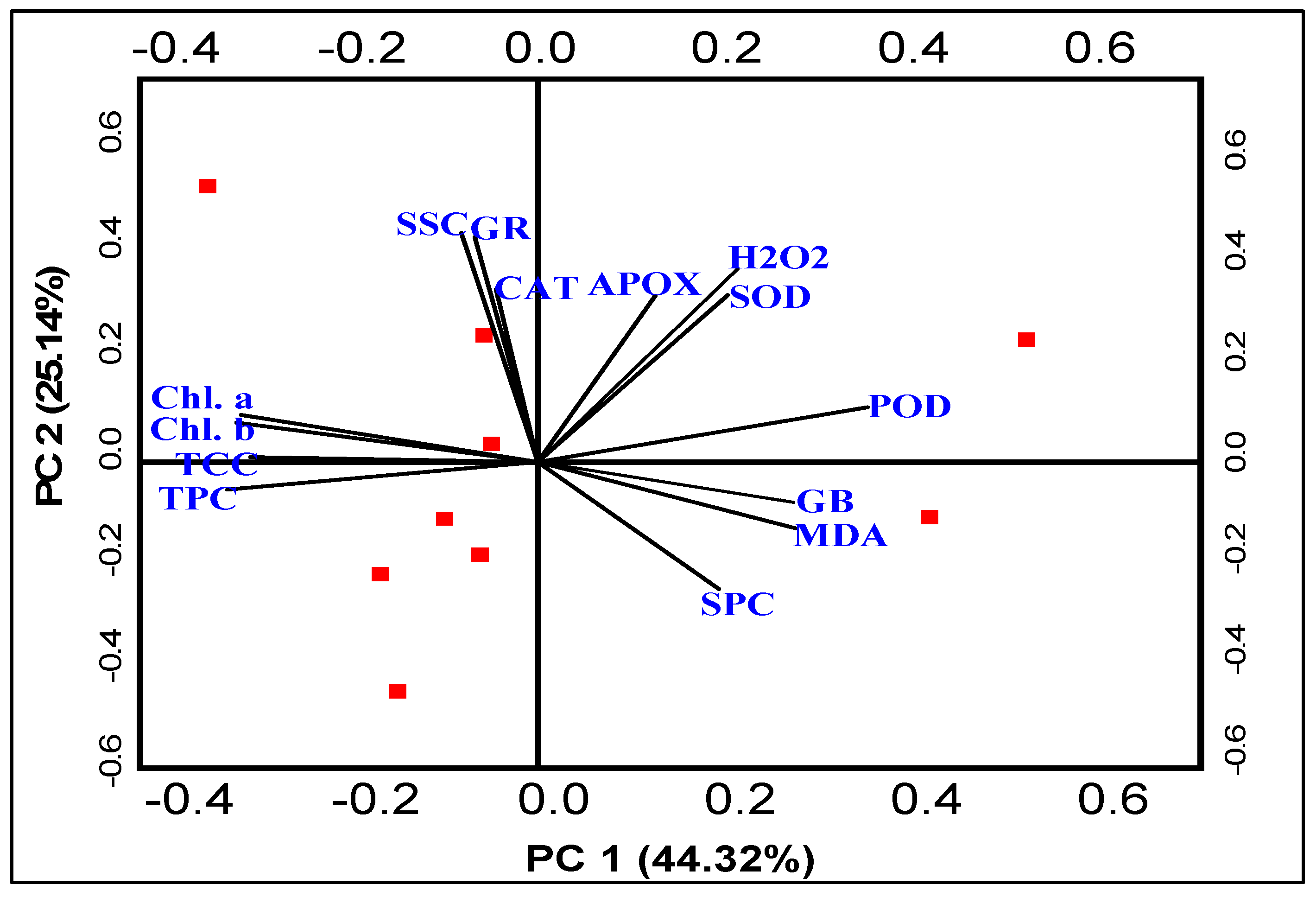
3.7. Pearson’s Correlation and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramzan, S. Oat: A novel therapeutic ingredient for food applications. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 756–760. [Google Scholar] [CrossRef]
- Ahmad, M.; Jehangir, I.; Rizvan, R.; Dar, S.A.; Iqbal, S.; Wani, S.; Mehraj, U.; Hassan, R. Phylogenetic Relationship of Oats (Avena sativa L): A Guide to Conservation and Utilisation of Genetic Resources. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 831–845. [Google Scholar] [CrossRef]
- Mehraj, U.; Abidi, I.; Ahmad, M.; Wani, B.; Mir, S. Genotype x environment interaction for forage yield and its components in oats (Avena sativa L.). Electron. J. Plant Breed. 2017, 8, 157–162. [Google Scholar] [CrossRef]
- Shoaib, M.; Khan, M.N.; Akhtar, N.; Ayub, M.; Ashraf, M.S.; Ghaffar, A.; Ullah, S. Productivity of oat (Avena sativa L.)-berseem (Trifolium alexandrinum L.) forage mixture in irrigated plain of Pakistan. Pak. J. Agric. Sci. 2018, 55, 303–312. [Google Scholar]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Hossain, M.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous melatonin modulates the physiological and biochemical mechanisms of drought tolerance in tartary buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N. Biostimulant-treated seedlings under sustainable agriculture: A global perspective facing climate change. Agronomy 2020, 11, 14. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Wiley: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Sadiq, M.; Akram, N.A.; Ashraf, M. Impact of exogenously applied tocopherol on some key physio-biochemical and yield attributes in mungbean [Vigna radiata (L.) Wilczek] under limited irrigation regimes. Acta Physiol. Plant. 2018, 40, 131. [Google Scholar] [CrossRef]
- Ali, Q.; Tariq Javed, M.; Haider, M.Z.; Habib, N.; Rizwan, M.; Perveen, R.; Ali, S.; Nasser Alyemeni, M.; El-Serehy, H.A.; Al-Misned, F.A. α-Tocopherol foliar spray and translocation mediates growth, photosynthetic pigments, nutrient uptake, and oxidative defense in maize (Zea mays L.) under drought stress. Agronomy 2020, 10, 1235. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Shah, S.M.; Sadaf; Amin, M.; Gul, B.; Begum, M. Ethnoecological, Elemental, and Phytochemical Evaluation of Five Plant Species of Lamiaceae in Peshawar, Pakistan. Scientifica 2020, 2020, 2982934. [Google Scholar] [CrossRef]
- Nafees, M.; Ullah, S.; Ahmed, I. Morphological and elemental evaluation of biochar through analytical techniques and its combined effect along with plant growth promoting rhizobacteria on Vicia faba L. under induced drought stress. Microsc. Res. Tech. 2021, 84, 2947–2959. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ullah, S.; Ali, S.; Khan, A.; Ali, M.; Hassan, S. Using mathematical models to evaluate germination rate and seedlings length of chickpea seed (Cicer arietinum L.) to osmotic stress at cardinal temperatures. PLoS ONE 2021, 16, e0260990. [Google Scholar] [CrossRef] [PubMed]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Ahmad, A.; Anis, M. Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium Roxb.: A potential drug-yielding tree. J. Plant Growth Regul. 2019, 38, 1007–1016. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Hitz, W.D.; Hanson, A.D. Determination of glycine betaine by pyrolysis-gas chromatography in cereals and grasses. In Genetic Engineering of Osmoregulation; Springer: Cham, Switzerland, 1980; p. 362. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F.; Tsonev, T.; Brilli, F.; Edreva, A. Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct. Plant Biol. 2006, 33, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.A.; Taha, N.A.; Rakha, M.T.; El-Beltagi, H.S.; Shehata, W.F.; Ramadan, K.M.A.; El-Ramady, H.; Bayoumi, Y.A. CanGrafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress? Plants 2022, 11, 1147. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Shah, S.; Ullah, S.; Sulaiman; Mansour, A.T.; Shalaby, T.A. Impacts of ascorbic acid and alpha-tocopherol on Chickpea (Cicer arietinum L.) grown in water deficit regimes for sustainable production. Sustainability 2022, 14, 8861. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. Changes in phenolic compounds profile and glutathione status in raspberry fruit during storage in ozone-enriched atmosphere. Postharvest Biol. Technol. 2020, 168, 111277. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Newiry, N.A.; El-Tarawy, M.; El-Mahrouk, M.E.; Shala, A.Y.; El-Beltagi, H.S.; Rezk, A.A.; Ramadan, K.M.A.; Shehata, W.F.; El-Ramady, H. Biochemical and physiological response of Marigold (Tagetes Erecta L.) to foliar application ofsalicylic acid and potassium humate in different soil growth media. Gesunde Pflanz. 2022, 11, 14. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell Dev. Biol. Plant 2022, 58, 615–629. [Google Scholar] [CrossRef]
- Rojas, E.R.; Huang, K.C. Regulation of microbial growth by turgor pressure. Curr. Opin. Microbiol. 2018, 42, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; Akladious, S.A.; El-Beltagi, H.S. Mitigation the harmful effect of salt stress on physiological, biochemical andanatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Env. Bull. 2018, 27, 7054–7065. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Hameed, A.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Abdullah Alsahli, A.; Alyemeni, M.N. Seed treatment with α-tocopherol regulates growth and key physio-biochemical attributes in carrot (Daucus carota L.) plants under water limited regimes. Agronomy 2021, 11, 469. [Google Scholar] [CrossRef]
- Ali, A.; Beshir Issa, A.; Rahut, D.B. Adoption and impact of the maize hybrid on the livelihood of the maize growers: Some policy insights from Pakistan. Scientifica 2020, 2020, 5959868. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant. 2021, 172, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Shahid, S.; Perveen, R.; Alamri, S.A.; Alyemeni, M.N. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 12924. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Ge, G.; Han, G.; Jia, Y. Influence of drought stress on afalfa yields and nutritional composition. BMC Plant Biol. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Ahmed, S.; Ali, S. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.R.; Abdel-Aziz, S.M. Comparative response of drought tolerant and drought sensitive maize genotypes to water stress. Aust. J. Crop Sci. 2008, 1, 31–36. [Google Scholar]
- Shalaby, T.A.; Taha, N.A.; Taher, D.I.; Metwaly, M.M.; El-Beltagi, H.S.; Rezk, A.A.; El-Ganainy, S.M.; Shehata, W.F.; El-Ramady, H.R.; Bayoumi, Y.A. Paclobutrazol improves the quality of tomato seedlings to be resistant to Alternaria solani blight disease:Biochemical and histological perspectives. Plants 2022, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Abd El-Lateef, H.M.; Yasir, M.; Shah, S.T.; Ullah, I.; Mohamed, M.E.M.; Ali, I.; Ali, F. Effect of azospirillum and azotobacter species on the performance of cherry tomato under different salinity levels. Gesunde Pflanz. 2022, 74, 487–499. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A.; et al. Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequencecombination in improving chickpea plant through physiological change and antioxidant defense under different levels ofirrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed]
- Kobeasy, M.I.; El-Beltagi, H.S.; El-Shazly, M.A.; Khattab, E.A.H. Induction of resistance in Arachis hypogaea L. Against Peanut mottle virus by nitric oxide and salicylic acid. Physiol. Mol. Plant Pathol. 2011, 76, 112–118. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ismail, S.A.; Ibrahim, N.M.; Shehata, W.F.; Alkhateeb, A.A.; Ghazzawy, H.S.; El-Mogy, M.M.; Sayed, E.G. Unravelling the effect of triacontanol in combating drought stress by improving growth, productivity, and physiological performance in Strawberry plants. Plants 2022, 11, 1913. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ali, M.R.; Ramadan, K.M.A.; Anwar, R.; Shalaby, T.A.; Rezk, A.A.; El-Ganainy, S.M.; Mahmoud, S.F.; Alkafafy, M.; El-Mogy, M.M. Exogenous postharvest application of calcium chloride and salicylic acid to maintain the quality of broccoli florets. Plants 2022, 11, 1513. [Google Scholar] [CrossRef]
- Shah, S.; Khan, S.; Bussmann, R.W.; Ali, M.; Hussain, D.; Hussain, W. Quantitative ethnobotanical study of Indigenous knowledge on medicinal plants used by the tribal communities of Gokand Valley, District Buner, Khyber Pakhtunkhwa, Pakistan. Plants 2020, 9, 1001. [Google Scholar]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- El-Beltagi, H.S.; Maraei, R.W.; Shalaby, T.A.; Aly, A.A. Metabolites, nutritional quality and antioxidant activity of red radish roots affected by gamma rays. Agronomy 2022, 12, 1916. [Google Scholar] [CrossRef]
- Afify, A.M.M.R.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, T.A.; El-Gabry, Y.A.E.-G.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef] [PubMed]

| Treatments | T (°C) | pH | ORP (mV) | R (Ω·m) | EC (mS m−1) | TDS (mg L−1) | Salinity | DO | SDW (g) | SMC | FC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 17.8 | 7.6 | 109.2 | 1653 | 605 | 303 | 0.29 | 10.2 | 6.81 | 31.9 | 46.8 |
| T1 | 20.5 | 7.1 | 96.1 | 2688 | 372 | 148 | 0.18 | 11.1 | 6.89 | 31.1 | 45.1 |
| T2 | 19.6 | 7.1 | 82.5 | 3984 | 252 | 126 | 0.12 | 11.2 | 7.13 | 28.7 | 40.3 |
| T3 | 19.6 | 7.2 | 88.2 | 3367 | 296 | 148 | 0.14 | 11.2 | 7.43 | 25.7 | 34.6 |
| T4 | 19.6 | 7.0 | 83 | 3704 | 270 | 135 | 0.13 | 11.2 | 7.18 | 28.2 | 39.3 |
| T5 | 23.7 | 6.3 | 108.7 | 1675 | 597 | 298 | 0.29 | 10.2 | 7.82 | 21.8 | 27.9 |
| T6 | 19.4 | 7.2 | 103 | 2857 | 350 | 175 | 0.17 | 10.1 | 6.53 | 34.7 | 53.1 |
| T7 | 19.4 | 7.5 | 87.9 | 3268 | 306 | 153 | 0.15 | 11.2 | 6.3 | 37 | 58.7 |
| T8 | 19.4 | 7.2 | 91 | 3012 | 332 | 166 | 0.16 | 10.4 | 6.83 | 31.7 | 46.4 |
| Treatments | TGI | CVG | GE | MGT | GRI | WUE |
|---|---|---|---|---|---|---|
| T0 | 64.33 ± 3.0 a | 3.82 ± 0.4 a | 3.83 ± 0.4 ab | 4.5 ± 0.3 a | 130.3 ± 11.5 ab | 5824.2 ± 702.6 a |
| T1 | 50.33 ± 3.4 ac | 5.19 ± 0.5 ab | 3.04 ± 0.5 b | 5.9 ± 0.3 b | 96.8 ± 14.4 c | 8295.7 ± 2748.4 b |
| T2 | 51.67 ± 2.6 b | 5.06 ± 0.4 a | 3.08 ± 0.3 ac | 5.8 ± 0.2 ab | 99.4 ± 8.2 a | 5849.4 ± 527.4 cd |
| T3 | 49.33 ± 1.2 a | 5.67 ± 0.3 b | 2.65 ± 0.3 c | 6.0 ± 0.1 c | 88.4 ± 7.5 d | 5479.2 ± 1370.4 d |
| T4 | 50.33 ± 2.0 bc | 4.97 ± 0.3 cd | 3.37 ± 0.3 d | 5.9 ± 0.2 d | 104.6 ± 9.5 de | 8540.2 ± 1877.2 cd |
| T5 | 54.00 ± 3.7 d | 4.54 ± 0.3 c | 3.38 ± 0.2 cd | 5.6 ± 0.3 de | 107.0 ± 7.2 cd | 12,696.1 ± 10,168.7 de |
| T6 | 51.67 ± 4.1 cd | 5.05 ± 0.4 b | 3.21 ± 0.3 e | 5.8 ± 0.4 a | 102.5 ± 11.0 b | 7873.6 ± 3189.1 e |
| T7 | 54.67 ± 2.4 d | 5.00 ± 0.6 a | 3.31 ± 0.3 d | 5.5 ± 0.2 b | 108.7 ± 9.2 a | 6902 ± 3802 d |
| T8 | 51.00 ± 2.1 e | 5.12 ± 0.3 de | 3.14 ± 0.4 de | 5.9 ± 0.2 cd | 100.5 ± 11.4 d | 7270.9 ± 1044.1 b |
| Treatments | RMC | MGR | T50% | SVI-I | SVI-II |
|---|---|---|---|---|---|
| T0 | 47.7 ± 5.08 b | 0.22 ± 0.01 a | 3.4 ± 0.46 bc | 5516.6 ± 812.92 ab | 3760.0 ± 371.57 a |
| T1 | 42.4 ± 8.33 cd | 0.17 ± 0.02 b | 3.9 ± 0.29 c | 6110.0 ± 10.00 b | 3100.0 ± 353.27 ab |
| T2 | 52.6 ± 8.73 b | 0.17 ± 0.01 bc | 3.9 ± 0.29 bc | 5536.6 ± 609.29 bc | 3793.3 ± 245.54 c |
| T3 | 51.8 ± 4.99 c | 0.16 ± 0.00 d | 4.2 ± 0.23 c | 5713.3 ± 990.07 d | 4130.0 ± 201.16 bc |
| T4 | 49.6 ± 9.46 a | 0.17 ± 0.01 c | 3.7 ± 0.29 de | 5043.3 ± 1110.20 de | 4196.6 ± 310.09 cd |
| T5 | 48.5 ± 10.88 cd | 0.18 ± 0.01 de | 3.7 ± 0.29 d | 4243.3 ± 516.17 e | 3603.3 ± 530.18 b |
| T6 | 30.6 ± 9.33 d | 0.17 ± 0.00 b | 3.9 ± 0.29 ab | 4316.6 ± 343.85 cde | 3603.3 ± 575.63 c |
| T7 | 36.9 ± 5.67 dc | 0.18 ± 0.02 ab | 3.9 ± 0.29 a | 4680.0 ± 980.15 b | 3346.6 ± 616.68 de |
| T8 | 38.8 ± 3.7 c | 0.17 ± 0.01 b | 3.9 ± 0.29 bc | 4643.3 ± 914.40 a | 3796.6 ± 244.99 c |
| Variables | Source of Variation | SS | DF | MS | F | p |
|---|---|---|---|---|---|---|
| Chl. a | Treatment | 0.169 | 12 | 0.435 | 4.374 | 0.001 *** |
| AsA + α-toc | 0.125 | 5 | 0.767 | 3.685 | 0.002 ** | |
| Treatment × AsA + α-toc | 0.182 | 12 | 1.434 | 7.574 | 0.000 ** | |
| Error | 0.154 | 57 | 1.314 | - | - | |
| Chl. b | Treatment | 0.672 | 12 | 0.985 | 14.335 | 0.002 *** |
| AsA + α-toc | 0.212 | 5 | 1.085 | 7.465 | 0.001 ** | |
| Treatment × AsA + α-toc | 0.169 | 12 | 1.442 | 10.905 | 0.000 *** | |
| Error | 0.191 | 57 | 1.404 | - | - | |
| TCC | Treatment | 0.483 | 12 | 2.338 | 3.575 | 0.005 *** |
| AsA + α-toc | 0.284 | 5 | 0.918 | 3.01 | 0.002 * | |
| Treatment × AsA + α-toc | 0.218 | 12 | 1.095 | 3.575 | 0.002 ** | |
| Error | 0.769 | 57 | 0.318 | - | - | |
| SSC | Treatment | 0.938 | 12 | 1.047 | 5.908 | 0.005 *** |
| AsA + α-toc | 0.882 | 5 | 0.545 | 3.344 | 0.001 ** | |
| Treatment × AsA + α-toc | 0.303 | 12 | 1.553 | 9.124 | 0.011 ** | |
| Error | 0.154 | 57 | 1.424 | - | - | |
| TPC | Treatment | 0.548 | 12 | 2.193 | 13.214 | 0.002 *** |
| AsA + α-toc | 0.845 | 5 | 0.435 | 4.012 | 0.005 ** | |
| Treatment × AsA + α-toc | 0.992 | 12 | 1.432 | 9.125 | 0.000 *** | |
| Error | 0.622 | 57 | 0.435 | - | - | |
| SPC | Treatment | 0.769 | 12 | 0.871 | 9.453 | 0.005 *** |
| AsA + α-toc | 0.313 | 5 | 1.767 | 10.454 | 0.002 ** | |
| Treatment × AsA + α-toc | 0.422 | 12 | 1.435 | 3.901 | 0.000 *** | |
| Error | 0.622 | 57 | 1.993 | - | - | |
| H2O2 | Treatment | 0.391 | 12 | 2.656 | 12.465 | 0.011 |
| AsA + α-toc | 0.313 | 5 | 1.765 | 3.015 | 0.002 *** | |
| Treatment × AsA + α-toc | 0.958 | 12 | 2.096 | 4.01 | 0.016 ** | |
| Error | 0.154 | 57 | 0.757 | - | - | |
| GB | Treatment | 0.213 | 12 | 1.079 | 8.123 | 0.002 *** |
| AsA + α-toc | 0.311 | 5 | 0.371 | 4.901 | 0.011 ** | |
| Treatment × AsA + α-toc | 0.902 | 12 | 1 | 4.125 | 0.002 ** | |
| Error | 0.877 | 57 | 0.435 | - | - | |
| MDA | Treatment | 0.655 | 12 | 0.427 | 10.224 | 0.002 ** |
| AsA + α-toc | 0.32 | 5 | 0.394 | 6.015 | 0.018 *** | |
| Treatment × AsA + α-toc | 0.146 | 12 | 1.435 | 4.224 | 0.001 ** | |
| Error | 0.664 | 57 | 1.885 | - | - | |
| APOX | Treatment | 0.341 | 12 | 1.194 | 4.121 | 0.011 * |
| AsA + α-toc | 1.02 | 5 | 1.434 | 6.105 | 0.002 ** | |
| Treatment × AsA + α-toc | 0.904 | 12 | 1.076 | 2.325 | 0.016 *** | |
| Error | 0.123 | 57 | 0.868 | - | - | |
| SOD | Treatment | 0.324 | 12 | 0.536 | 4.232 | 0.005 ** |
| AsA + α-toc | 0.283 | 5 | 0.217 | 4.105 | 0.002 ** | |
| Treatment × AsA + α-toc | 0.374 | 12 | 0.327 | 8.123 | 0.000 ** | |
| Error | 0.755 | 57 | 0.975 | - | - | |
| POD | Treatment | 0.662 | 12 | 0.214 | 19.104 | 0.002 ** |
| AsA + α-toc | 0.332 | 5 | 0.655 | 14.995 | 0.001 ** | |
| Treatment × AsA + α-toc | 0.182 | 12 | 0.291 | 9.125 | 0.014 *** | |
| Error | 0.154 | 57 | 0.375 | - | - | |
| CAT | Treatment | 0.623 | 12 | 1.075 | 5.885 | 0.005 *** |
| AsA + α-toc | 0.359 | 5 | 0.98 | 5.215 | 0.001 ** | |
| Treatment × AsA + α-toc | 0.973 | 12 | 2.085 | 4.225 | 0.002 ** | |
| Error | 0.335 | 57 | 1.965 | - | - | |
| GR | Treatment | 0.893 | 12 | 1.114 | 22.434 | 0.002 ** |
| AsA + α-toc | 0.372 | 5 | 0.538 | 14.034 | 0.005 ** | |
| Treatment × AsA + α-toc | 0.182 | 12 | 0.212 | - | - | |
| Error | 0.553 | 57 | 0.634 | 5.104 | 0.002 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Sulaiman; Mohamed, M.E.M.; Ullah, S.; Shah, S. Effects of Ascorbic Acid and/or α-Tocopherol on Agronomic and Physio-Biochemical Traits of Oat (Avena sativa L.) under Drought Condition. Agronomy 2022, 12, 2296. https://doi.org/10.3390/agronomy12102296
El-Beltagi HS, Sulaiman, Mohamed MEM, Ullah S, Shah S. Effects of Ascorbic Acid and/or α-Tocopherol on Agronomic and Physio-Biochemical Traits of Oat (Avena sativa L.) under Drought Condition. Agronomy. 2022; 12(10):2296. https://doi.org/10.3390/agronomy12102296
Chicago/Turabian StyleEl-Beltagi, Hossam S., Sulaiman, Maged Elsayed Mohamed Mohamed, Sami Ullah, and Sikandar Shah. 2022. "Effects of Ascorbic Acid and/or α-Tocopherol on Agronomic and Physio-Biochemical Traits of Oat (Avena sativa L.) under Drought Condition" Agronomy 12, no. 10: 2296. https://doi.org/10.3390/agronomy12102296









