A Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils
Abstract
:1. Introduction
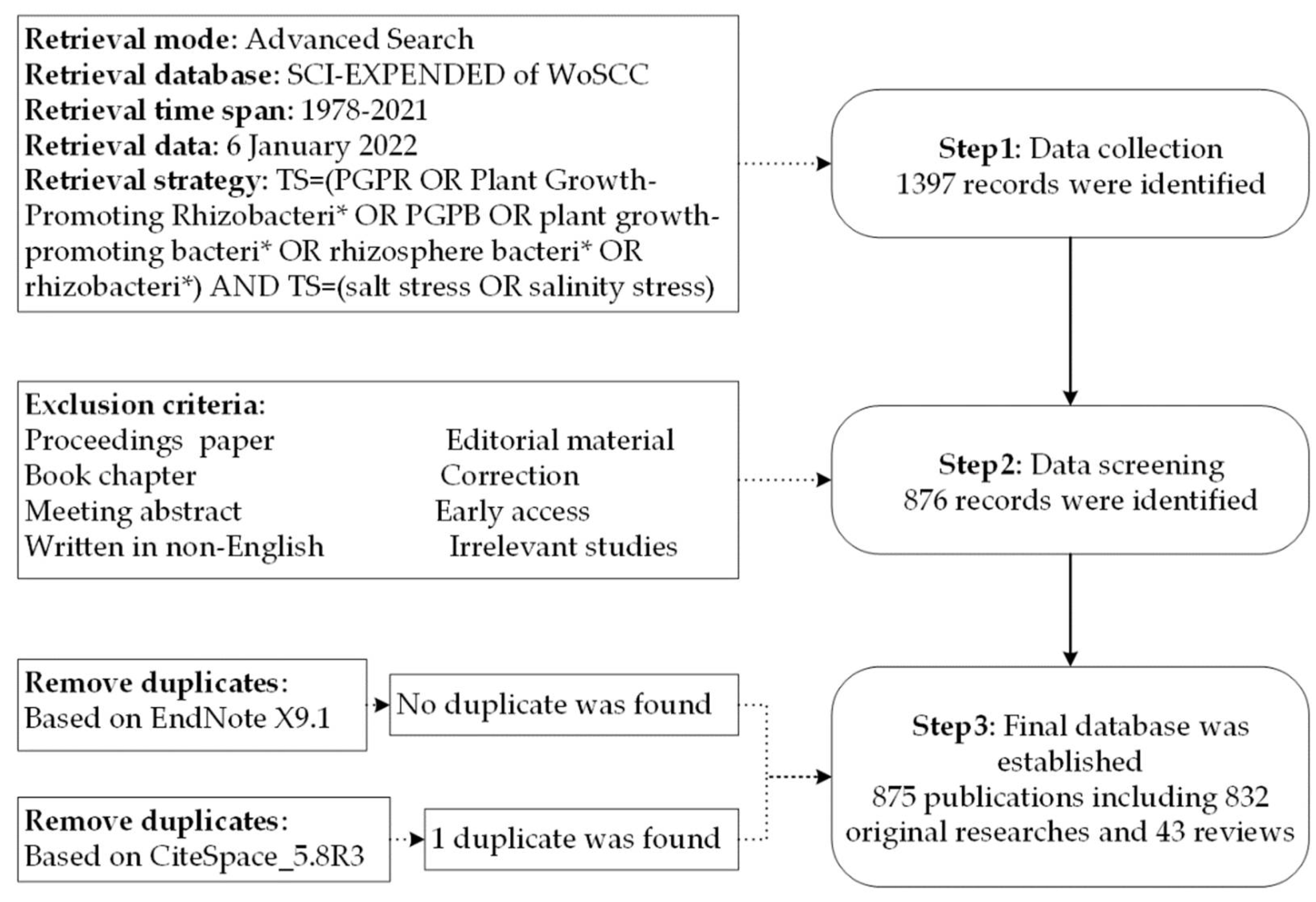
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Data Extraction
2.3. Data Analysis and Knowledge Mapping
3. Results
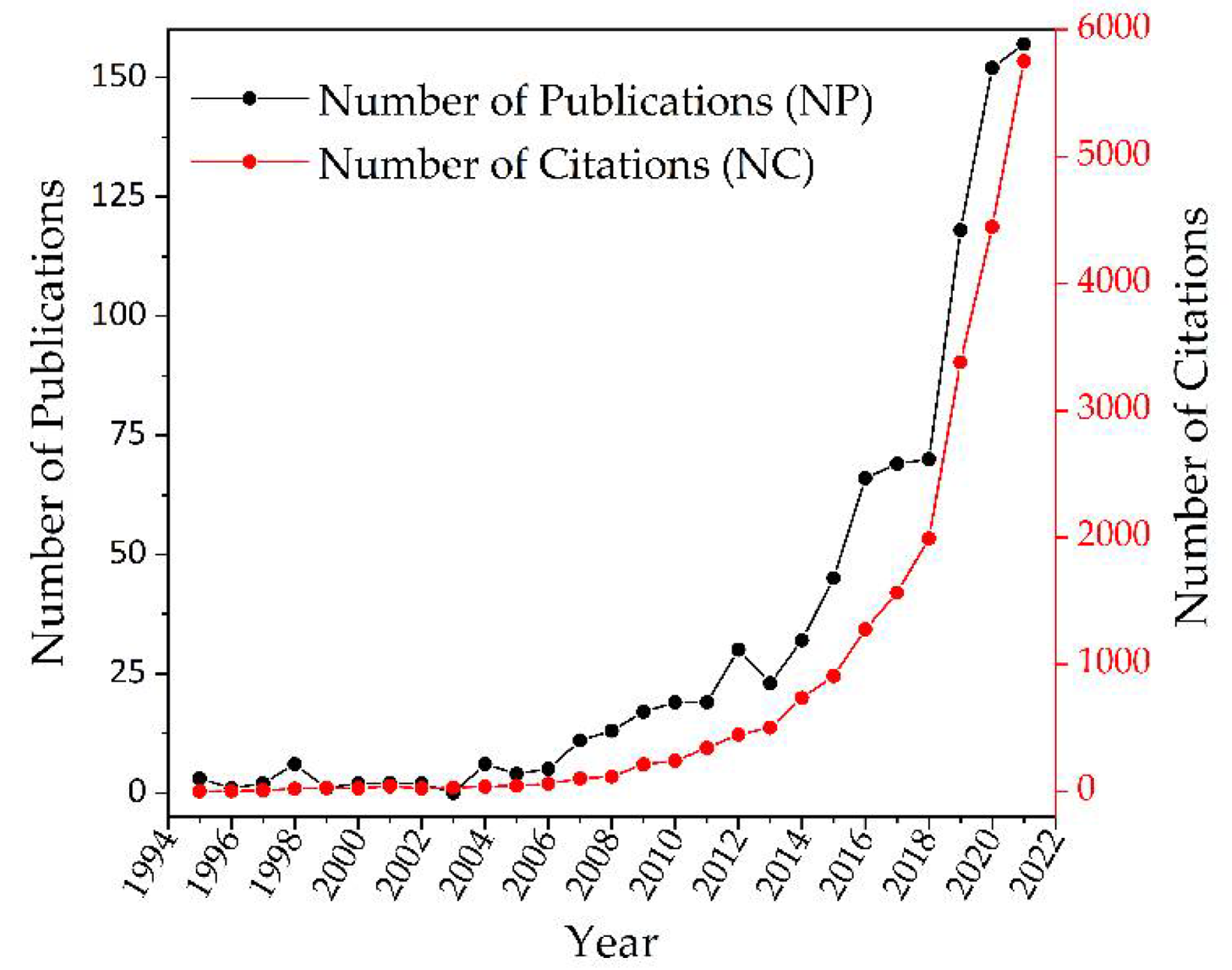
3.1. Number of Publications and Citations
3.2. Knowledge Structures
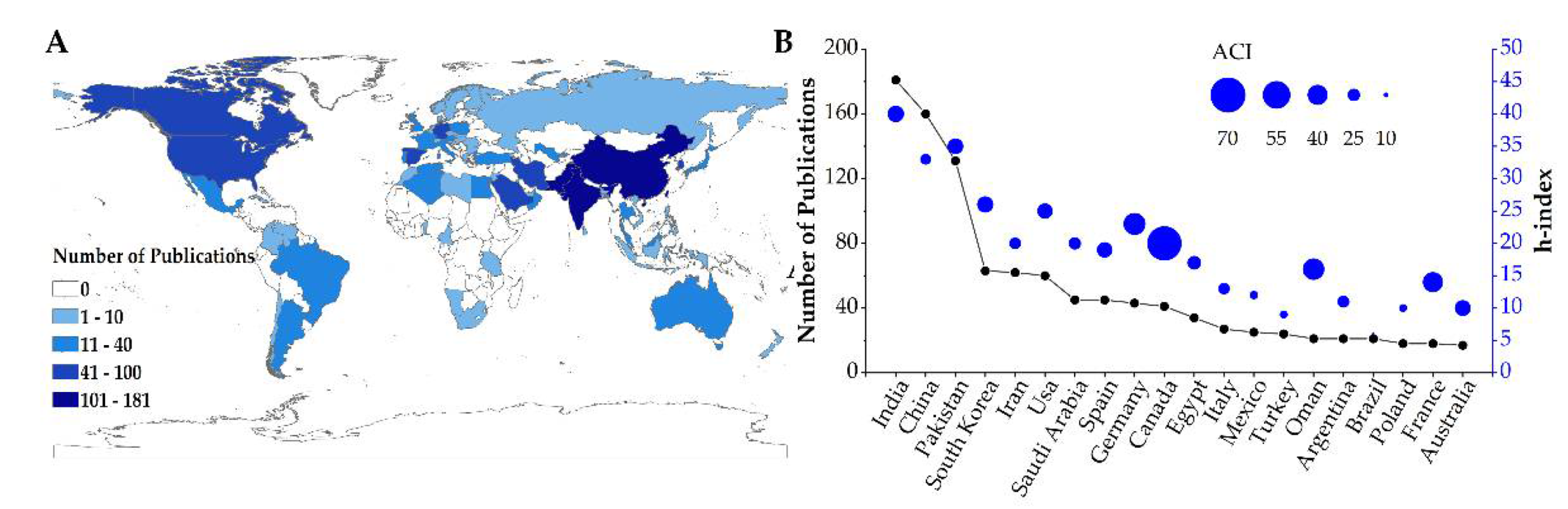
3.2.1. Most Productive Regions/Countries
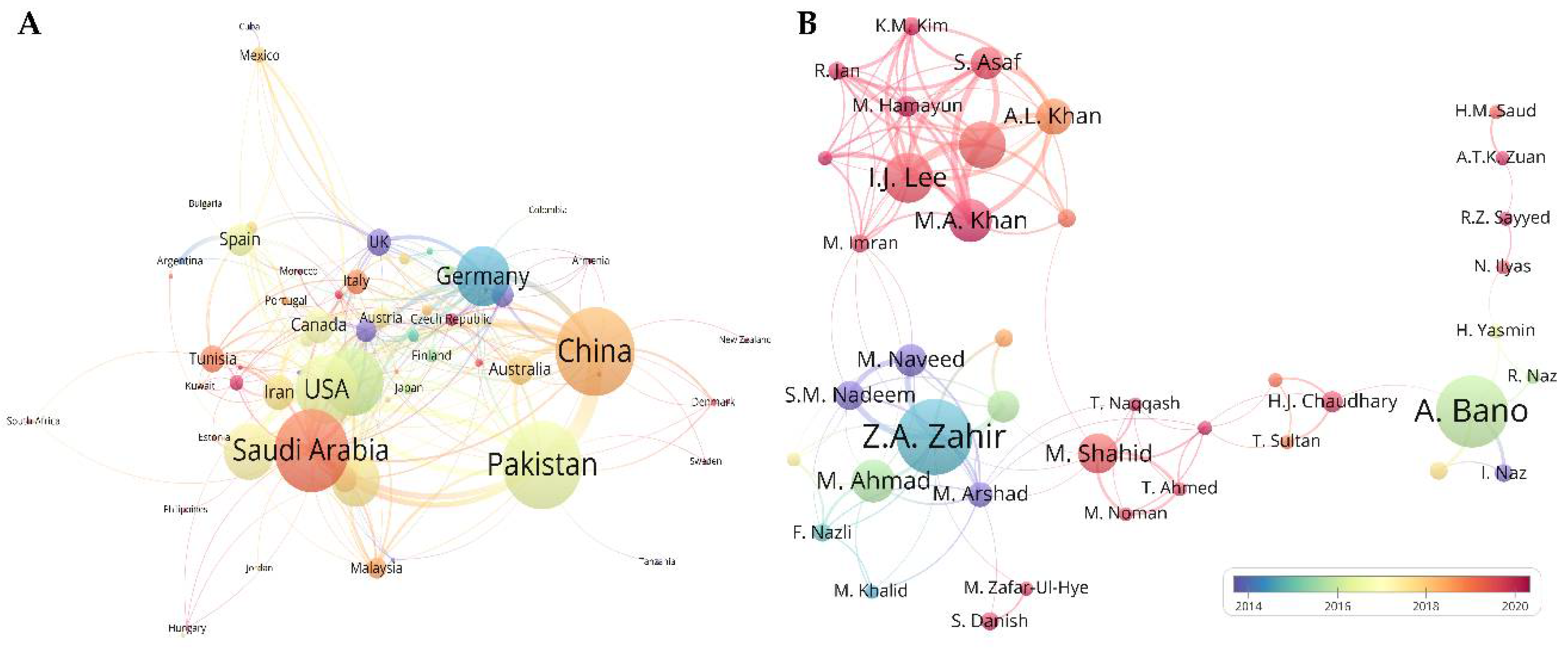
3.2.2. Most Influential Researchers
3.2.3. Network of Collaboration
3.3. Review and Hot Topics
3.3.1. Highly-Cited Publications
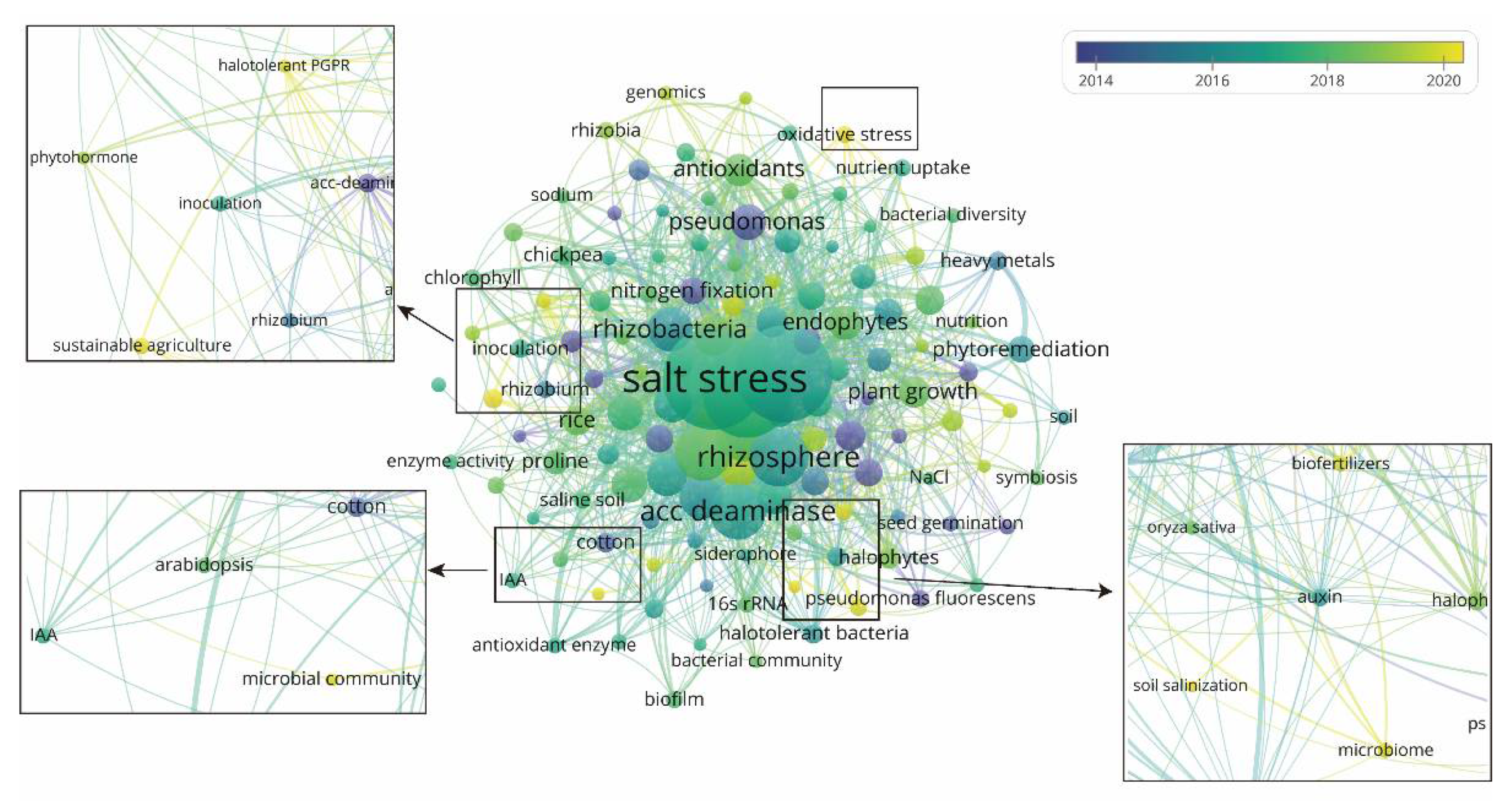
3.3.2. Keyword Co-Occurrence
4. Discussion
4.1. The Effectiveness of Employing PGPR to Enhance Salt Tolerance in Plants Is Attracting Attention
4.2. Intensive Research Has Had Weak Academic Influence among Asian Countries
4.3. Rich Research Themes and Outstanding Research Results
4.4. Future Research Hotspots
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Wu, L. Breeding for salinity tolerance in plants. Crit. Rev. Plant Sci. 1994, 13, 17–42. [Google Scholar] [CrossRef]
- Szabolcs, I. Salinization of soil and water and its relation to desertification. Desertific. Control Bull. 1992, 21, 32–37. [Google Scholar]
- Li, J.; Pu, L.; Zhu, M.; Zhang, R. The present situation and hot issues in the salt-affected soil research. Acta Geogr. Sin. 2012, 67, 1233–1245. [Google Scholar]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Alkhatib, M.; McNeilly, T.; Collins, J.C. The potential of selection and breeding for improved salt tolerance in lucerne (Medicago-sativa L.). Euphytica 1993, 65, 43–51. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D.; Schubert, S.; Noble, A.D.; Sahrawat, K.L. Phytoremediation of sodic and saline-sodic soils. Adv. Agron. 2007, 96, 197–247. [Google Scholar]
- Fita, A.; Rodriguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Neubert, A.; Schierholt, A.; Suemer, A.; Zoerb, C. Development of salt-resistant maize hybrids: The combination of physiological strategies using conventional breeding methods. Plant Sci. 2009, 177, 196–202. [Google Scholar] [CrossRef]
- Dodd, I.C.; Perez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [PubMed]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Asaf, S.; Khan, M.A.; Lee, I.J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, E.M.; Osman, H.S.; Gowayed, S.M.; Okasha, S.A.; Omara, A.E.-D.; Sami, R.; Abd El-Monem, A.M.; Abd El-Razek, U.A. Minimizing the adversely impacts of water deficit and soil salinity on maize growth and productivity in response to the application of plant growth-promoting rhizobacteria and silica nanoparticles. Agronomy 2021, 11, 676. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Burdman, S.; Jurkevitch, E.; Helman, Y. From the lab to the field: Combined application of plant-growth-promoting bacteria for mitigation of salinity stress in melon plants. Agronomy 2022, 12, 408. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Angers, France, 27 August–2 September 1978; pp. 879–882. [Google Scholar]
- Burr, T.J.; Caesar, A. Beneficial plant bacteria. Crit. Rev. Plant Sci. 1984, 2, 1–20. [Google Scholar] [CrossRef]
- Davison, J. Plant beneficial bacteria. Nat. Biotechnol. 1988, 6, 282–286. [Google Scholar] [CrossRef]
- Lambert, B.; Joos, H. Fundamental-aspects of rhizobacterial plant-growth promotion research. Trends Biotechnol. 1989, 7, 215–219. [Google Scholar] [CrossRef]
- Gupta, S.; Arora, D.K.; Srivastava, A.K. Growth promotion of tomato plants by rhizobacteria and imposition of energy stress on Rhizoctonia solani. Soil Biol. Biochem. 1995, 27, 1051–1058. [Google Scholar] [CrossRef]
- Sakai, M.; Futamata, H.; Urashima, Y.; Matsuguchi, T. Effect of cations on the growth of fluorescent Pseudomonad isolates from spinach roots grown in soils with different salinity levels. Soil Sci. Plant Nutr. 1995, 41, 605–611. [Google Scholar] [CrossRef]
- Agrawal, M.; Archana, G. Phenotypic display of plant growth-promoting traits in individual strains and multispecies consortia of plant growth promoting rhizobacteria and rhizobia under salinity stress. Rhizosphere 2021, 20, 100443. [Google Scholar] [CrossRef]
- Haroon, U.; Khizar, M.; Liaquat, F.; Ali, M.; Akbar, M.; Tahir, K.; Batool, S.S.; Kamal, A.; Chaudhary, H.J.; Munis, M.F.H. Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J. Plant Growth Regul. 2022, 41, 2435–2448. [Google Scholar] [CrossRef]
- Nigam, B.; Dubey, R.S.; Rathore, D. Protective role of exogenously supplied salicylic acid and PGPB (stenotrophomonas sp.) on spinach and soybean cultivars grown under salt stress. Sci. Hortic. 2022, 293, 110654. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Guo, Y.; Zhu, D.; Porter, A.L. Four dimensional science and technology planning: A new approach based on bibliometrics and technology roadmapping. Technol. Forecast. Soc. Chang. 2014, 81, 39–48. [Google Scholar] [CrossRef]
- Aznar-Sanchez, J.A.; Belmonte-Urena, L.J.; Lopez-Serrano, M.J.; Velasco-Munoz, J.F. Forest ecosystem services: An analysis of worldwide research. Forests 2018, 9, 453. [Google Scholar] [CrossRef]
- Aznar-Sanchez, J.A.; Garcia-Gomez, J.J.; Velasco-Munoz, J.F.; Carretero-Gomez, A. Mining waste and its sustainable management: Advances in worldwide research. Minerals 2018, 8, 284. [Google Scholar] [CrossRef]
- Wu, H.Y.; Cheng, K.M.; Guo, Q.; Yang, W.G.; Tong, L.J.; Wang, Y.L.; Sun, Z.M. Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: A bibliometric analysis. Front. Med. 2021, 8, 787228. [Google Scholar] [CrossRef]
- Lucio-Arias, D.; Leydesdorff, L. Main-path analysis and path-dependent transitions in HistCite (TM)-based historiograms. JASIST 2008, 59, 1948–1962. [Google Scholar] [CrossRef]
- Garfield, E. From the science of science to scientometrics visualizing the history of science with HistCite software. J. Informetr. 2009, 3, 173–179. [Google Scholar] [CrossRef]
- Verasoundarapandian, G.; Lim, Z.S.; Radziff, S.B.M.; Taufik, S.H.; Puasa, N.A.; Shaharuddin, N.A.; Merican, F.; Wong, C.-Y.; Lalung, J.; Ahmad, S.A. Remediation of pesticides by microalgae as feasible approach in agriculture: Bibliometric strategies. Agronomy 2022, 12, 117. [Google Scholar] [CrossRef]
- Ochoa-Noriega, C.A.; Aznar-Sanchez, J.A.; Velasco-Munoz, J.F.; Alvarez-Bejar, A. The use of water in agriculture in mexico and its sustainable management: A bibliometric review. Agronomy 2020, 10, 1957. [Google Scholar] [CrossRef]
- Han, L.; Mei, Q.; Lu, Y. Analysis and study on AHP-fuzzy comprehensive evaluation. China Saf. Sci. J. 2004, 14, 86–89. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Al-Harrasi, A. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.B.; Hou, Z.K.; Liu, F.B.; Zhang, M.; Hu, W.; Xu, S.F. Scientometric analysis of medicinal and edible plant coptis. Front. Pharmacol. 2021, 12, 725162. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Asaf, S.; Kamran, M.; Shahzad, R.; Bilal, S.; Khan, M.A.; Kang, S.M.; Kim, Y.H.; Yun, B.W.; et al. Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum Pimpinellifolium. Environ. Exp. Bot. 2017, 133, 58–69. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.W.; Kang, S.M.; Lee, I.J. Rhizospheric Bacillus spp. Rescues plant growth under salinity stress via regulating gene expression, endogenous hormones, and antioxidant system of Oryza sativa L. Front. Plant Sci. 2021, 12, 17. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The halotolerant rhizobacterium-Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant rhizobacterial strains mitigate the adverse effects of nacl stress in soybean seedlings. BioMed. Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.M.; Kim, K.M.; Jan, R.; Lee, I.J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can. J. Microbiol. 2007, 53, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Arshad, M. Rhizobacteria containing ACC-deaminase confer salt tolerance in maize grown on salt-affected fields. Can. J. Microbiol. 2009, 55, 1302–1309. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Arshad, M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 2012, 62, 1321–1330. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Akhtar, S.S.; Ahmad, M.; Saifullah; Nadeem, S.M. Comparative effectiveness of Enterobacter aerogenes and Pseudomonas fluorescens for mitigating the depressing effect of brackish water on maize. Int. J. Agric. Biol. 2012, 14, 337–344. [Google Scholar]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N.I. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Nawaz, S. Mitigation of salinity-induced negative impact on the growth and yield of wheat by plant growth-promoting rhizobacteria in naturally saline conditions. Ann. Microbiol. 2013, 63, 225–232. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Khalid, M.; Zahir, Z.A.; Ahmad, R. Auxin producing plant growth promoting rhizobacteria improve growth, physiology and yield of maize under saline field conditions. Int. J. Agric. Biol. 2016, 18, 37–45. [Google Scholar] [CrossRef]
- Shahzad, S.; Khan, M.Y.; Zahir, Z.A.; Asghar, H.N.; Chaudhry, U.K. Comparative effectiveness of different carriers to improve the efficacy of bacterial consortium for enhancing wheat production under salt affected field conditions. Pak. J. Bot. 2017, 49, 1523–1530. [Google Scholar]
- Barbieri, P.; Zanelli, T.; Galli, E.; Zanetti, G. Wheat inoculation with Azospirillum brasilense Sp6 and some mutants altered in nitrogen fixation and indole-3-acetic acid production. FEMS Microbiol. Lett. 1986, 36, 87–90. [Google Scholar] [CrossRef]
- Barbieri, P.; Galli, E. Effect on wheat root development of inoculation with an Azospirillum brasilense mutant with altered indole-3-acetic-acid production. Res. Microbiol. 1993, 144, 69–75. [Google Scholar] [CrossRef]
- Yao, L.X.; Wu, Z.S.; Zheng, Y.Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Osullivan, D.J.; Ogara, F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 1992, 56, 662–676. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Oliveira, A.L.M.; Santos, O.J.A.P.; Marcelino, P.R.F.; Milani, K.M.L.; Zuluaga, M.Y.A.; Zucareli, C.; Goncalves, L.S.A. Maize inoculation with Azospirillum brasilense ab-v5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front. Microbiol. 2017, 8, 1873. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.Z.; Roberto, L.D.A.; Hungria, M.; Correa, R.S.; Magri, E.; Correia, T.D. Meta-analysis of maize responses to Azospirillum brasilense inoculation in Brazil: Benefits and lessons to improve inoculation efficiency. Appl. Soil Ecol. 2022, 170, 104276. [Google Scholar] [CrossRef]
- Abd El-Fattah, D.A.; Eweda, W.E.; Zayed, M.S.; Hassanein, M.K. Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann. Agric. Sci. 2013, 58, 111–118. [Google Scholar] [CrossRef]
- Etesami, H. Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric. Ecosyst. Environ. 2018, 253, 98–112. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Pan, J.; Xue, X.; Zhang, J.; Guo, Q. A Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils. Agronomy 2022, 12, 2304. https://doi.org/10.3390/agronomy12102304
Ma X, Pan J, Xue X, Zhang J, Guo Q. A Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils. Agronomy. 2022; 12(10):2304. https://doi.org/10.3390/agronomy12102304
Chicago/Turabian StyleMa, Xixi, Jing Pan, Xian Xue, Jun Zhang, and Qi Guo. 2022. "A Bibliometric Review of Plant Growth-Promoting Rhizobacteria in Salt-Affected Soils" Agronomy 12, no. 10: 2304. https://doi.org/10.3390/agronomy12102304





