Breeding Peaches for Brown Rot Resistance in Embrapa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design and Treatments
2.3. Pathogen Culture, Conidia Production, and Inoculation
2.4. Brown Rot Evaluation
2.5. Statistical and Genetic Analysis
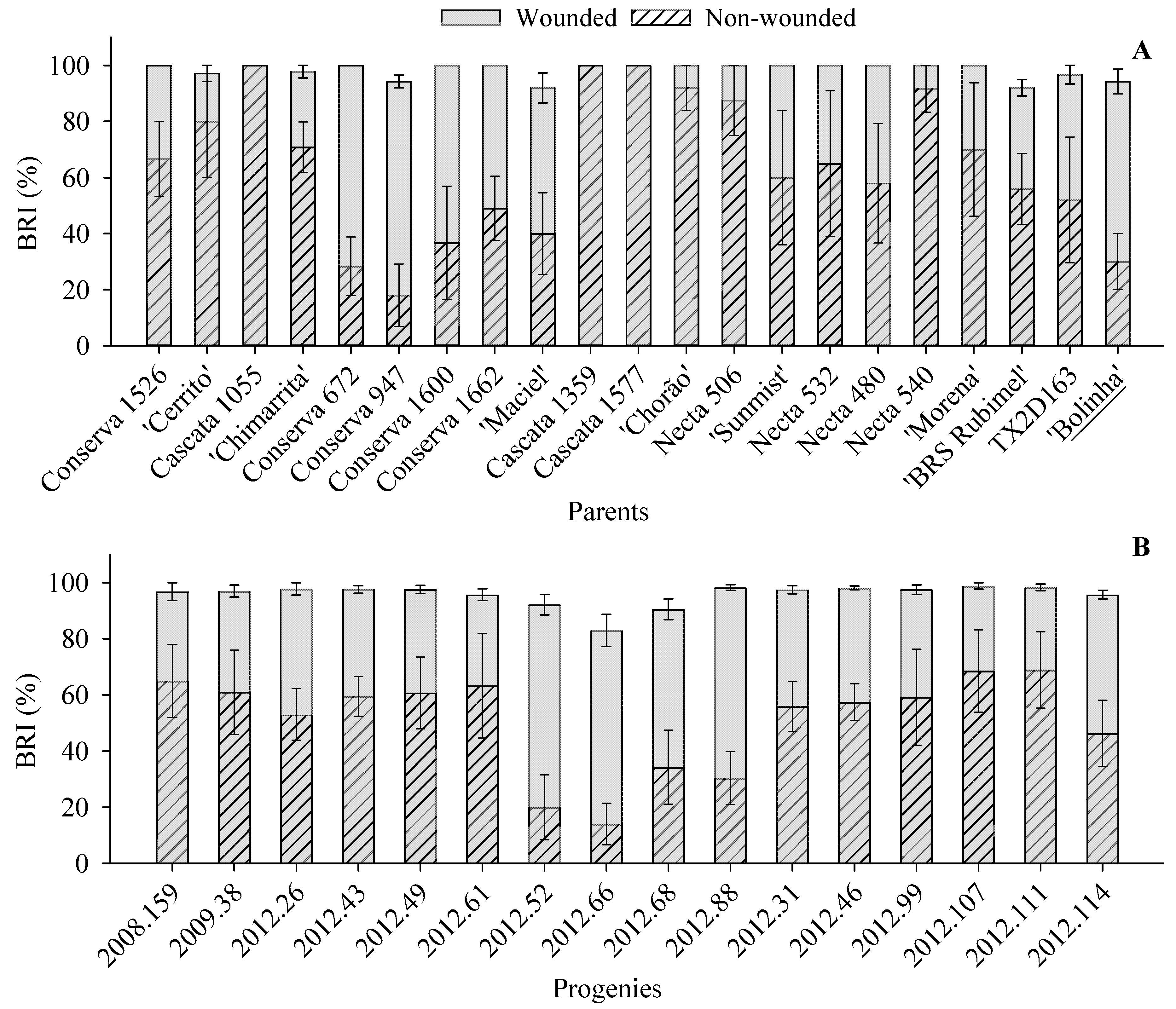
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raseira, M.C.B.; Franzon, R. Melhoramento genético. In Pessegueiro; Raseira, M.C.B., Pereira, J.F.M., Carvalho, F.L.C., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 57–72. [Google Scholar]
- IBGE, Instituto Brasileiro de Pesquisas e Estatísticas. Available online: https://cidades.ibge.gov.br/brasil/pesquisa/15/12028 (accessed on 29 July 2022).
- Adaskaveg, J.E.; Schnabel, G.; Forster, H. Diseases of peach caused by fungi and fungal-like organisms: Biology, epidemiology and management. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CAB International: Wallingford, UK, 2008; pp. 352–406. [Google Scholar]
- May-De-Mio, L.L.; Moreira, L.M.; Monteiro, L.B.; Justiniano Júnior, P.R. Infection of Monilinia fructicola in budding stages and incidence of brown rot on fruits in two peach production systems. Trop. Plant Pathol. 2008, 33, 227–234. [Google Scholar] [CrossRef]
- May-De-Mio, L.L.; Garrido, L.R.; Ueno, B.; Fajardo, T.V.M. Doenças da cultura do pessegueiro e métodos de controle. In Pessegueiro; Raseira, M.C.B., Pereira, J.F.M., Carvalho, F.L.C., Eds.; Embrapa: Brasília, Brazil, 2014; pp. 355–432. [Google Scholar]
- Thomidis, T.; Michailides, T.; Exadaktylou, E. Contribution of pathogens to peach fruit rot in northern Greece and their sensitivity to iprodione, carbendazim, thiophanate-methyl and tebuconazole fungicides. J. Phytopathol 2009, 157, 194–200. [Google Scholar] [CrossRef]
- Luo, C.X.; Hu, M.J.; Jin, X.; Yin, L.F.; Bryson, P.K.; Schnabel, G. An intron in the cytochrome b gene of Monilinia fructicola mitigates the risk of resistance development to Qol fungicides. Pest Manag. Sci. 2010, 66, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Hall, R. Pathogenicity of Monilinia fructicola. Part II. Penetration of peach leaf and fruit. J. Phytopathol. 1971, 72, 281–290. [Google Scholar] [CrossRef]
- Zhu, F.; Bryson, P.K.; Schnabel, G. Influence of storage approaches on instability of propiconazole resistance in Monilinia fructicola. Pest Manag. Sci. 2012, 68, 1003–1009. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, N.; Schnabel, G.; Luo, C. Function of the genetic element ‘Mona’ associated with fungicide resistance in Monilinia fructicola. Mol. Plant. Pathol. 2017, 18, 90–97. [Google Scholar] [CrossRef]
- Fu, W.; Tian, G.; Pei, Q.; Ge, X.; Tian, P. Evaluation of berberine as a natural compound to inhibit peach brown rot pathogen Monilinia fructicola. Crop Prot. 2017, 91, 20–26. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Camele, I.; De Martino, L.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crops Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Eduardo, I.; Usall, J.; Casals, C.; Arús, P.; Teixidó, N.; Torres, R. Exploring sources of resistance to brown rot in an interspecific almond × peach population. J. Sci. Food Agric. 2019, 99, 4105–4113. [Google Scholar] [CrossRef]
- Feliciano, A.; Feliciano, A.J.; Ogawa, J.M. Monilinia fructicola resistance in peach cultivar Bolinha. Phytopathology 1987, 77, 776–780. [Google Scholar] [CrossRef]
- Santos, J.; Raseira, M.C.B.; Zanandrea, I. Resistance to brown rot in peach plants. Bragantia 2012, 71, 219–225. [Google Scholar] [CrossRef]
- Scariotto, S.; Santos, J.; Raseira, M.C.B. Search for resistance sources to brown rot in Brazilian peach genotypes. Acta Hortic. 2015, 1084, 211–216. [Google Scholar] [CrossRef]
- Scariotto, S. Variabilidade Genética e Abordagem Bioquímica da Resistência do Fruto à Podridão-Parda em Diferentes Genótipos de Prunus persica. Ph.D. Thesis, Faculty of Agronomy Eliseu Maciel, Federal University of Pelotas, Pelotas, Brazil, 2016. [Google Scholar]
- Dini, M.; Scariotto, S.; Raseira, M.C.B.; Ueno, B. Heritability and segregation of resistance to brown rot in peach fruits. Acta Hortic. 2021, 1304, 339–346. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.E.; Bostock, R.M.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Gradziel, T.M.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidate resistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L.; Vecchietti, A. QTL mapping for brown rot (Monilinia fructigena) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Kushalappa, A.C.; Gunnaiah, R. Metabolo-proteomics to discover plant biotic stress resistance genes. Trends Plant Sci. 2013, 18, 522–531. [Google Scholar] [CrossRef]
- Dallagnol, L.J. Resistência Genética de Plantas a Patógenos; UFPel: Pelotas, Brazil, 2018; 437p. [Google Scholar]
- Ramalho, M.A.P.; Santos, J.B.; Pinto, C.B.P.; Souza, E.A.; Gonçalves, F.M.A.; Souza, J.C. Genética na Agropecuária: Genética Quantitativa, 5th ed.; UFLA: Lavras, Brazil, 2012; 565p. [Google Scholar]
- Dini, M.; Raseira, M.C.B.; Scariotto, S.; Carpenedo, S.; Ueno, B. Peach and nectarine susceptibility to brown rot and protocol optimization to evaluate Monilinia fructicola sporulation. Acta Sci. Agron. 2022, 44, e55850. [Google Scholar] [CrossRef]
- Fu, W.; Burrell, R.; Linge, C.S.; Schnabel, G.; Gasic, K. Breeding for brown rot (Monilinia spp.) tolerance in Clemson University Peach Breeding Program. J. Am. Pom. Soc. 2018, 72, 94–100. [Google Scholar]
- Obi, V.I.; Barriuso, J.J.; Moreno, M.A.; Giménez, R.; Gogorcena, Y. Optimizing protocols to evaluate brown rot (Monilinia laxa) susceptibility in peach and nectarine fruits. Australas. Plant Path. 2017, 46, 183–189. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Usall, J.; Gogorcena, Y. Breeding strategies for identifying superior peach genotypes resistant to brown rot. Sci. Hort. 2019, 246, 1028–1036. [Google Scholar] [CrossRef]
- Londero, P.M.G.; Ribeiro, N.D.; Cerutti, T.; Mazeiro, S.M.; Rosa, D.P.; Rosa, S.S. Maternal effect in sulfur amino acids content expression in common bean grains. Ciência Rural. 2009, 39, 1884–1887. [Google Scholar] [CrossRef] [Green Version]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Harlow, UK, 1996. [Google Scholar]
- Dirlewanger, E.; Quero-García, J.; Le Dantec, L.; Lambert, P.; Ruiz, D.; Dondini, L.; Illa, E.; Quilot-Turion, B.; Audergon, J.M.; Tartarini, S.; et al. Comparison of the genetic determinism of two key phenological traits, flowering and maturity dates, in three Prunus species: Peach, apricot and sweet cherry. Heredity 2012, 109, 280–292. [Google Scholar] [CrossRef]
- Griffiths, A.J.F.; Wessler, S.R.; Carroll, S.B.; Doebley, J. Introduction to Genetic Analysis, 10th ed.; W. H. Freeman and Company: New York, NY, USA, 2015; 862p. [Google Scholar]
- Michailides, T.J.; Morgan, D.P. Influence of fruit-to-fruit contact on the susceptibility of French prune to infection by Monilinia fructicola. Plant Dis. 1997, 81, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Bostock, R.M.; Adaskaveg, J.E. Resistance to brown rot disease in peach is determined by multiple structural and biochemical components. Acta Hort. 2003, 622, 347–352. [Google Scholar] [CrossRef]
- Gibert, C.; Chadœuf, J.; Vercambre, G.; Génard, M.; Lescourret, F. Cuticular cracking on nectarine fruit surface: Spatial distribution and development in relation to irrigation and thinning. J. Amer. Soc. Hort. Sci. 2007, 132, 583–591. [Google Scholar] [CrossRef]
- Kappel, F.; Sholberg, P.L. Screening sweet cherry cultivars from the Pacific Agri-Food Research Centre Summerland breeding program for resistance to brown rot (Monilinia fructicola). Can. J. Plant Sci. 2008, 88, 747–752. [Google Scholar] [CrossRef]
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Incidence and type of cracking in sweet cherry (Prunus avium L.) are affected by genotype and season. Crop Pasture Sci. 2009, 60, 1002–1008. [Google Scholar] [CrossRef]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown rot strikes Prunus fruit: An ancient fight almost always lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef]
- Fernandez, V.; Khayet, M.; Montero-Prado, P.; Heredia-Guerrero, J.A.; Liakopoulos, G.; Karabourniotis, G.; del Río, V.; Domínguez, E.; Tacchini, I.; Nerín, C.; et al. New insights into the properties of pubescent surfaces: Peach fruit as a model. Plant Physiol. 2011, 156, 2098–2108. [Google Scholar] [CrossRef]
- Garcia-Benitez, C.; Melgarejo, P.; De Cal, A.; Fontaniella, B. Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS ONE 2016, 11, e0160675. [Google Scholar] [CrossRef]
- Wu, B.H.; Zhao, J.B.; Chen, J.; Xi, H.F.; Jiang, Q.; Li, S.H. Maternal inheritance of sugars and acids in peach (P. persica (L.) Batsch) fruit. Euphytica 2012, 188, 333–345. [Google Scholar] [CrossRef]
- Dini, M.; Raseira, M.C.B.; Scariotto, S.; Marchi, P.M.; Mello-Farias, P. Peach phenological characters: Heritability, maternal effect and correlation with brown rot. Genet. Mol. Res. 2021, 20, gmr18684. [Google Scholar] [CrossRef]
- Panda, S.; Martín, J.P.; Aguinagalde, I. Chloroplast DNA study in sweet cherry cultivars (Prunus avium L.) using PCR-RFLP method. Genet. Resour. Crop. Ev. 2003, 50, 489–495. [Google Scholar] [CrossRef]
- Bouhadida, M.; Martín, J.P.; Eremin, G.; Pinochet, J.; Moreno, M.Á.; Gogorcena, Y. Chloroplast DNA diversity in Prunus and its implication on genetic relationships. J. Amer. Soc. Hort. Sci. 2007, 132, 670–679. [Google Scholar] [CrossRef]
- Bielsa, B.; Jiwan, D.; Marti, A.I.F.; Dhingra, A.; Rubio-Cabetas, M.J. Detection of SNP and validation of a SFP InDel (deletion) in inverted repeat region of the Prunus species chloroplast genome. Sci. Hort. 2014, 168, 108–112. [Google Scholar] [CrossRef]
- Wagner Júnior, A. Avaliação de Germoplasma de Pessegueiro, Quanto à Reação à Monilinia fructicola (Wint.) Honey. Master’s Thesis, Faculty of Agronomy Eliseu Maciel, Federal University of Pelotas, Pelotas, Brazil, 2003. [Google Scholar]
- Souza, V.A.B.; Byrne, D.H.; Taylor, J.F. Heritability, genetic and phenotypic correlations, and predicted selection response of quantitative traits in peach: II. An analysis of several fruits traits. J. Am. Soc. Hortic. Sci. 1998, 123, 604–611. [Google Scholar] [CrossRef]
- Chandrababu, R.J.; Sharma, R.K. Heritability estimates in almond [Prunus dulcis (Miller) D.A. Webb]. Sci. Hort. 1999, 79, 237–243. [Google Scholar] [CrossRef]
- Willimas, W.; Brown, A.G. Genetic response to selection in cultivated plants: Gene frequencies in varieties of Prunus persica. Proc. R. Soc. Lond. B 1956, 145, 337–347. [Google Scholar] [CrossRef]



| Progeny | Parent ♀ | Parent ♂ | N° Seedlings |
|---|---|---|---|
| 2008.159 | Conserva 1526 | ‘Cerrito’ | 7 |
| 2009.38 | ‘Cerrito’ | Conserva 1526 | 23 |
| 2012.26 | Cascata 1055 | ‘Chimarrita’ | 18 |
| 2012.43 | ‘Chimarrita’ | Cascata 1055 | 25 |
| 2012.49 | Conserva 672 | Conserva 1526 | 18 |
| 2012.61 | Conserva 1526 | Conserva 672 | 7 |
| 2012.52 | Conserva 947 | Conserva 1600 | 17 |
| 2012.66 | Conserva 1600 | Conserva 947 | 12 |
| 2012.68 | Conserva 1662 | ‘Maciel’ | 24 |
| 2012.88 | ‘Maciel’ | Conserva 1662 | 17 |
| 2012.31 | Cascata 1359 | Cascata 1577 | 31 |
| 2012.46 | Chorão | ‘Maciel’ | 25 |
| 2012.99 | Necta 506 | ‘Sunmist’ | 20 |
| 2012.107 | Necta 532 | Necta 480 | 25 |
| 2012.111 | Necta 540 | ‘Morena’ | 25 |
| 2012.114 | ‘Rubimel’ | TX2D163 | 21 |
| Fruits | Traits * | H2 (%) | h2 (%) | |||
|---|---|---|---|---|---|---|
| 2015–2016 | 2016–2017 | 2017–2018 | Mean | |||
| Wounded | BRI | 42.5 | 35.8 | 37.6 | 31.1 | 34.8 |
| LD | 51.7 | 47.1 | 42.1 | 35.5 | 41.6 | |
| LA | 44.7 | 33.5 | 41.1 | 25.5 | 33.4 | |
| SPP | 27.5 | 23.2 | 18.0 | 13.8 | 18.3 | |
| SPD | 41.2 | 33.0 | 39.3 | 35.9 | 36.1 | |
| SPA | 30.1 | 21.0 | 28.9 | 18.5 | 22.8 | |
| Non-wounded | BRI | 26.7 | - | 20.2 | 17.1 | 18.7 |
| LD | 40.5 | - | 34.6 | 30.1 | 32.4 | |
| LA | 29.8 | - | 28.6 | 20.2 | 24.4 | |
| SPP | 25.0 | - | 20.1 | 11.8 | 16.0 | |
| SPD | 31.1 | - | 29.3 | 19.9 | 24.6 | |
| SPA | 23.6 | - | 21.8 | 16.9 | 19.4 | |
| Wounded | Non-Wounded | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRI | LD | LA | SPP | SPD | SPA | BRI | LD | LA | SPP * | SPD * | SPA * | |
| 96.3 | 29.5 | 39.7 | 60.6 | 14.6 | 15.9 | 54.0 | 12.8 | 16.6 | 19.4 | 4.5 | 5.4 | |
| ‘Bolinha’ | 94.3 | 20.2 | 13.3 | 37.1 | 5.5 | 2.1 | 30.0 | 6.5 | 4.0 | 0 | 0 | 0 |
| 81.8 | 14.5 | 8.1 | 15.8 | 2.4 | 0.5 | 11.0 | 1.9 | 0.9 | 0 | 0 | 0 | |
| SD | −14.5 | −15.0 | −31.6 | −44.8 | −12.2 | −15.5 | −43.0 | −10.9 | −15.7 | −19.4 | −4.5 | −5.4 |
| SGN | 58 | 21 | 11 | 76 | 63 | 41 | 53 | 59 | 56 | 93 | 93 | 93 |
| h2 | 0.35 | 0.42 | 0.33 | 0.18 | 0.36 | 0.23 | 0.19 | 0.32 | 0.24 | 0.16 | 0.25 | 0.19 |
| GA (direct) | −5.0 | −6.2 | −10.5 | −8.2 | −4.4 | −3.5 | −8.1 | −3.5 | −3.8 | −3.2 | −1.1 | −1.0 |
| GA% | −5.2 | −21.1 | −26.5 | −13.5 | −30.2 | −22.1 | −15.0 | −27.3 | −22.8 | −16.4 | −25.0 | −18.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, M.; Raseira, M.d.C.B.; Scariotto, S.; Ueno, B. Breeding Peaches for Brown Rot Resistance in Embrapa. Agronomy 2022, 12, 2306. https://doi.org/10.3390/agronomy12102306
Dini M, Raseira MdCB, Scariotto S, Ueno B. Breeding Peaches for Brown Rot Resistance in Embrapa. Agronomy. 2022; 12(10):2306. https://doi.org/10.3390/agronomy12102306
Chicago/Turabian StyleDini, Maximiliano, Maria do Carmo Bassols Raseira, Silvia Scariotto, and Bernardo Ueno. 2022. "Breeding Peaches for Brown Rot Resistance in Embrapa" Agronomy 12, no. 10: 2306. https://doi.org/10.3390/agronomy12102306






