Breeding Long Shelf-Life (LSL) Tomato Landraces to Non-Trellised Culture and Water Deficit Irrigation: The Effect on Yield and Postharvest Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Breeding Program
2.2. Plant Genotyping
2.3. Growth Conditions and Irrigation Scheduling
2.4. Plant Phenotyping
2.4.1. Agronomic traits and Water Use Efficiency
2.4.2. Quality Traits
2.5. Postharvest Conservation and Analysis of Shelf Life
2.6. Statistical Analysis
3. Results
3.1. Plants Obtained in the Breeding Program Contain a Known Allele of the SP Gene and a New Allele of the S Gene
3.2. Performance of Penjar Tomato under Non-Trellised Culture Depends on Irrigation and Genotypic Factors
3.3. Harvest Index Is Neither Affected by Environmental Conditions nor by the Introgression of the Double sp/s Mutation
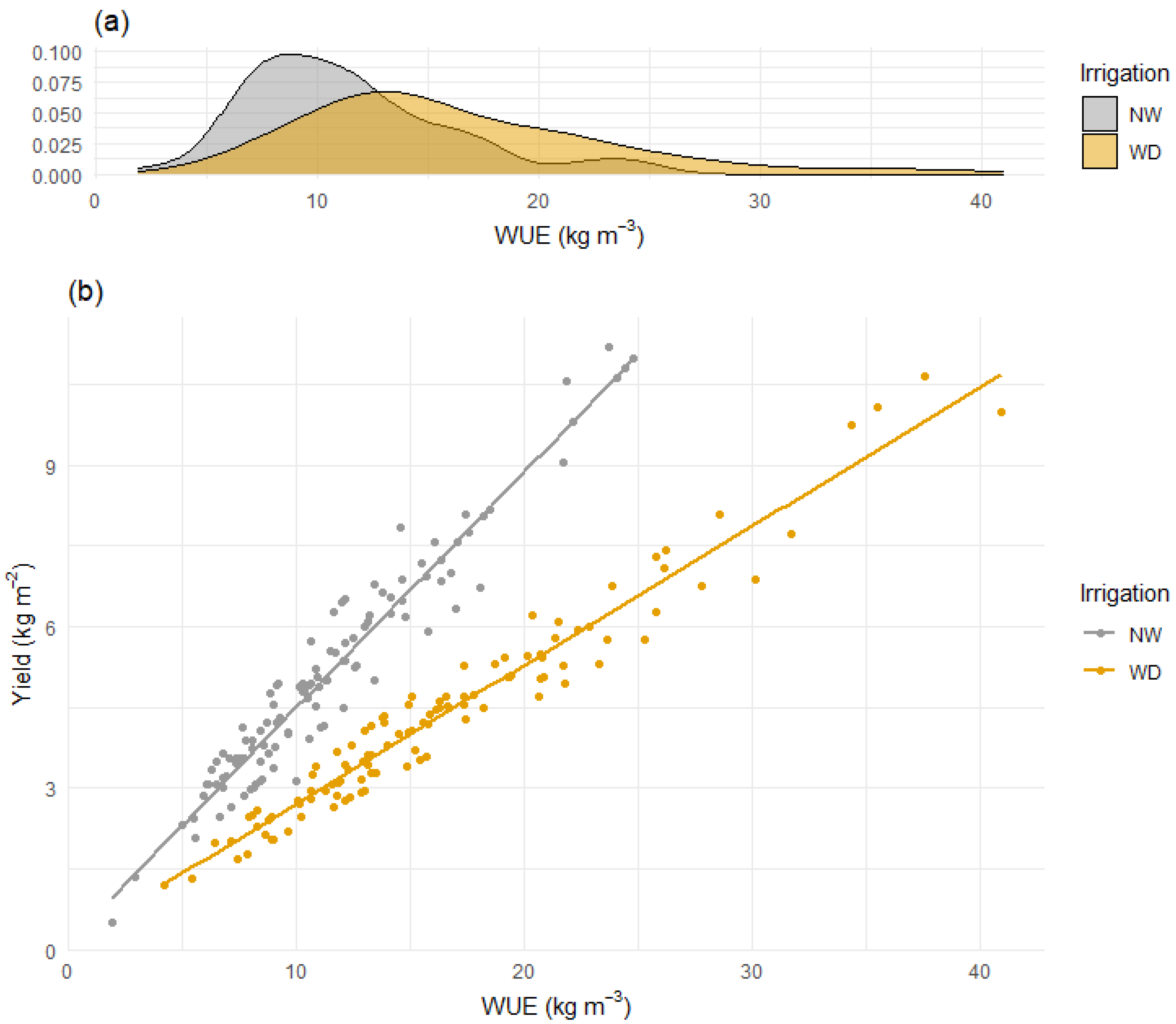
3.4. Deficit Irrigation Has the Potential to Combine High WUE and Yield in Penjar Tomato
3.5. Induced Water Stress Increases Fruit Quality While Genotype-Specific Characteristics Are Retained
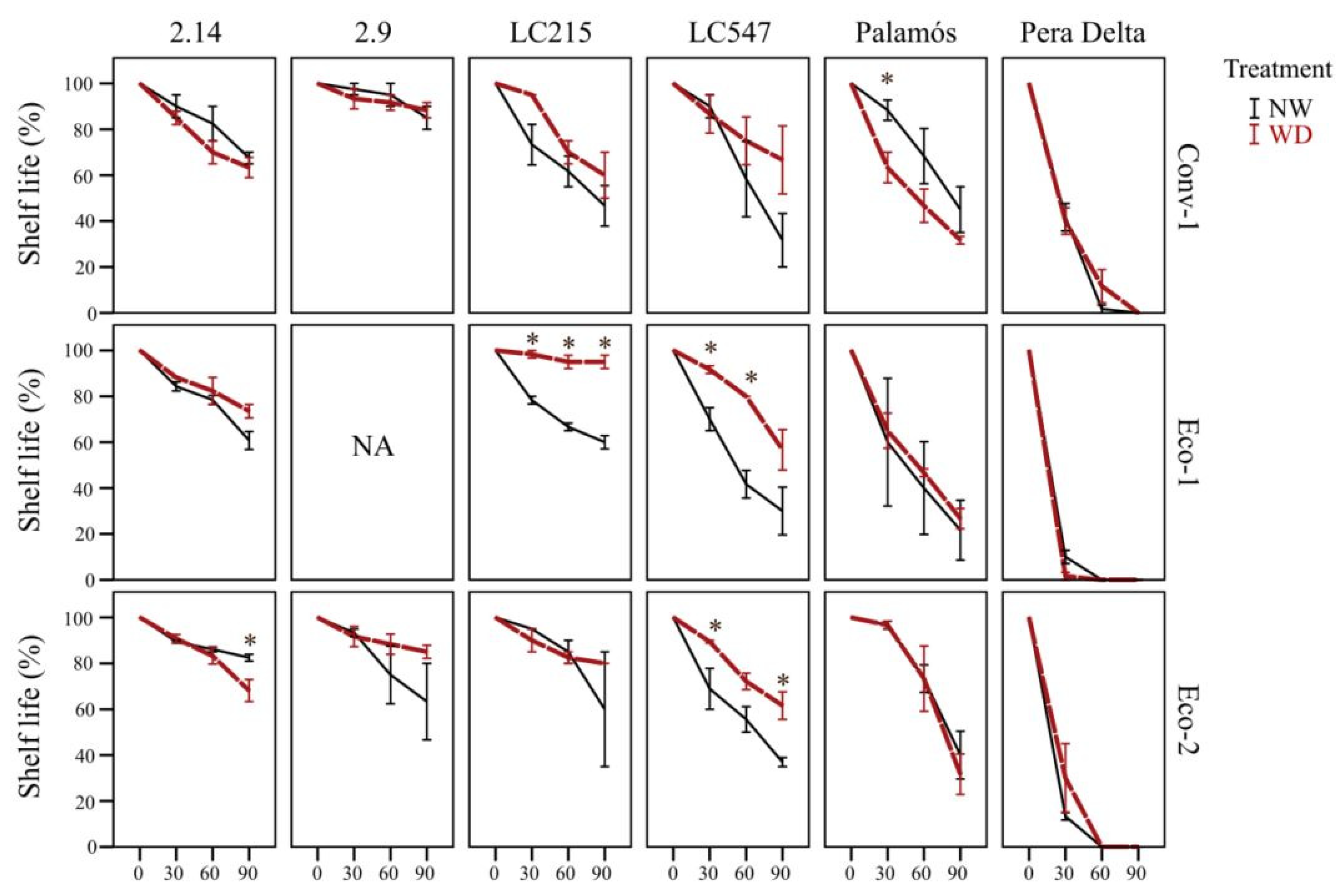
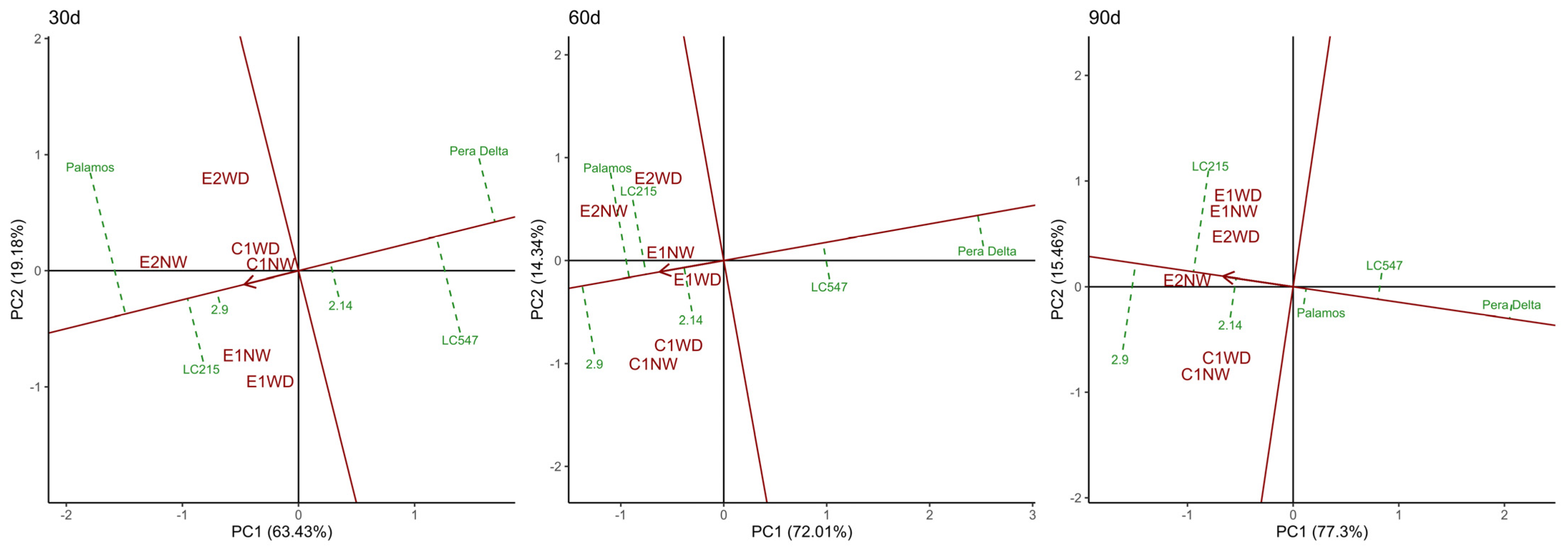
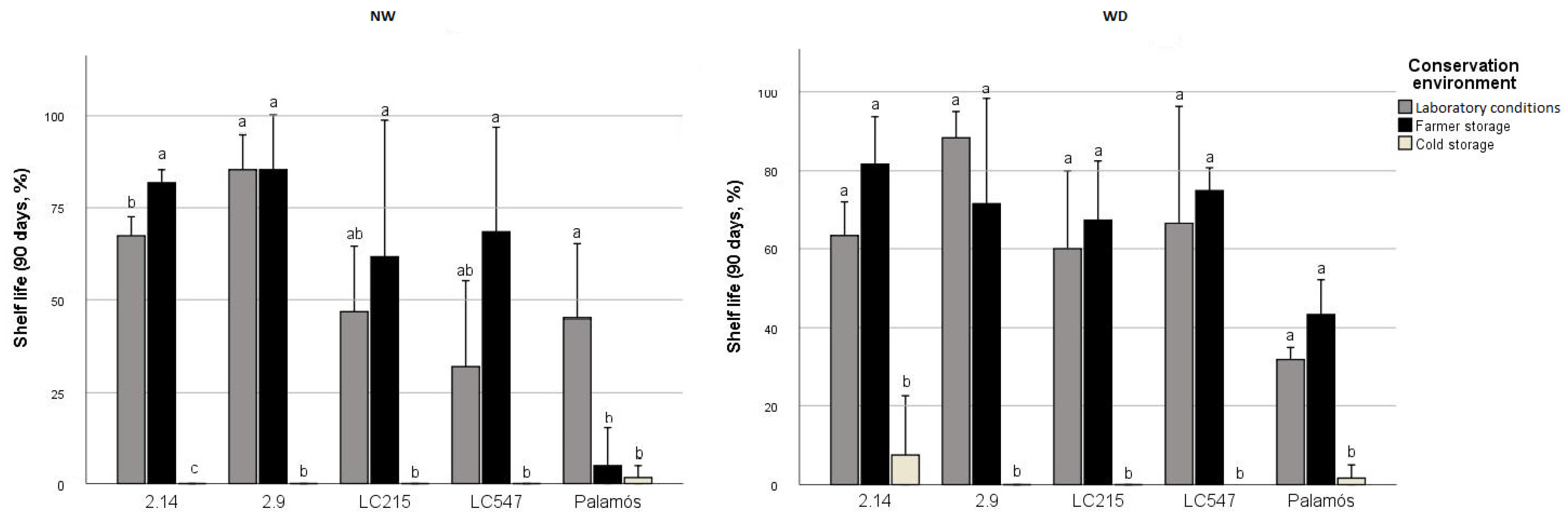
3.6. Specific Genotype*Preharvest Conditions Drive High Shelf Life in the Short Term, While Genotype Is Critical for Long-Storage
3.7. Cold Storage Impedes LSL Phenotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozturk, T.; Ceber, Z.P.; Türkeş, M.; Kurnaz, M.L. Projections of Climate Change in the Mediterranean Basin by Using Downscaled Global Climate Model Outputs. Int. J. Climatol. 2015, 35, 4276–4292. [Google Scholar] [CrossRef]
- Savé, R.; de Herralde, F.; Aranda, X.; Pla, E.; Pascual, D.; Funes, I.; Biel, C. Potential Changes in Irrigation Requirements and Phenology of Maize, Apple Trees and Alfalfa under Global Change Conditions in Fluvià Watershed during XXIst Century: Results from a Modeling Approximation to Watershed-Level Water Balance. Agric. Water Manag. 2012, 114, 78–87. [Google Scholar] [CrossRef]
- Harmanny, K.S.; Malek, Ž. Adaptations in Irrigated Agriculture in the Mediterranean Region: An Overview and Spatial Analysis of Implemented Strategies. Reg. Environ. Chang. 2019, 19, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, S.; Costa, L.D.; Vallone, S.; Barbieri, G.; Maggio, A. Increasing Water Use Efficiency in Vegetable Crop Production: From Plant to Irrigation Systems Efficiency. Horttechnology 2011, 21, 301–308. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Passioura, J. Increasing Crop Productivity When Water Is Scarce—From Breeding to Field Management. Agric. Water Manag. 2006, 80, 176–196. [Google Scholar] [CrossRef]
- Li, M.; Peterson, C.A.; Tautges, N.E.; Scow, K.M.; Gaudin, A.C.M. Yields and Resilience Outcomes of Organic, Cover Crop, and Conventional Practices in a Mediterranean Climate. Sci. Rep. 2019, 9, 12283. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Gallardo, M.; Thompson, R.B. Water and Fertilization Management of Vegetables: State of Art and Future Challenges. Eur. J. Hortic. Sci. 2018, 83, 306–318. [Google Scholar] [CrossRef]
- Causse, M.; Zhao, J.; Diouf, I.; Qang, J.; Lefebvre, V.; Caromel, B.; Génard, M.; Bertin, N. Genomic designing for climate-smart tomato. In Genomic Designing of Climate-Smart Vegetable Crops; Kole, C., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 47–159. ISBN 978-3-319-97415-6. [Google Scholar]
- Mekonnen, M.M.; Hoekstra, A.Y. The Green, Blue and Grey Water Footprint of Crops and Derived Crop Products. Hydrol. Earth Syst. Sci. 2011, 15, 1577–1600. [Google Scholar] [CrossRef]
- Page, G.; Ridoutt, B.; Bellotti, B. Carbon and Water Footprint Tradeoffs in Fresh Tomato Production. J. Clean. Prod. 2012, 32, 219–226. [Google Scholar] [CrossRef]
- Santos, B.M.; Torres-Quezada, E.A. Irrigation and fertilization. In Tomatoes; Heuvelink, E., Ed.; CABI: Wallingford, UK, 2018; pp. 180–206. ISBN 9781780641935. [Google Scholar]
- Stanghellini, C. Horticultural Production in Greenhouses: Efficient Use of Water. Acta Hortic. 2014, 1034, 25–32. [Google Scholar] [CrossRef]
- Hanson, B.R.; May, D.M. Crop Coefficients for Drip-Irrigated Processing Tomato. Agric. Water Manag. 2006, 81, 381–399. [Google Scholar] [CrossRef]
- Valcárcel, M.; Lahoz, I.; Campillo, C.; Martí, R.; Leiva-Brondo, M.; Roselló, S.; Cebolla-Cornejo, J. Controlled Deficit Irrigation as a Water-Saving Strategy for Processing Tomato. Sci. Hortic. 2020, 261, 108972. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of Deficit Irrigation on Biomass, Yield, Water Productivity and Fruit Quality of Processing Tomato under Semi-Arid Mediterranean Climate Conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Lei, S.; Yunzhou, Q.; Fengchao, J.; Changhai, S.; Chao, Y.; Yuxin, L.; Mengyu, L.; Baodi, D. Physiological Mechanism Contributing to Efficient Use of Water in Field Tomato under Different Irrigation. Plant Soil Environ. 2009, 55, 128–133. [Google Scholar] [CrossRef]
- Blanca, J.; Pons, C.; Montero-Pau, J.; Sanchez-Matarredona, D.; Ziarsolo, P.; Fontanet, L.; Fisher, J.; Plazas, M.; Casals, J.; Rambla, J.L.; et al. European Traditional Tomatoes Galore: A Result of Farmers’ Selection of a Few Diversity-Rich Loci. J. Exp. Bot. 2022, 73, 3431–3445. [Google Scholar] [CrossRef]
- Pons, C.; Casals, J.; Palombieri, S.; Fontanet, L.; Riccini, A.; Rambla, J.L.; Ruggiero, A.; Figás, M.D.R.; Plazas, M.; Koukounaras, A.; et al. Atlas of phenotypic, genotypic and geographical diversity present in the European traditional tomato. Hortic. Res. 2022, 9, uhac112. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Conesa, M.À.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean Long Shelf-Life Landraces: An Untapped Genetic Resource for Tomato Improvement. Front. Plant Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Casals, J.; Pascual, L.; Canizares, J.; Cebolla-Cornejo, J.; Casanas, F.; Nuez, F. Genetic Basis of Long Shelf Life and Variability into Penjar Tomato. Genet. Resour. Crop Evol. 2012, 59, 219–229. [Google Scholar] [CrossRef]
- Bota, J.; Conesa, M.À.; Ochogavia, J.M.; Medrano, H.; Francis, D.M.; Cifre, J. Characterization of a Landrace Collection for Tomàtiga de Ramellet (Solanum lycopersicum L.) from the Balearic Islands. Genet. Resour. Crop Evol. 2014, 61, 1131–1146. [Google Scholar] [CrossRef]
- Rosa-Martínez, E.; Adalid, A.M.; Alvarado, L.E.; Burguet, R.; García-Martínez, M.D.; Pereira-Dias, L.; Casanova, C.; Soler, E.; Figàs, M.R.; Plazas, M.; et al. Variation for Composition and Quality in a Collection of the Resilient Mediterranean ‘de Penjar’ Long Shelf-Life Tomato under High and Low N Fertilization Levels. Front. Plant Sci. 2021, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Casals, J.; Cebolla-Cornejo, J.; Rosello, S.; Beltran, J.; Casanas, F.; Nuez, F. Long-Term Postharvest Aroma Evolution of Tomatoes with the Alcobaça (Alc) Mutation. Eur. Food Res. Technol. 2011, 233, 331–342. [Google Scholar] [CrossRef]
- Conesa, M.À.; Galmés, J.; Ochogavía, J.M.; March, J.; Jaume, J.; Martorell, A.; Francis, D.M.; Medrano, H.; Rose, J.K.C.; Cifre, J. The Postharvest Tomato Fruit Quality of Long Shelf-Life Mediterranean Landraces Is Substantially Influenced by Irrigation Regimes. Postharvest Biol. Technol. 2014, 93, 114–121. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Douthe, C.; El Aou-ouad, H.; Ribas-Carbó, M.; Galmés, J. Tomato Landraces as a Source to Minimize Yield Losses and Improve Fruit Quality under Water Deficit Conditions. Agric. Water Manag. 2019, 223, 105722. [Google Scholar] [CrossRef]
- Galmés, J.; Conesa, M.A.; Ochogavía, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-Carbó, M.; Savé, R.; Flexas, J.; Medrano, H.; Cifre, J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2010, 34, 245–260. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of Composition Traits Related to Organoleptic and Functional Quality for the Differentiation, Selection and Enhancement of Local Varieties of Tomato from Different Cultivar Groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef]
- Casals, J.; Martí, R.; Casañas, F.; Cebolla, J. Sugar-and-Acid Profile of Penjar Tomatoes and Its Evolution during Storage. Sci. Agric. 2015, 72, 314–321. [Google Scholar] [CrossRef]
- Casals, J.; Martí, M.; Rull, A.; Pons, C. Sustainable Transfer of Tomato Landraces to Modern Cropping Systems: The Effects of Environmental Conditions and Management Practices on Long-Shelf-Life Tomatoes. Agronomy 2021, 11, 533. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Pereira-Dias, L.; Casanova, C.; García-Martínez, M.D.; Rosa, E.; Soler, E.; Plazas, M.; Soler, S. Insights into the Adaptation to Greenhouse Cultivation of the Traditional Mediterranean Long Shelf-Life Tomato Carrying the Alc Mutation: A Multi-Trait Comparison of Landraces, Selections, and Hybrids in Open Field and Greenhouse. Front. Plant Sci. 2018, 9, 1774. [Google Scholar] [CrossRef] [Green Version]
- Pnueli, L.; Carmel-Goren, L.; Hareven, D.; Gutfinger, T.; Alvarez, J.; Ganal, M.; Zamir, D.; Lifschitz, E. The SELF-PRUNING Gene of Tomato Regulates Vegetative to Reproductive Switching of Sympodial Meristems and Is the Ortholog of CEN and TFL1. Development 1998, 125, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Lippman, Z.B.; Cohen, O.; Alvarez, J.P.; Abu-Abied, M.; Pekker, I.; Paran, I.; Eshed, Y.; Zamir, D. The Making of a Compound Inflorescence in Tomato and Related Nightshades. PLoS Biol. 2008, 6, e288. [Google Scholar] [CrossRef]
- Gur, A.; Osorio, S.; Fridman, E.; Fernie, A.R.; Zamir, D. Hi2-1, A QTL Which Improves Harvest Index, Earliness and Alters Metabolite Accumulation of Processing Tomatoes. Theor. Appl. Genet. 2010, 121, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Visa, S.; Cao, C.; Gardener, B.M.; van der Knaap, E. Modeling of Tomato Fruits into Nine Shape Categories Using Elliptic Fourier Shape Modeling and Bayesian Classification of Contour Morphometric Data. Euphytica 2014, 200, 429–439. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Allen, R.G.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 92-5-104219-5. [Google Scholar]
- Peet, M.M. Irrigation and fertilization. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing: Cambridge, UK, 2005; pp. 171–198. ISBN 9781780641935. [Google Scholar]
- Patanè, C.; Saita, A. Biomass, Fruit Yield, Water Productivity and Quality Response of Processing Tomato to Plant Density and Deficit Irrigation under a Semi-Arid Mediterranean Climate. Crop Pasture Sci. 2015, 66, 224. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Olivoto, T.; Lúcio, A.D. Metan: An R Package for Multi-Environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit Irrigation as an On-Farm Strategy to Maximize Crop Water Productivity in Dry Areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Hooshmand, M.; Albaji, M.; Nasab, S.B.; Ansari, N.A.Z. The Effect of Deficit Irrigation on Yield and Yield Components of Greenhouse Tomato (Solanum lycopersicum) in Hydroponic Culture in Ahvaz Region, Iran. Sci. Hortic. 2019, 254, 84–90. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, G.; Jia, D.; Wang, J.; Mota, M.; Pereira, L.S.; Huang, Q.; Xu, X.; Liu, H. Responses of Drip Irrigated Tomato (Solanum lycopersicum L.) Yield, Quality and Water Productivity to Various Soil Matric Potential Thresholds in an Arid Region of Northwest China. Agric. Water Manag. 2013, 129, 181–193. [Google Scholar] [CrossRef]
- Kumar, R.; Tamboli, V.; Sharma, R.; Sreelakshmi, Y. NAC-NOR Mutations in Tomato Penjar Accessions Attenuate Multiple Metabolic Processes and Prolong the Fruit Shelf Life. Food Chem. 2018, 259, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water Shortage and Quality of Fleshy Fruits—Making the Most of the Unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Quinet, M.; Dielen, V.; Batoko, H.; Boutry, M.; Havelange, A.; Kinet, J.-M. Genetic Interactions in the Control of Flowering Time and Reproductive Structure Development in Tomato (Solanum lycopersicum). New Phytol. 2006, 170, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Philouze, J. Comparaison Des Effets Des Gènes J et J-2 Conditionnant Le Caractère ‘Jointless’ Chez La Tomate et Les Relations d’épistasie Entre j et j-2 Dans Les Lignées de Même Type Variétal. Ann. Amélior. Plantes 1978, 28, 431–445. [Google Scholar]
- Rozek, E.; Nurzynska-Wierdak, R.; Kosior, M. Quality and Structure of Single Harvest Tomato Fruit Yield. Acta Sci. Pol. Hortorum Cultus 2011, 10, 319–329. [Google Scholar]
- Kedar, N.; Rabinowitch, H.D.; Budowski, P. Conditioning of Tomato Fruit against Sunscald. Sci. Hortic. 1975, 3, 83–87. [Google Scholar] [CrossRef]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ben Ahmed, H.; Bertin, N. Effects of Water Deficit on Leaves and Fruit Quality during the Development Period in Tomato Plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Hay, R. Harvest Index: A Review of Its Use in Plant Breeding and Crop Physiology. Ann. Appl. Biol. 1995, 126, 197–216. [Google Scholar] [CrossRef]
- van der Ploeg, A.; van der Meer, M.; Heuvelink, E. Breeding for a More Energy Efficient Greenhouse Tomato: Past and Future Perspectives. Euphytica 2007, 158, 129–138. [Google Scholar] [CrossRef]
- Higashide, T.; Heuvelink, E. Physiological and Morphological Changes over the Past 50 Years in Yield Components in Tomato. J. Am. Soc. Hortic. Sci. 2009, 134, 460–465. [Google Scholar] [CrossRef]
- Cavero, J.; Plant, R.E.; Shennan, C.; Williams, J.R.; Kiniry, J.R.; Benson, V.W. Application of Epic Model to Nitrogen Cycling in Irrigated Processing Tomatoes under Different Management Systems. Agric. Syst. 1998, 56, 391–414. [Google Scholar] [CrossRef]
- Heuvelink, E.; Li, T.; Dorais, M. Crop growth and yield. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing: Cambridge, UK, 2005; pp. 89–136. ISBN 9781780641935. [Google Scholar]
- Wang, X.; Xing, Y. Evaluation of the Effects of Irrigation and Fertilization on Tomato Fruit Yield and Quality: A Principal Component Analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, E.; Segura, V.; Gricourt, J.; Bonnefoi, J.; Derivot, L.; Causse, M. Association Mapping Reveals the Genetic Architecture of Tomato Response to Water Deficit: Focus on Major Fruit Quality Traits. J. Exp. Bot. 2016, 67, 6413–6430. [Google Scholar] [CrossRef] [PubMed]
- Galmes, J.; OchogavÍA, J.M.; Gago, J.; RoldÁN, E.J.; Cifre, J.; Conesa, M.À. Leaf Responses to Drought Stress in Mediterranean Accessions of Solanum lycopersicum: Anatomical Adaptations in Relation to Gas Exchange Parameters. Plant. Cell Environ. 2013, 36, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological Screening for Drought Tolerance in Mediterranean Long-Storage Tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fullana-Pericàs, M.; Conesa, M.À.; Ribas-Carbó, M.; Galmés, J. The Use of a Tomato Landrace as Rootstock Improves the Response of Commercial Tomato under Water Deficit Conditions. Agronomy 2020, 10, 748. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of Water Stress on Processing Tomatoes Yield, Quality and Water Use Efficiency with Plastic Mulched Drip Irrigation in Sandy Soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Du, T.; Qiu, R.; Guo, P.; Chen, R. Quantitative Response of Greenhouse Tomato Yield and Quality to Water Deficit at Different Growth Stages. Agric. Water Manag. 2013, 129, 152–162. [Google Scholar] [CrossRef]
- Favati, F.; Lovelli, S.; Galgano, F.; Miccolis, V.; Di Tommaso, T.; Candido, V. Processing Tomato Quality as Affected by Irrigation Scheduling. Sci. Hortic. 2009, 122, 562–571. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R.; May, D.M. Tomato Fruit Yields and Quality under Water Deficit and Salinity. J. Am. Soc. Hortic. Sci. 1991, 116, 215–221. [Google Scholar] [CrossRef]
- Saltveit, M.E. Postharvest biology and handling of tomatoes. In Tomatoes; Heuvelink, E., Ed.; CABI: Wallingford, UK, 2018; pp. 314–336. ISBN 9781780641935. [Google Scholar]
- Arah, I.; Amaglo, H.; Kumah, E.K.; Ofori, H. Preharvest and Postharvest Factors Affecting the Quality and Shelf Life of Harvested Tomatoes: A Mini Review. Int. J. Agron. 2015, 2015, 478041. [Google Scholar] [CrossRef] [Green Version]
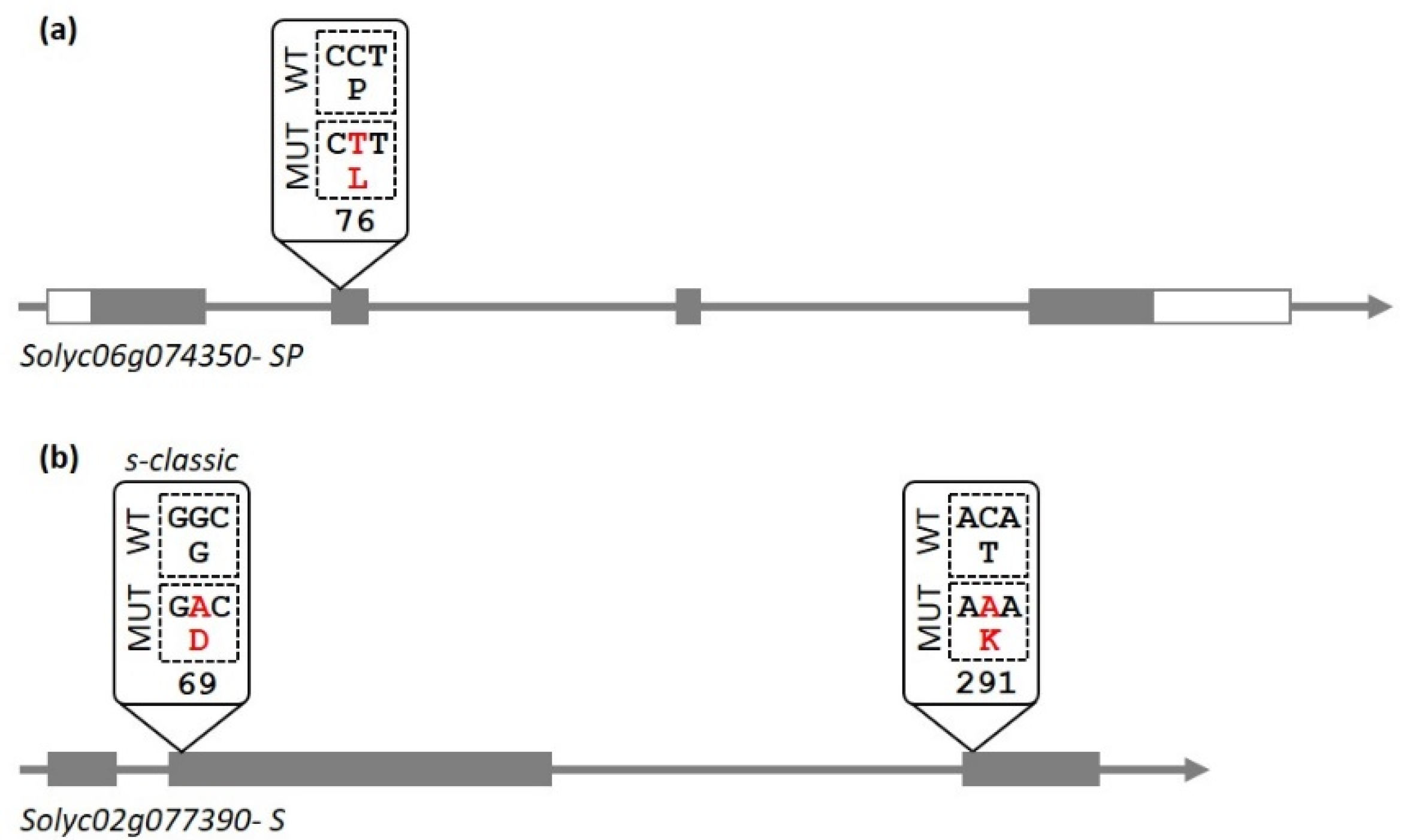
| Genotype | Varietal Type | Origin | Ripening 1 | Plant Architecture | Inflorescence Type | Fruit Shape 2 | Earliness (DAT) 3 |
|---|---|---|---|---|---|---|---|
| 2.9 | Penjar | Breeding line | LSL | Determinate | Compound | Heart | 133.3 ± 0.5 |
| 2.14 | Penjar | Breeding line | LSL | Determinate | Compound | Round | 133.3 ± 0.5 |
| LC215 | Penjar | Landrace (Origin: Catalonia) | LSL | Determinate | Regular | Round | 112.0 ± 9.9 |
| LC547 | Ramellet | Landrace (Origin Balearic Islands) | LSL | Determinate | Regular | Flat | 127.3 ± 1.9 |
| Palamós | Penjar | Commercial hybrid (Semillas Fitó) | LSL | Indeterminate | Regular | Round | 104.3 ± 4.9 |
| Pera Delta | Processing | Commercial inbred (Mas Pastoret) | Normal ripening | Determinate | Regular | Long rectangular | 104.3 ± 4.9 |
| Number of Fruits Per Plant | Yield (kg m−2) | Commercial Yield (%) | Cracking (%) | Sunscald (%) | Unripen Fruits (%) | Fruit Weight (g) | ||
|---|---|---|---|---|---|---|---|---|
| Irrigation regime (I) | NW | 66.4 | 5.1 a | 57.6 a | 4.7 | 13.1 b | 21.0 | 58.7 a |
| WD | 68.9 | 4.3 b | 55.2 b | 4.2 | 19.0 a | 18.9 | 47.9 b | |
| p value | ns | *** | ** | ns | *** | ns | *** | |
| Locality (L) | Eco-1 | 62.1 | 4.0 | 57.9 | 5.9 | 9.9 | 18.6 | 48.3 |
| Conv-1 | 75.1 | 4.9 | 56.5 | 3.2 | 19.8 | 21.6 | 49.9 | |
| Eco-2 | 66.5 | 5.5 | 54.4 | 4.1 | 19.2 | 19.7 | 63.2 | |
| p value | ** | *** | *** | ** | *** | ns | *** | |
| Genotype (G) | ‘2.9’ | 83.5 a | 4.7 ab | 52.4 bc | 4.2 ab | 24.0 a | 20.0 ab | 41.9 b |
| ‘2.14’ | 70.1 ab | 4.2 b | 55.6 bc | 3.8 ab | 17.9 ab | 17.9 ab | 45.2 b | |
| ‘LC215’ | 60.3 b | 4.4 b | 54.8 bc | 5.7 ab | 18.5 ab | 16.4 ab | 56.0 a | |
| ‘LC547’ | 55.6 b | 4.5 b | 34.0 c | 7.2 a | 27.1 a | 16.1 b | 62.1 a | |
| ‘Pera Delta’ | 67.4 ab | 6.1 a | 66.0 b | 3.5 ab | 7.1 bc | 20.1 ab | 67.0 a | |
| ‘Palamós’ | 67.7 ab | 4.3 b | 71.2 a | 3.2 b | 5.1 c | 27.1 a | 46.7 b | |
| p value | *** | *** | *** | *** | *** | *** | *** | |
| GxI | p value | ns | ns | ns | * | ns | ns | ns |
| Total Biomass (kg Plant−1, fw) | Harvest Index (%, fw) | Total Biomass (kg Plant−1, dw) | Harvest Index (%, dw) | ||
|---|---|---|---|---|---|
| Irrigation regime (I) | NW | 5.6 a | 69.3 | 0.51 a | 54.4 |
| WD | 4.7 b | 69.6 | 0.47 b | 53.8 | |
| p value | *** | ns | * | ns | |
| Locality (L) | Eco-1 | 4.0 | 71.2 | 0.39 | 54.9 |
| Conv-1 | 5.3 | 69.7 | 0.52 | 54.0 | |
| Eco-2 | 5.9 | 67.7 | 0.54 | 53.6 | |
| p value | *** | * | *** | ns | |
| Genotype (G) | ‘2.9’ | 5.0 b | 71.5 ab | 0.52 ab | 55.4 abc |
| ‘2.14’ | 4.8 b | 65.9 bc | 0.48 ab | 51.3 bc | |
| ‘LC215’ | 4.4 b | 73.9 ab | 0.40 b | 59.1 ab | |
| ‘LC547’ | 5.0 ab | 67.1 bc | 0.47 ab | 49.7 c | |
| ‘Pera Delta’ | 5.9 a | 77.5 a | 0.49 ab | 60.9 a | |
| ‘Palamós’ | 5.4 ab | 61.4 c | 0.54 a | 48.8 c | |
| p value | *** | *** | *** | *** | |
| GxI | p value | ns | ** | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schober, P.; Buil, J.; Rivera, A.; Campo, S.; Roig-Villanova, I.; Casals, J. Breeding Long Shelf-Life (LSL) Tomato Landraces to Non-Trellised Culture and Water Deficit Irrigation: The Effect on Yield and Postharvest Storage. Agronomy 2022, 12, 2312. https://doi.org/10.3390/agronomy12102312
Schober P, Buil J, Rivera A, Campo S, Roig-Villanova I, Casals J. Breeding Long Shelf-Life (LSL) Tomato Landraces to Non-Trellised Culture and Water Deficit Irrigation: The Effect on Yield and Postharvest Storage. Agronomy. 2022; 12(10):2312. https://doi.org/10.3390/agronomy12102312
Chicago/Turabian StyleSchober, Philipp, Júlia Buil, Ana Rivera, Sonia Campo, Irma Roig-Villanova, and Joan Casals. 2022. "Breeding Long Shelf-Life (LSL) Tomato Landraces to Non-Trellised Culture and Water Deficit Irrigation: The Effect on Yield and Postharvest Storage" Agronomy 12, no. 10: 2312. https://doi.org/10.3390/agronomy12102312





