Rubisco Small Subunits’ Genome-Wide Identification and Their Function from Gene Expression to Rubisco Activity and Photosynthesis among Peanut Genotypes under Different Nitrogen Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of RBCS Gene Family and Multiple Sequence Alignment and Phylogenetic Analysis
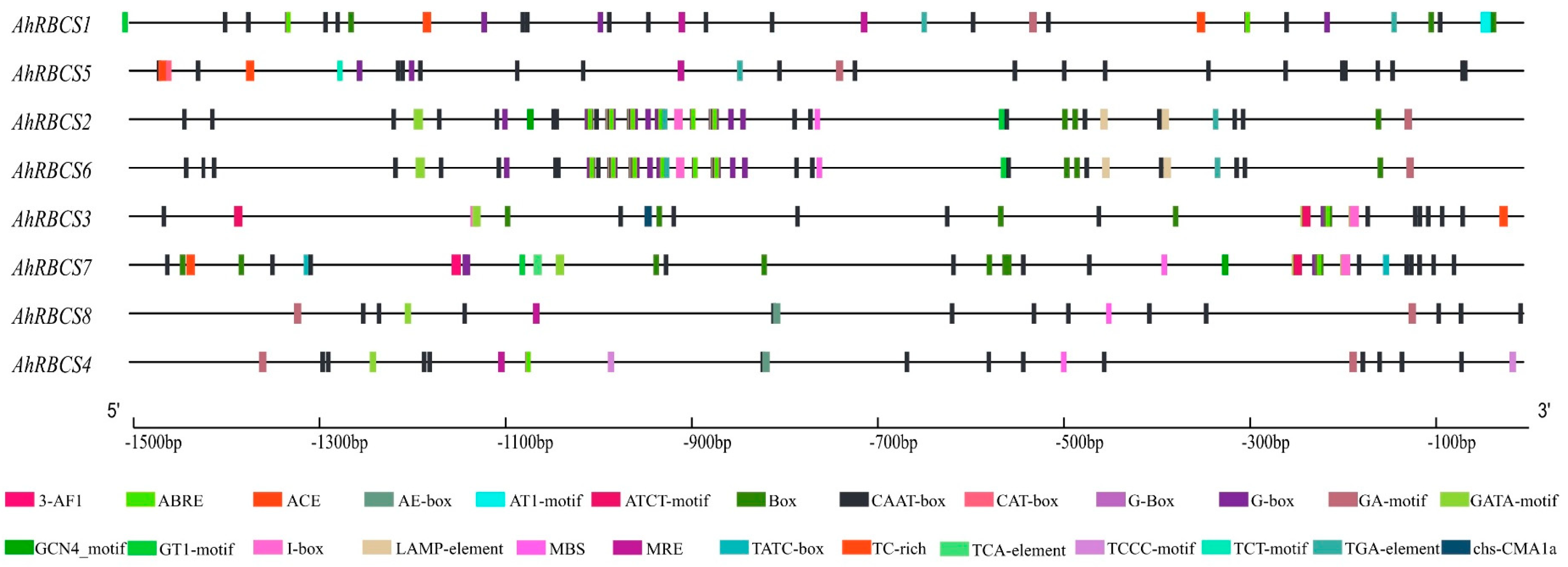
2.2. Gene Structure, Conserved Motif, and Cis-Acting Regulatory Element Analysis
2.3. Plant Materials and Treatments
2.4. Morphological Indexes and Photosynthesis Characteristics’ Measurement
2.5. Rubisco Enzyme Activity Assay
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
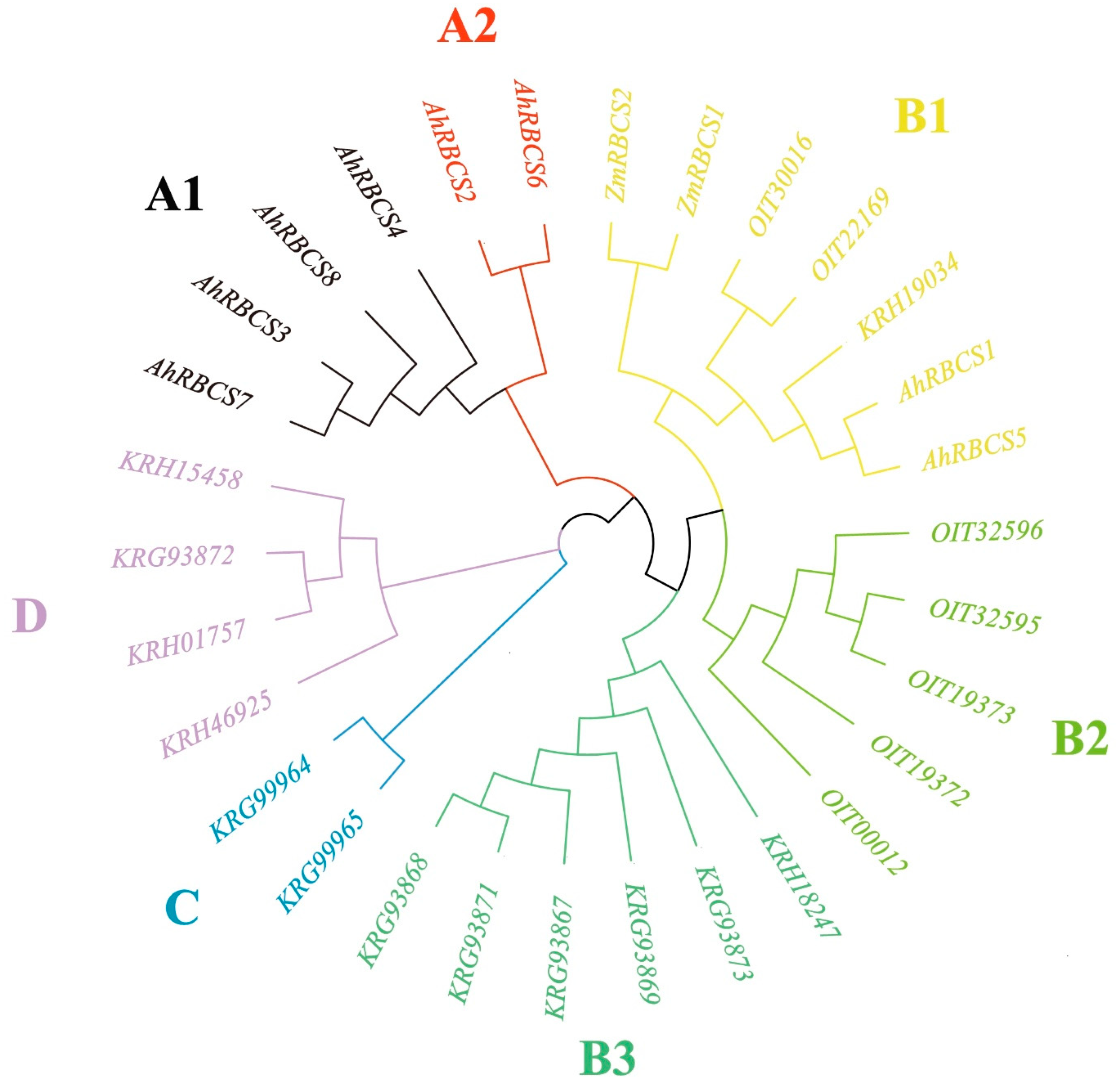
3.1. Identification of RBCS Genes
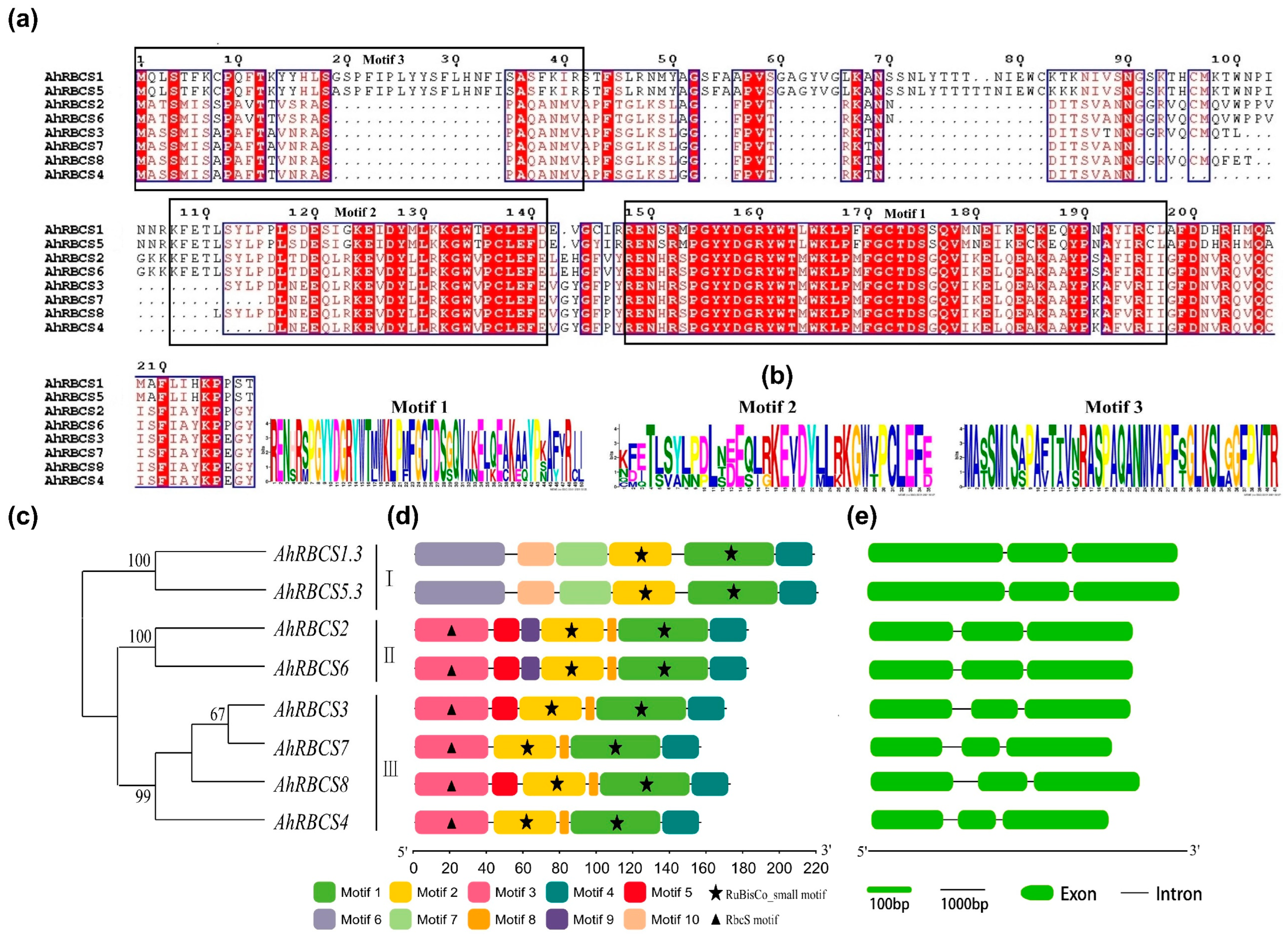
3.2. Sequence Characteristics of RBCS Family Genes
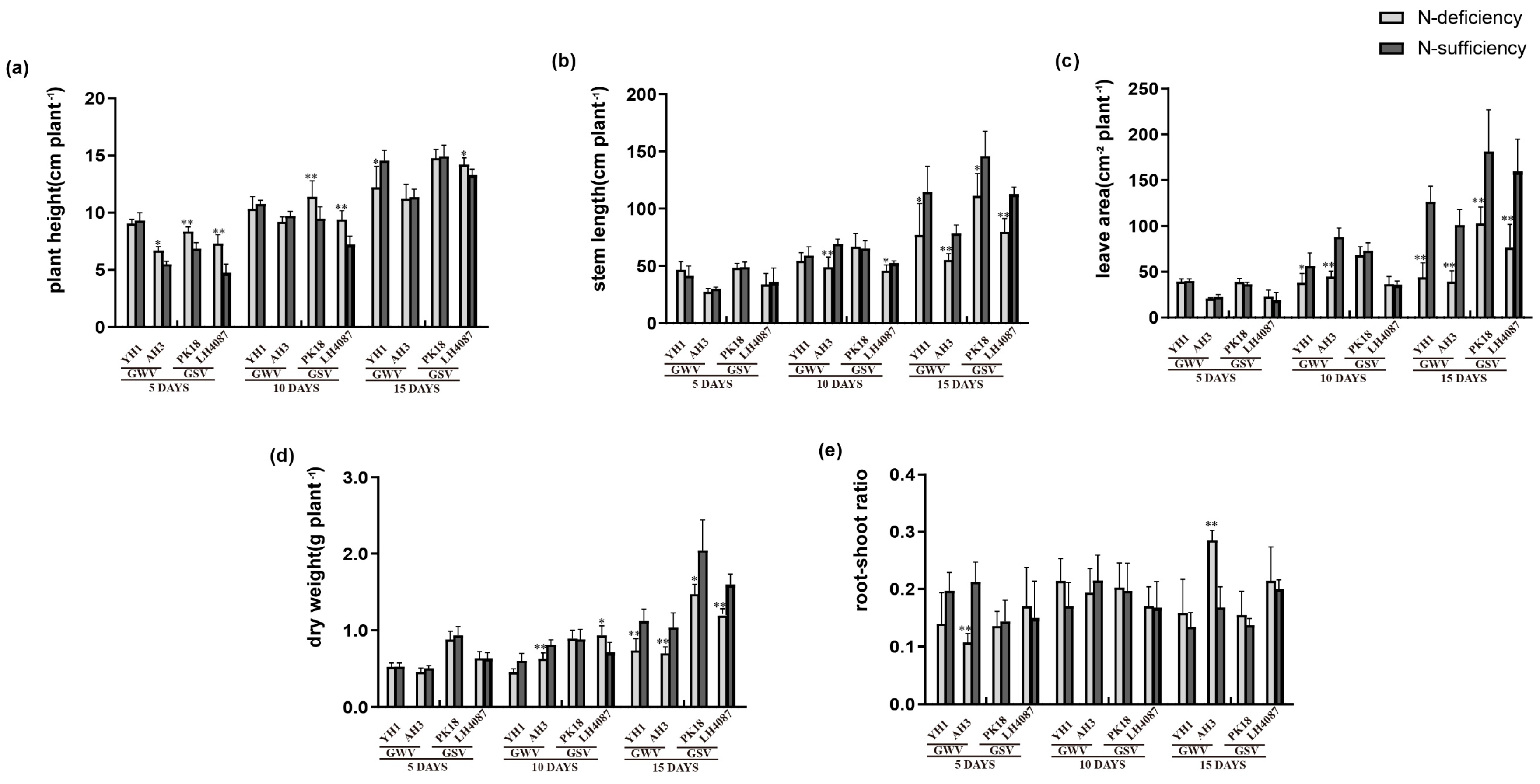
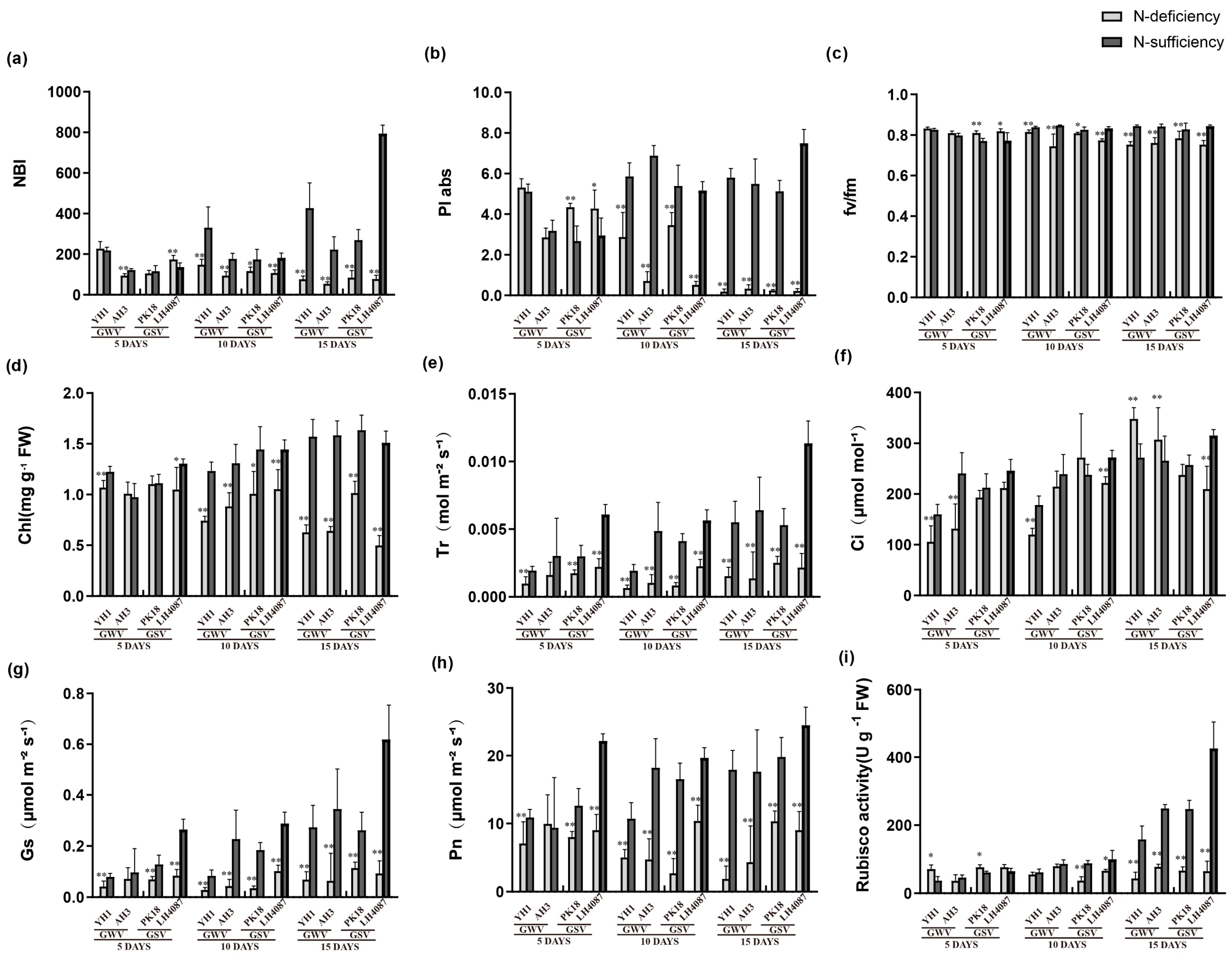
3.3. Morphological Changes under N-deficiency
3.4. Peanut Seedlings’ Leaf Gas Exchange Parameters under N-deficiency
3.5. Rubisco Carboxylation Activity Assay
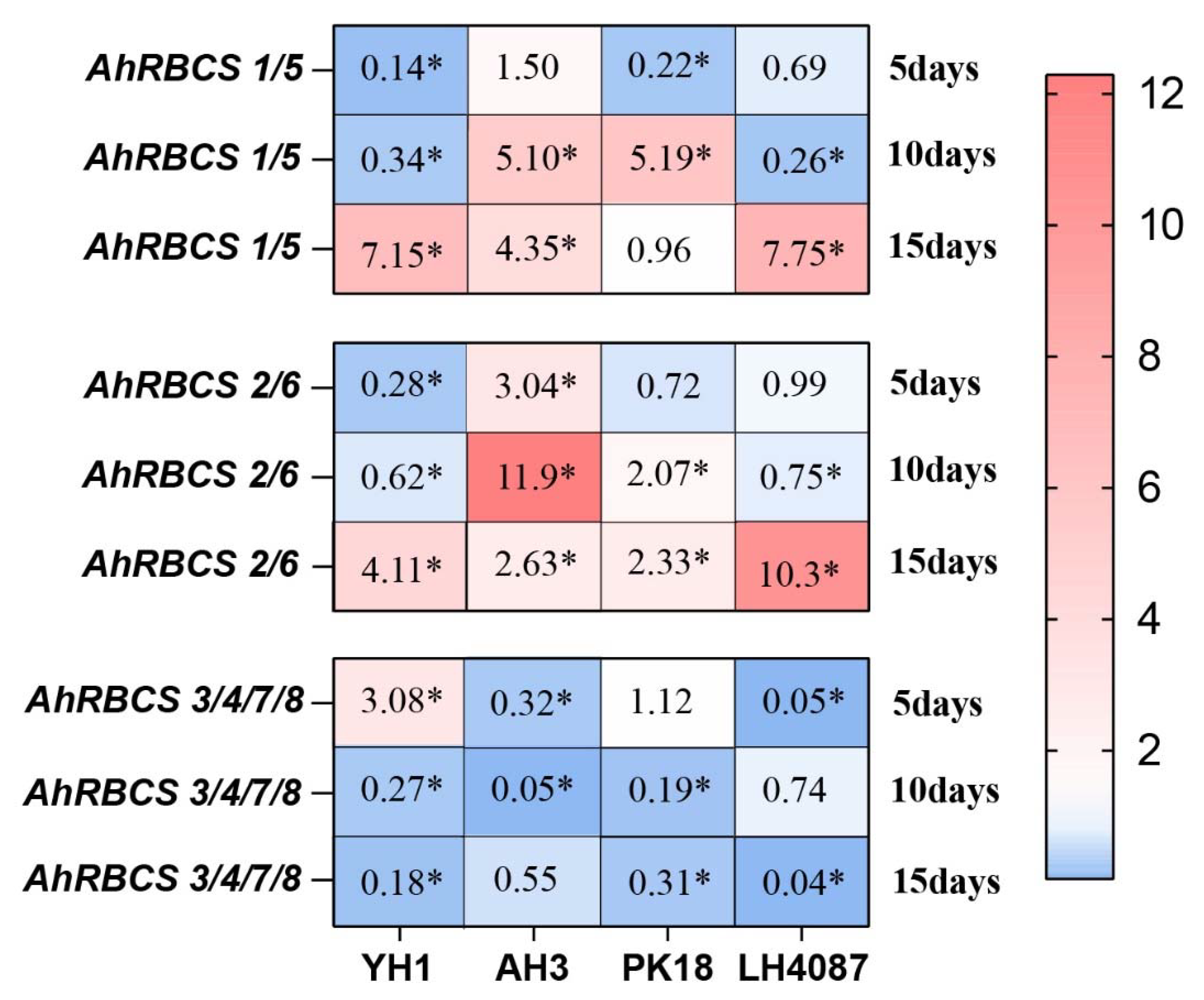
3.6. Gene Expression of RBCS Response to N-deficiency
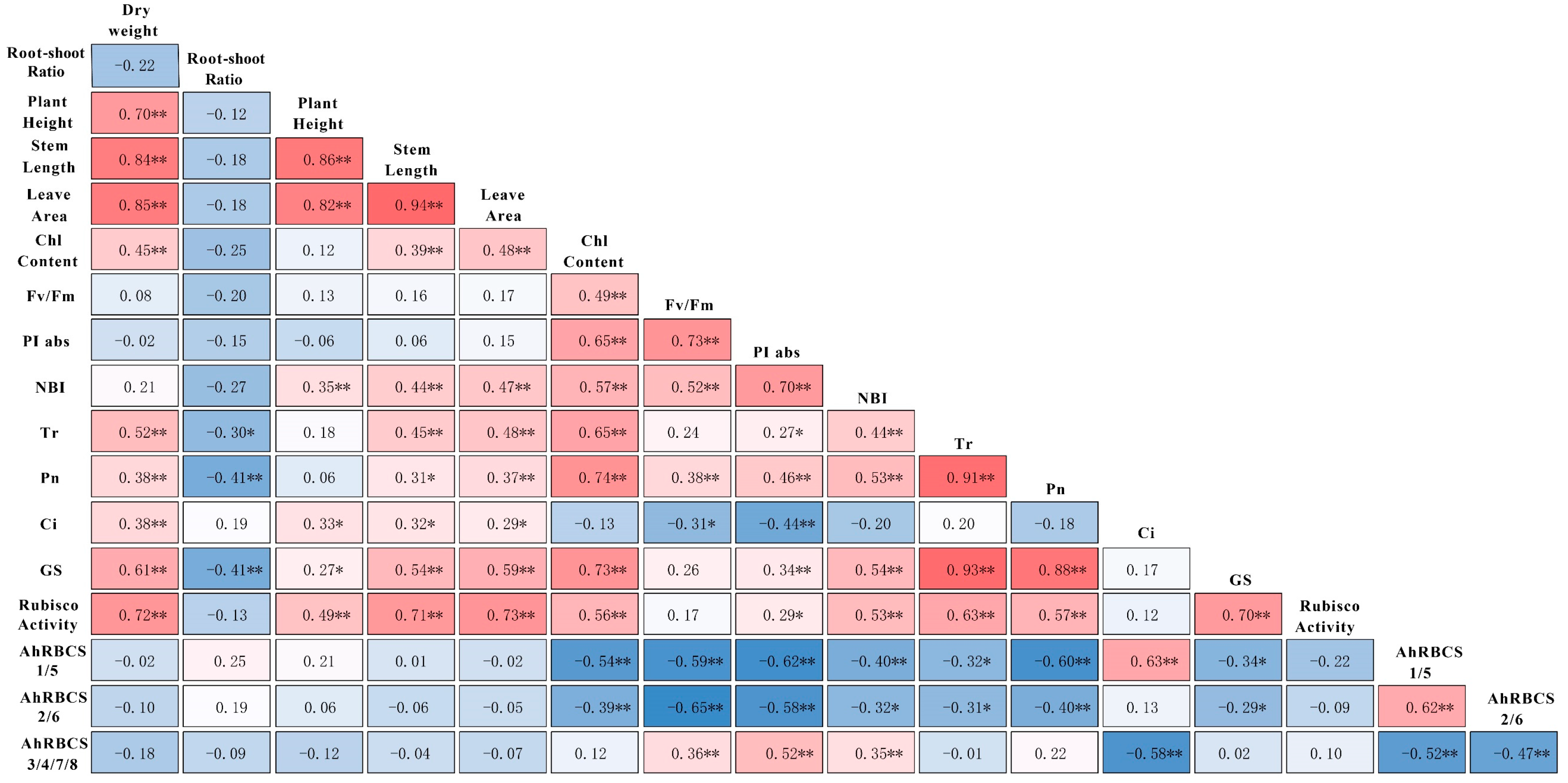
3.7. Correlation Analysis
4. Discussion
4.1. RBCS Gene Characteristics in Peanut
4.2. The Morpho-Physiological Changes of Peanut under Low-N Stress
4.3. The Expressions of AhRBCSs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008, 59, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Suzuki, Y.; Yoshizawa, R.; Kanno, K.; Makino, A. Effect of individual suppression of RBCS multigene family on Rubisco contents in rice leaves. Plant Cell Environ. 2012, 35, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.V.; Hub, J.S.; Spoel, D.; Andersson, I. CO2 and O2 Distribution in Rubisco Suggests the Small Subunit Functions as a CO2 Reservoir. J. Am. Chem. Soc. 2014, 136, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Stasolla, C.; Lé-Babel, A.B.; Ayele, B.T. Isolation and characterization of rubisco small subunit gene promoter from common wheat (Triticum aestivum L.). Plant Signal Behav. 2015, 10, e989033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tai, W.Y.; Mak, Y.M.; Ho, K.K. Rubisco small subunit gene family in cassava. DNA Seq. 2009, 10, 189–194. [Google Scholar]
- Fritz, C.C.; Wolter, F.P.; Schenkemeyer, V.; Herget, T.; Schreier, P.H. The gene family encoding the ribulose-(1,5)-bisphosphate carboxylase/oxygenase (Rubisco) small subunit of potato. Gene 1993, 137, 271–274. [Google Scholar] [CrossRef]
- Qin, L.; Xue, Y.; Fei, Y.; Zeng, L.; Yang, S.; Deng, X. Identification, evolution and expression analyses of Ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene family in wheat (Triticum aestivum L.). Acta Physiol. Plant 2018, 40, 85. [Google Scholar] [CrossRef]
- Donald, R.; Cashmore, A.R. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990, 9, 1717–1726. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Xu, N.; Sun, X. Changes in Growth, Carbon and Nitrogen Enzyme Activity and mRNA Accumulation in the Halophilic Microalga Dunaliella viridis in Response to NaCl Stress. J. Ocean Univ. China 2016, 15, 1094–1110. [Google Scholar] [CrossRef]
- Doron, L.; Xu, L.; Rachmilevitch, S.; Stern, D.B. Transgenic overexpression of rubisco subunits and the assembly factor RAF1 are beneficial to recovery from drought stress in maize. Environ. Exp. Bot. 2020, 177, 104126. [Google Scholar] [CrossRef]
- Hao, J.; Liu, L.; Yang, Y.; Zhou, R. The influence of HCO3−, K+ and HSO3− on RUBISCO large-subunit (rbcL) and small-subunit (rbcS) genes expression. Afr. J. Agric. Res. 2011, 6, 2280–2284. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Z.; Dong, Z.; Shi, S.; Zhang, J. Effects of Nntrogen Application Rate on the Yelds, Yutritive Value and Silage Fermentation Quality of Whole-crop Wheat. Asian-Austral. J. Anim. Sci. 2016, 29, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, S. Effectiveness analysis of investment in chemical fertilizer to increase grain crops yield and countermeasures in Tianjin. J. Tianjin Agric. Coll. 1999, 6, 35–39. [Google Scholar]
- Amane, M.; Barry, O. Effects of Nitrogen Nutrition on Nitrogen Partitioning Between Chloroplasts and Mitochondria in Pea and Wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Meng, L.I.; Qing, M.A.; Wang, H.; Wei, X.X.; Yong-Sheng, W.U. Characterization and evaluation of sensitivity to nitrogen input across maize inbreds based on the relative chlorophyll content. J. Plant Genet. Resour. 2015, 16, 1264–1271. [Google Scholar]
- Gao, J.; Feng, W.; Sun, J.; Tian, Z.; Dai, T. Enhanced Rubisco activation associated with maintenance of electron transport alleviates inhibition of photosynthesis under low nitrogen conditions in winter wheat seedlings. J. Exp. Bot. 2018, 69, 5477–5488. [Google Scholar] [CrossRef]
- Tantray, A.Y.; Bashir, S.S.; Ahmad, A. Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice. Physiol. Mol. Biol. Plants 2020, 26, 83–94. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Liu, L.; Wang, Q.; Li, Q.; Du, Y.; Zhang, J. Protein Contents in Different Peanut Varieties and Their Relationship to Gel Property. Int. J. Food Prop. 2014, 17, 1560–1576. [Google Scholar] [CrossRef]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and Transcriptome Analysis of Wheat Seedlings Response to Low Nitrogen Stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Davis, K.E.; Patterson, C.; Zhang, B. Response of Root Growth and Development to Nitrogen and Potassium Deficiency as well as microRNA-Mediated Mechanism in Peanut (Arachis hypogaea L.). Front. Plant Sci. 2021, 12, 695234. [Google Scholar] [CrossRef] [PubMed]
- Chandna, R.; Kaur, G.; Iqbal, M.; Khan, I.; Ahmad, A. Differential response of wheat genotypes to applied nitrogen: Biochemical and molecular analysis. Arch. Agron. Soil Sci. 2012, 58, 915–929. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Song, M. Variations in Nitrogen Metabolism are Closely Linked with Nitrogen Uptake and Utilization Efficiency in Cotton Genotypes under Various Nitrogen Supplies. Plants 2020, 9, 250. [Google Scholar] [CrossRef]
- Lozano, R.; Hamblin, M.T.; Prochnik, S.; Jannink, J.L. Identification and distribution of the NBS-LRR gene family in the Cassava genome. BMC Genom. 2015, 16, 360. [Google Scholar] [CrossRef]
- Fontana, J.E.; Wang, G.; Sun, R.; Xue, H.; Pan, X. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol. Biochem. 2020, 153, 72–80. [Google Scholar] [CrossRef]
- Chang, M.; Wei, X.; Wang, Q.; Hu, Y.; Li, C.; Tang, Y. University G. A Comparative Study on Different Extraction Methods for Plant Chlorophyll. Chin. Agric. Sci. Bull. 2016, 32, 177–180. [Google Scholar]
- Fargašová, A.; Molnárová, M. Assessment of Cr and Ni phytotoxicity from cutlery-washing waste-waters using biomass and chlorophyll production tests on mustard Sinapis alba L. seedlings. Environ. Sci. Pollut. Res. 2009, 17, 187–194. [Google Scholar] [CrossRef]
- Zhou, F. An improved CTAB Method for Extraction of Total RNA From Mature Leaves in Oil Sunflower. Heilongjiang Agric. Sci. 2013, 7, 14–16. [Google Scholar]
- Wang, B.; Yingjuan, S.; Wang, T. Molecular cloning of RBCS genes in Selaginella and the evolution of the rbcS gene family. Arch. Biol. Sci. 2015, 67, 373–383. [Google Scholar] [CrossRef]
- Brenchley, R.; Spannagl, M.; Pfeifer, M.; Barker, G.L.A.; D’Amore, R.; Allen, A.M.; Mckenzie, N.; Kramer, M.; Kerhornou, A.; Bolser, D. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 2012, 491, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low Light Stress Down-Regulated Rubisco Gene Expression and Photosynthetic Capacity During Cucumber (Cucumis sativus L.) Leaf Development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Morelli, G.; Nagy, F.; Fraley, R.T.; Rogers, S.G.; Chua, N.H. A short conserved sequence is involved in the light-inducibility of a gene encoding ribulose 1,5-bisphosphate carboxylase small subunit of pea. Nature 1985, 315, 200–204. [Google Scholar] [CrossRef]
- Giuliano, G.; Pichersky, E.; Malik, V.S.; Timko, M.P.; Scolnik, P.A.; Cashmore, A.R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc. Natl. Acad. Sci. USA 1988, 85, 7089–7093. [Google Scholar] [CrossRef] [PubMed]
- Schindler, U.; Cashmore, A.R. Photoregulated gene expression may involve ubiquitous DNA binding proteins. EMBO J. 1990, 9, 3415–3427. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Fujikawa, Y.; Esaka, M. Light Regulation of Ascorbic Acid Biosynthesis in Rice via Light Responsive cis-Elements in Genes Encoding Ascorbic Acid Biosynthetic Enzymes. J. Agric. Chem. Soc. Jpn. 2010, 74, 888–891. [Google Scholar]
- Perdomo, J.A.; Buchner, P.; Carmo-Silva, E. The relative abundance of wheat Rubisco activase isoforms is post-transcriptionally regulated. Photosynth. Res. 2021, 148, 47–56. [Google Scholar] [CrossRef]
- Garai, S.; Joshi, N.C.; Tripathy, B.C. Phylogenetic analysis and photoregulation of siroheme biosynthesis genes: Uroporphyrinogen III methyltransferase and sirohydrochlorin ferrochelatase of Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2016, 22, 351–359. [Google Scholar] [CrossRef]
- Xin, S.; Tao, C.; Li, H.; Fang, D.D. Cloning and Functional Analysis of the Promoter of An Ascorbate Oxidase Gene from Gossypium hirsutum. PLoS ONE 2016, 11, e161695. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, Y.; Wang, S.; Wu, G.; Xu, H.; Wei, J.; Ju, L.; Huang, Y.; Chen, H. Interspecies Evolution and Networks Investigation of the Auxin Response Protein (AUX/IAA) Family Reveals the Adaptation Mechanisms of Halophytes Crops in Nitrogen Starvation Agroecological Environments. Agriculture 2021, 11, 780. [Google Scholar] [CrossRef]
- Lin, Y.L.; Chao, Y.Y.; Huang, W.D.; Kao, C.H. Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul. 2011, 64, 263–273. [Google Scholar] [CrossRef]
- Wei, S.; Wang, X.; Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. The mechanisms of low nitrogen induced weakened photosynthesis in summer maize (Zea mays L.) under field conditions. Plant Physiol. Biochem. 2016, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, Z.; Liu, C.; Ting, H.E.; Guo, G.; Gao, R.; Hongwei, X.U.; Yingbo, L.I.; Ruiju, L.U.; Huang, J. Screening and Identification Indices of Low-nitrogen Tolerance for Barley Landraces at Seedling Stage. Acta Agric. Boreali-Sin. 2019, 34, 148–155. [Google Scholar]
- Liu, C.; Gong, X.; Wang, H.; Dang, K.; Deng, X.; Feng, B. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant Physiol. Biochem. 2020, 151, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Xiong, Q.; Zhong, L.; Shi, X.; Chen, X. Analysis of main metabolisms during nitrogen deficiency and compensation in rice. Acta Physiol. Plant 2019, 41, 68. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, X.; Khan, A.; Tan, D.K.Y.; Luo, H. Biomass Accumulation, Photosynthetic Traits and Root Development of Cotton as Affected by Irrigation and Nitrogen-Fertilization. Front. Plant Sci. 2018, 9, 173. [Google Scholar] [CrossRef]
- Ma, F.J.; Li, D.D.; Cai, J.; Jiang, D.; Dai, T.B. Responses of wheat seedlings root growth and leaf photosynthesis to drought stress. J. Appl. Ecol. 2012, 23, 724–730. [Google Scholar]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Liu, W. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Levine, R.P. The mechanism of photosynthesis. Sci. Am. 1969, 221, 58–70. [Google Scholar] [CrossRef]
- Yuasa, K.; Shikata, T.; Kuwahara, Y.; Nishiyama, Y. Adverse effects of strong light and nitrogen deficiency on cell viability, photosynthesis, and motility of the red-tide dinoflagellate Karenia mikimotoi. Phycologia 2018, 57, 525–533. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Deng, X.; Zhang, Z.; Yin, L. Comprehensive evaluation of physiological traits under nitrogen stress and participation of linolenic acid in nitrogen-deficiency response in wheat seedlings. BMC Plant Biol. 2020, 20, 501. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. Imaging of the Blue, Green, and Red Fluorescence Emission of Plants: An Overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Hiroshi, F.; Akina, M.; Chiaki, U.; Jun, K.; Ryutaro, M.; Daisuke, S.; Tomoko, H.; Tetsushi, A. Expression level of Rubisco activase negatively correlates with Rubisco content in transgenic rice. Photosynth. Res. 2018, 137, 465–474. [Google Scholar]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.Y.; Gabriel, T.P.; Ulrich, H.F.; Manajit, H.H. Rubisco Activases: AAA+ Chaperones Adapted to Enzyme Repair. Front. Mol. Biosci. 2017, 4, 20. [Google Scholar] [CrossRef]
- Mao, S.; Suzuki, Y.; Kondo, E.; Nishida, S.; Makino, A. Effects of Overproduction of Rubisco Activase on Rubisco Content in Transgenic Rice Grown at Different N Levels. Int. J. Mol. Sci. 2020, 21, 1626. [Google Scholar]
- Suzuki, Y.; Nakabayashi, K.; Yoshizawa, R.; Mae, T.; Makino, A. Differences in Expression of the RBCS Multigene Family and Rubisco Protein Content in Various Rice Plant Tissues at Different Growth Stages. Plant Cell Physiol. 2009, 50, 1851–1855. [Google Scholar] [CrossRef]
- Kudo, N.; Mano, K.; Suganami, M.; Kondo, E.; Suzuki, Y.; Makino, A. Effects of overexpression of the Rubisco small subunit gene under the control of the Rubisco activase promoter on Rubisco contents of rice leaves at different positions. Soil Sci. Plant Nutr. 2020, 66, 569–578. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Chen, J.; Wang, X.; Wang, R.; Peng, S.; Chen, L.; Ma, L.; Luo, J. Identification of Rubisco rbcL and rbcS in Camellia oleifera and their potential as molecular markers for selection of high tea oil cultivars. Front. Plant Sci. 2015, 6, 189. [Google Scholar] [CrossRef]
| Gene Name | Translation Product | Chromosome | aa | MW | pI |
|---|---|---|---|---|---|
| AhRBCS1 | ATg2a1.7U0T6N.3 | 1 | 218 | 25.23 | 9.03 |
| AhRBCS2 | ATg2a1.K6N5RU.1 | 4 | 182 | 20.50 | 9.03 |
| AhRBCS3 | ATg2a1.97RMNS.1 | 10 | 170 | 19.16 | 8.87 |
| AhRBCS4 | ATg2a1.FHUH7B.1 | 10 | 156 | 17.63 | 8.74 |
| AhRBCS5 | ATg2a1.53KNFG.3 | 11 | 220 | 25.54 | 9.14 |
| AhRBCS6 | ATg2a1.HCT4KE.1 | 14 | 182 | 20.50 | 9.03 |
| AhRBCS7 | ATg2a1.XT6T0B.1 | 20 | 156 | 17.60 | 8.74 |
| AhRBCS8 | ATg2a1.I56F9F.1 | 20 | 172 | 19.44 | 8.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kong, X.; Li, L.; Jia, P.; Cheng, X.; Zhang, X.; Zhang, L.; Xue, H.; Khan, A.; Zhang, Z. Rubisco Small Subunits’ Genome-Wide Identification and Their Function from Gene Expression to Rubisco Activity and Photosynthesis among Peanut Genotypes under Different Nitrogen Levels. Agronomy 2022, 12, 2316. https://doi.org/10.3390/agronomy12102316
Wang X, Kong X, Li L, Jia P, Cheng X, Zhang X, Zhang L, Xue H, Khan A, Zhang Z. Rubisco Small Subunits’ Genome-Wide Identification and Their Function from Gene Expression to Rubisco Activity and Photosynthesis among Peanut Genotypes under Different Nitrogen Levels. Agronomy. 2022; 12(10):2316. https://doi.org/10.3390/agronomy12102316
Chicago/Turabian StyleWang, Xiaohui, Xiangjun Kong, Lijie Li, Peipei Jia, Xiangguo Cheng, Xiaotian Zhang, Lei Zhang, Huiyun Xue, Aziz Khan, and Zhiyong Zhang. 2022. "Rubisco Small Subunits’ Genome-Wide Identification and Their Function from Gene Expression to Rubisco Activity and Photosynthesis among Peanut Genotypes under Different Nitrogen Levels" Agronomy 12, no. 10: 2316. https://doi.org/10.3390/agronomy12102316
APA StyleWang, X., Kong, X., Li, L., Jia, P., Cheng, X., Zhang, X., Zhang, L., Xue, H., Khan, A., & Zhang, Z. (2022). Rubisco Small Subunits’ Genome-Wide Identification and Their Function from Gene Expression to Rubisco Activity and Photosynthesis among Peanut Genotypes under Different Nitrogen Levels. Agronomy, 12(10), 2316. https://doi.org/10.3390/agronomy12102316








