Thifluzamide, Fludioxonil, and Clothianidin as Seed Treatment Can Efficiently Control Major Soil-Borne Diseases, Aphids (Aphidoidea spp.), and Residue Distribution in the Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Conditions
2.3. Preparation of the TFC Seed Coating Agent
2.4. Laboratory Experiments
2.5. Field Experiments
2.6. Analysis of Pesticide Residues in the Soil and Plant Samples
2.7. Biosafety of TFC in Earthworms
2.8. Data Analyses
3. Results and Discussion
3.1. Preparation of the TFC Seed Coating Agent
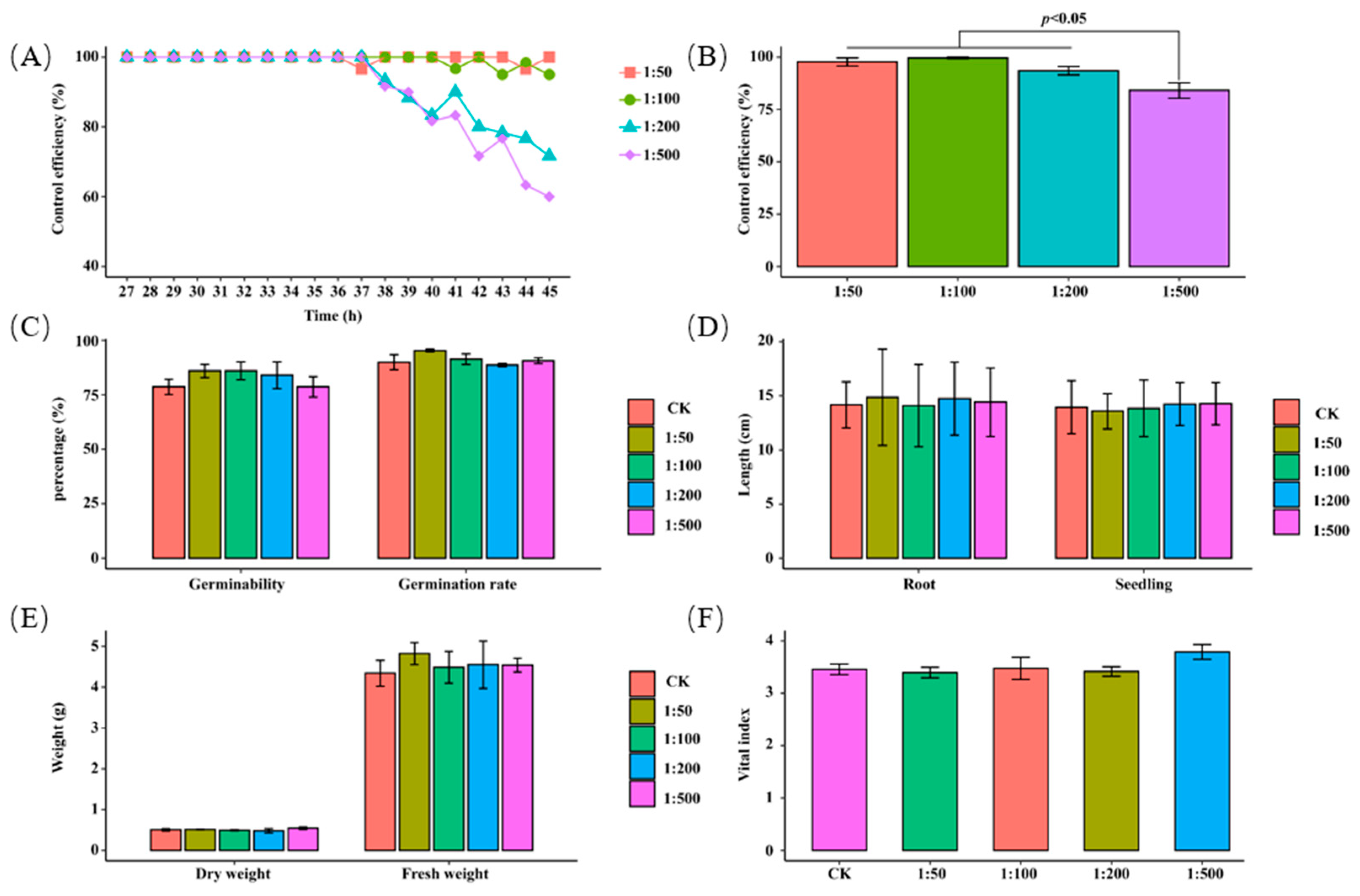
3.2. Laboratory Tests
3.3. Field Tests
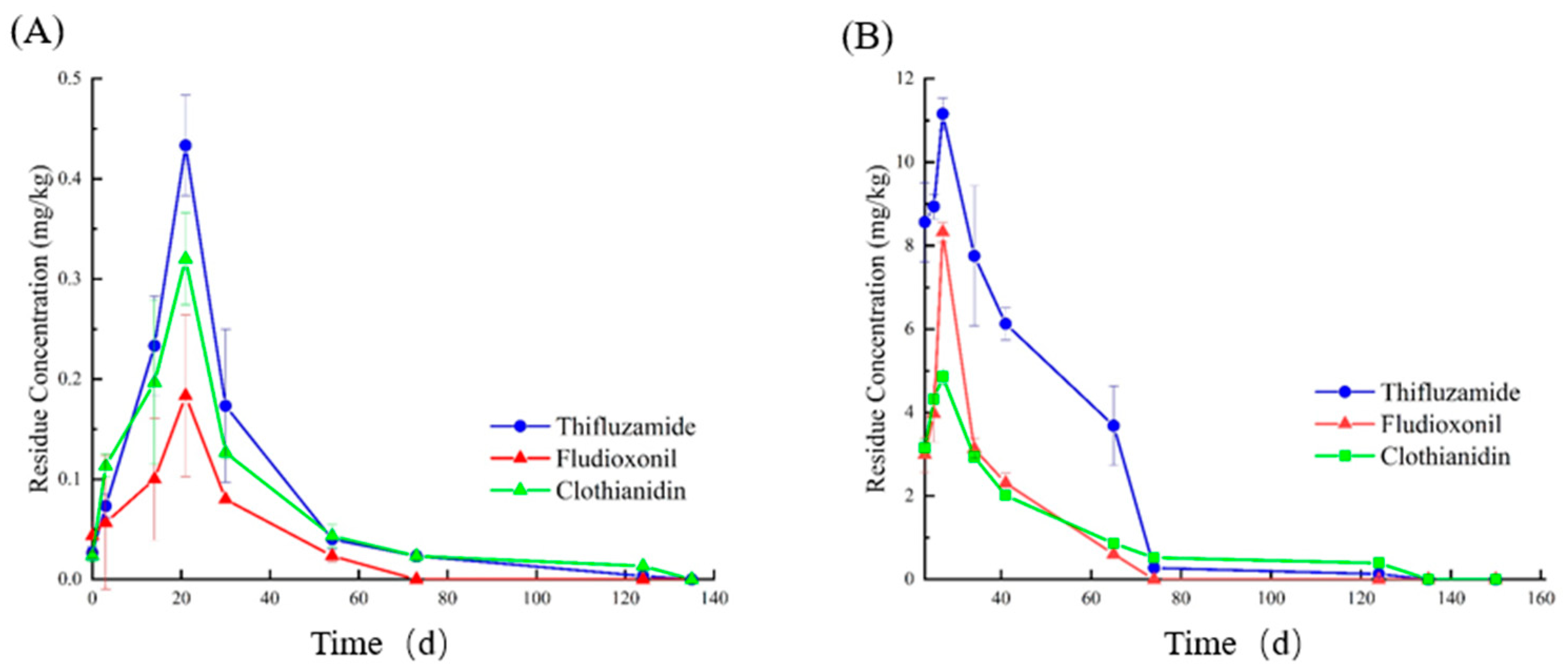
3.4. Dynamic Changes in Pesticide Residues in the Soil and Plants
3.5. Effect of TFC on Earthworm Survival
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, D.-F.; Wang, H. Preparation of a novel highly effective and environmental friendly wheat seed coating agent. Agric. Sci. China 2010, 9, 937–941. [Google Scholar] [CrossRef]
- Hamada, M.S.; Yin, Y.; Chen, H.; Ma, Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 2011, 67, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.E.; Kanan, H.O.; Inanaga, S.; Ma, Y.Q.; Sugimoto, Y. Impact of pesticide seed treatments on aphid control and yield of wheat in the Sudan. Crop Prot. 2001, 20, 929–934. [Google Scholar] [CrossRef]
- Capo, L.; Zappino, A.; Reyneri, A.; Blandino, M. Role of the fungicide seed dressing in controlling seed-borne Fusarium spp. Infection and in enhancing the early development and grain yield of maize. Agronomy 2020, 10, 784. [Google Scholar] [CrossRef]
- Angus, J.F.; Kirkegaard, J.A.; Hunt, J.R.; Ryan, M.H.; Ohlander, L.; Peoples, M.B. Break crops and rotations for wheat. Crop Pasture Sci. 2015, 66, 523–552. [Google Scholar] [CrossRef]
- Li, Y.-F.; An, J.-J.; Dang, Z.-H.; Pan, W.-L.; Gao, Z.-L. Systemic control efficacy of neonicotinoids seeds dressing on English grain aphid (Hemiptera: Aphididae). J. Asia-Pac. Entomol. 2018, 21, 430–435. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Liang, H.; Liu, H.; Du, L.; Xu, H.; Xin, Z. Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat. J. Exp. Bot. 2008, 59, 4195–4204. [Google Scholar] [CrossRef]
- McMillan, V.E.; Canning, G.; Moughan, J.; White, R.P.; Gutteridge, R.J.; Hammond-Kosack, K.E. Exploring the resilience of wheat crops grown in short rotations through minimising the build-up of an important soil- borne fungal pathogen. Sci. Rep. 2018, 8, 9550. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Li, B.; Wang, K.; Xia, X. Resistance detection and synergism of enzyme inhibitors on neonicotinoids to Aphis gossypii in Shandong Province. Chin. J. Pestic. Sci. 2014, 16, 673–680. [Google Scholar]
- Huang, J.; Hu, R.; Rozelle, S.; Qiao, F.; Pray, C.E. Transgenic varieties and productivity of smallholder cotton farmers in China. Aust. J. Agric. Resour. Econ. 2002, 46, 367–387. [Google Scholar] [CrossRef]
- Zhang, L.; Greenberg, S.M.; Zhang, Y.; Liu, T.-X. Effectiveness of thiamethoxam and imidacloprid seed treatments against Bemisia tabaci (Hemiptera: Aleyrodidae) on cotton. Pest Manag. Sci. 2011, 67, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Zhao, Y.; Wei, Y.; Mu, W.; Liu, F. Effects of imidacloprid and clothianidin seed treatments on wheat aphids and their natural enemies on winter wheat. Pest Manag. Sci. 2016, 72, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Neonicotinoid metabolism: Compounds, substituents, pathways, enzymes, organisms, and relevance. J. Agric. Food Chem. 2011, 59, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Richard, M. Application of latex emulsion polymers in seed coating technology. Pestic. Formul. Appl. Syst. 2005, 23, 55–67. [Google Scholar]
- Song, W.-J.; Hu, J.; Qiu, J.; Geng, H.-Y. Primary study on the development of special seed coating agents and their application in rice (Oryza saliva L.) cultivated by direct seeding. J. Zhejiang Univ. 2005, 31, 368–373. (In Chinese) [Google Scholar]
- Gesch, R.W.; Archer, D.W. Influence of sowing date on emergence characteristics of maize seed coated with a temperature-activated polymer. Agron. J. 2005, 97, 1543–1550. [Google Scholar] [CrossRef]
- Nault, B.A.; Taylor, A.G.; Urwiler, M.; Rabaey, T.; Hutchison, W.D. Neonicotinoid seed treatments for managing potato leafhopper infestations in snap bean. Crop Prot. 2004, 23, 147–154. [Google Scholar] [CrossRef]
- Magalhaes, L.C.; Hunt, T.E.; Siegfried, B.D. Efficacy of neonicotinoid seed treatments to reduce soybean aphid populations under field and controlled conditions in Nebraska. J. Econ. Entomol. 2009, 102, 187–195. [Google Scholar] [CrossRef]
- Saeed, R.; Razaq, M.; Hardy, I.C. Impact of neonicotinoid seed treatment of cotton on the cotton leafhopper, Amrasca devastans (Hemiptera: Cicadellidae), and its natural enemies. Pest Manag. Sci. 2016, 72, 1260–1267. [Google Scholar] [CrossRef]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef]
- Wettstein, F.E.; Kasteel, R.; Garcia Delgado, M.F.; Hanke, I.; Huntscha, S.; Balmer, M.E.; Poiger, T.; Bucheli, T.D. Leaching of the neonicotinoids thiamethoxam and imidacloprid from sugar beet seed dressings to subsurface tile drains. J. Agric. Food Chem. 2016, 64, 6407–6415. [Google Scholar] [CrossRef]
- Zhu, H. A summary of researches on main cotton diseases. Cotton Sci. 2007, 19, 391–398. [Google Scholar]
- Goertz, H.M. Technology development in coated fertilizer. In Proceedings of the Workshop on Controlled/Slow Release Fertilizers, Haifa, Israel, 1993; pp. 158–164. [Google Scholar]
- Raban, S.; Shaviv, A. Controlled release characteristics of coated urea fertilisers. In Proceedings of the 22nd Internal Symposium on Controlled Release of Bio-Active Materials, Seattle, WA, USA, 30 July–2 August 1995; pp. 105–106. [Google Scholar]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; der Sluijs, J.P.V. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Choung, C.B.; Hyne, R.V.; Stevens, M.M.; Hose, G.C. The ecological effects of a herbicide-insecticide mixture on an experimental freshwater ecosystem. Environ. Pollut. 2013, 172, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Liang, S.; Li, Y.; Liu, S.; Yu, Y.; Yang, Y.; Fang, A.; Bi, C. The sensitivity of Didymella bryoniae to difenoconazole and boscalid and the synergistic mechanism of fungicide co-formulation. Eur. J. Plant Pathol. 2021, 161, 865–879. [Google Scholar] [CrossRef]
- Badaw, M.E.I.; Abd-Elnabi, A.D.; Saad, A.-F.S.A. Insecticidal ctivity of nanoemulsions of organophosphorus insecticides against cotton leafworm (Spodoptera littoralis) and molecular docking studies. Int. J. Trop. Insect Science. 2022, 42, 293–313. [Google Scholar] [CrossRef]
- Aydin, H.; Gürkan, M.O. The efcacy of spinosad on diferent strains of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Turk. J. Biol. 2006, 30, 5–9. [Google Scholar]
- Wu, X.H.; Zhang, W.H.; Liu, P.F. Research and development trend of the seed coating agent in China. Plant Prot. Promot. Technol. 2003, 10, 36–38. [Google Scholar]
- Delgado, A.; Franco, G.M.; Páez, J.I.; Vega, J.M.; Carmona, E.; Aviles, M. Incidence of cotton seedling diseases caused by Rhizoctonia solani and Thielaviopsis basicola in relation to previous crop, residue management and nutrients availability in soils in SW Spain. J. Phytopathol. 2005, 153, 710–714. [Google Scholar] [CrossRef]
- Standardization Administration of the People’s Republic of China. Part 15: Earthworm acute toxicity test GB/T 3127015-2014, Test Guidelines on Environmental Safety Assessment for Chemical Pesticides; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2014. (In Chinese)
- OECD. Earthworm Acute Toxicity Tests. Guidelines for the Testing of Chemicals; Test No. 207; Organization for Economic Cooperation and Development: Paris, France, 1984.
- OECD. Guideline for Testing of Chemicals: Earthworm, Acute Toxicity Tests; Organization for Economic Cooperation and Development: Paris, France, 1984; Volume 207.
- Ren, X.-X.; Chen, C.; Ye, Z.-H.; Su, X.-Y.; Xiao, J.-J.; Liao, M.; Cao, H.-Q. Development and application of seed coating agent for the control of major soil-borne diseases infecting wheat. Agronomy 2019, 9, 413. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.-J.; Fan, Z.-J.; Guo, X.-F.; Zhang, Z.-M.; Xu, J.-H.; Song, Y.-Q.; Yurievich, M.Y.; Belskaya, N.P.; Bakulev, V.A. Synthesis of 1,2,3-thiadiazole and thiazole-based strobilurins as potent fungicide candidates. J. Agric. Food Chem. 2017, 65, 745–751. [Google Scholar] [CrossRef]
- Chen, X.; Ren, B.; Chen, M.; Wang, Q.; Zhang, L.; Yan, G. NLLSS: Predicting synergistic drug combinations based on semi-supervised learning. PLoS Comput. Biol. 2016, 12, e1004975. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-X.; Liu, Y.; Zhang, P.; Li, X.-X.; Pang, X.-Y.; Zhao, Y.-H.; Li, H.; Lin, J.; Mu, W. Selection of organosilicone surfactants for tank-mixed pesticides considering the balance between synergistic effects on pests and environmental risks. Chemosphere 2018, 217, 591–598. [Google Scholar] [CrossRef]
- Cox, W.J.; Shields, E.; Cherney, J.H. The effect of clothianidin seed treatments on corn growth following soybean. Crop Sci. 2007, 47, 2482–2485. [Google Scholar] [CrossRef]
- da Costa, C.M.; dos Santos, R.S.; Marini, N.; da Maia, L.C.; Vanier, N.L.; Elias, M.C.; de Oliveira, M.; de Oliveira, A.C. Seed coating and rice grain stickiness. Trop. Plant Biology 2020, 13, 225–235. [Google Scholar] [CrossRef]
- SANCO (Directorate General for Health and Consumer Affairs). Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed; Document No. SANCO/10684/2011; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Sánchez-Bayo, F. The trouble with neonicotinoids. Science 2014, 346, 806–807. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Wang, Y.; Zhao, Y.; Lin, J.; Liu, F.; Mu, W. Nitenpyram, Dinotefuran, and Thiamethoxam Used as Seed Treatments Act as Efficient Controls against Aphis gossypii via High Residues in Cotton Leaves. J. Agric. Food Chem. 2016, 64, 9276–9285. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Wheat, R.; McGahan, D.G.; Mitchell, F. Evaluation of leaching potential of three systemic neonicotinoid insecticides in vineyard soil. J. Contam. Hydrol. 2014, 170, 86–94. [Google Scholar] [CrossRef]
- Stamm, M.D.; Heng-Moss, T.M.; Baxendale, F.P.; Siegfried, B.D.; Blankenship, E.E.; Nauen, R. Uptake and translocation of imidacloprid, clothianidin and flupyradifurone in seed-treated soybeans. Pest Manag. Sci. 2016, 72, 1099–1109. [Google Scholar] [CrossRef]

| Fungicide | Mass Ratio | R. Cerealis | B. Sorokiniana | ||
|---|---|---|---|---|---|
| EC50(mg L−1) | CTC | EC50 (mg L−1) | CTC | ||
| Thifluzamide | - | 0.0189 | - | 1346.7960 | - |
| Fludioxonil | - | 0.0651 | - | 0.0101 | - |
| Thifluzamide: Fludioxonil | 10:1 | 0.0240 | 84.18 | 0.1550 | 71.67 |
| 5:1 | 0.0210 | 102.07 | 0.0600 | 101.00 | |
| 2:1 | 0.0210 | 117.89 | 0.0220 | 137.73 | |
| 1:1 | 0.0270 | 108.50 | 0.0330 | 61.21 | |
| 1:2 | 0.0320 | 112.10 | 0.0190 | 79.74 | |
| 1:5 | 0.0440 | 105.13 | 0.0230 | 52.70 | |
| 1:10 | 0.0520 | 102.43 | 0.0170 | 65.35 | |
| Neonicotinoid | Regression equation | LC50 (mg·L−1) | 95% CL (mg·L−1) | χ2 |
|---|---|---|---|---|
| Clothianidin | 0.900 + 1.746x | 0.3050 | 0.231–0.390 | 8.016 |
| Imidacloprid | 0.344 + 1.819x | 0.6470 | 0.493–0.819 | 6.247 |
| Nitenpyram | 0.351 + 1.483x | 0.5800 | 0.411–0.768 | 2.195 |
| Thiacloprid | 0.057 + 1.773x | 0.9820 | 0.723–1.182 | 3.417 |
| Acetamiprid | 0.146 + 1.646x | 0.8160 | 0.620–1.052 | 4.067 |
| Thiamethoxam | 0.379 + 1.506x | 0.5600 | 0.396–0.740 | 3.802 |
| Component | Content % (g/g) | Property |
|---|---|---|
| Thifluzamide | 4.00 | Active ingredient |
| Fludioxonil | 2.00 | Active ingredient |
| Clothianidin | 4.00 | Active ingredient |
| NNO | 1.70 | Wetting dispersant |
| LAE-9 | 2.00 | Nonionic surfactant |
| S-20 | 2.00 | Emulsifier |
| XG | 0.15 | Thickener |
| Magnesium aluminum silicate | 1.70 | Thickening agent |
| Octanol | 0.30 | Anti-foaming agent |
| Ethylene glycol | 3.00 | Antifreeze agent |
| Pigment red | 6.00 | Dye agent |
| Polyacrylamide carboxymethyl cellulose | 4.00 | Film-forming agent |
| Deionized water | 69.15 | -- |
| Agent | Parameter | Control Efficiency on Sharp Eyespot | Control Efficiency on Aphid Infestation | |||
|---|---|---|---|---|---|---|
| Seedling Height (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | Control Effect (%) | Control Effect (%) | |
| TFC | 32.27 ± 0.37 a | 12.9 ± 0.06 a | 75.60 ± 3.30 a | 26.54 ± 0.74 a | 86.7 a | 96.7 a |
| DFT | 31.40 ± 0.32 ab | 11.57 ± 0.43 ab | 63.19 ± 2.3 a | 25.37 ± 1.27 a | 81.3 a | 98.7 a |
| CK | 30.67 ± 0.26 b | 10.6 ± 0.55 b | 55.71 ± 1.28 a | 20.77 ± 1.32 b | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wang, X.; Yin, S.; Wang, C.; Ren, X.; Gao, Q.; Cao, H. Thifluzamide, Fludioxonil, and Clothianidin as Seed Treatment Can Efficiently Control Major Soil-Borne Diseases, Aphids (Aphidoidea spp.), and Residue Distribution in the Field. Agronomy 2022, 12, 2330. https://doi.org/10.3390/agronomy12102330
Chen C, Wang X, Yin S, Wang C, Ren X, Gao Q, Cao H. Thifluzamide, Fludioxonil, and Clothianidin as Seed Treatment Can Efficiently Control Major Soil-Borne Diseases, Aphids (Aphidoidea spp.), and Residue Distribution in the Field. Agronomy. 2022; 12(10):2330. https://doi.org/10.3390/agronomy12102330
Chicago/Turabian StyleChen, Chao, Xumiao Wang, Shanshan Yin, Chao Wang, Xuexiang Ren, Quan Gao, and Haiqun Cao. 2022. "Thifluzamide, Fludioxonil, and Clothianidin as Seed Treatment Can Efficiently Control Major Soil-Borne Diseases, Aphids (Aphidoidea spp.), and Residue Distribution in the Field" Agronomy 12, no. 10: 2330. https://doi.org/10.3390/agronomy12102330
APA StyleChen, C., Wang, X., Yin, S., Wang, C., Ren, X., Gao, Q., & Cao, H. (2022). Thifluzamide, Fludioxonil, and Clothianidin as Seed Treatment Can Efficiently Control Major Soil-Borne Diseases, Aphids (Aphidoidea spp.), and Residue Distribution in the Field. Agronomy, 12(10), 2330. https://doi.org/10.3390/agronomy12102330







