The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Modified Hedley Sequential Fractionation
2.3. Synchrotron-Based P K-Edge XANES Spectroscopy
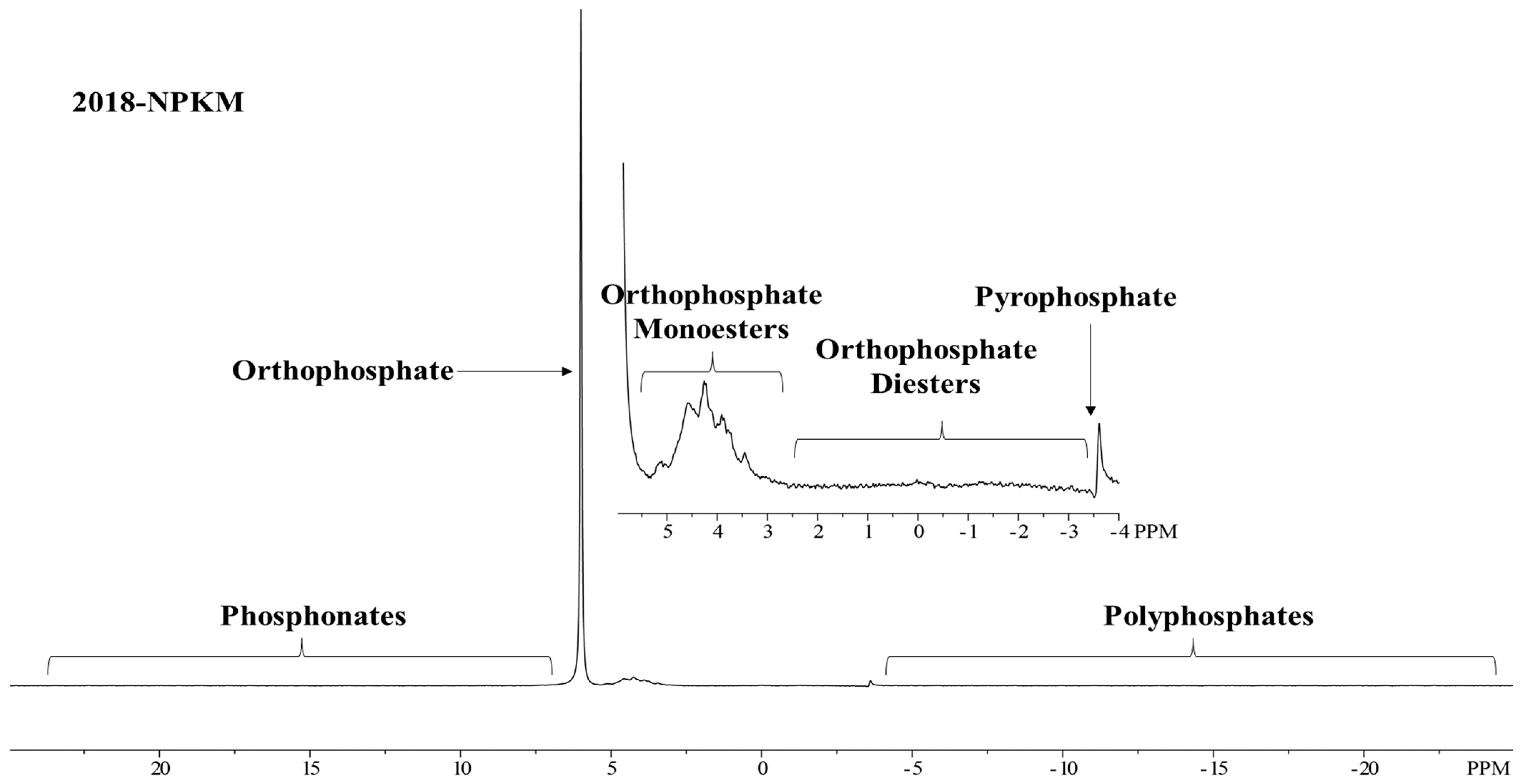
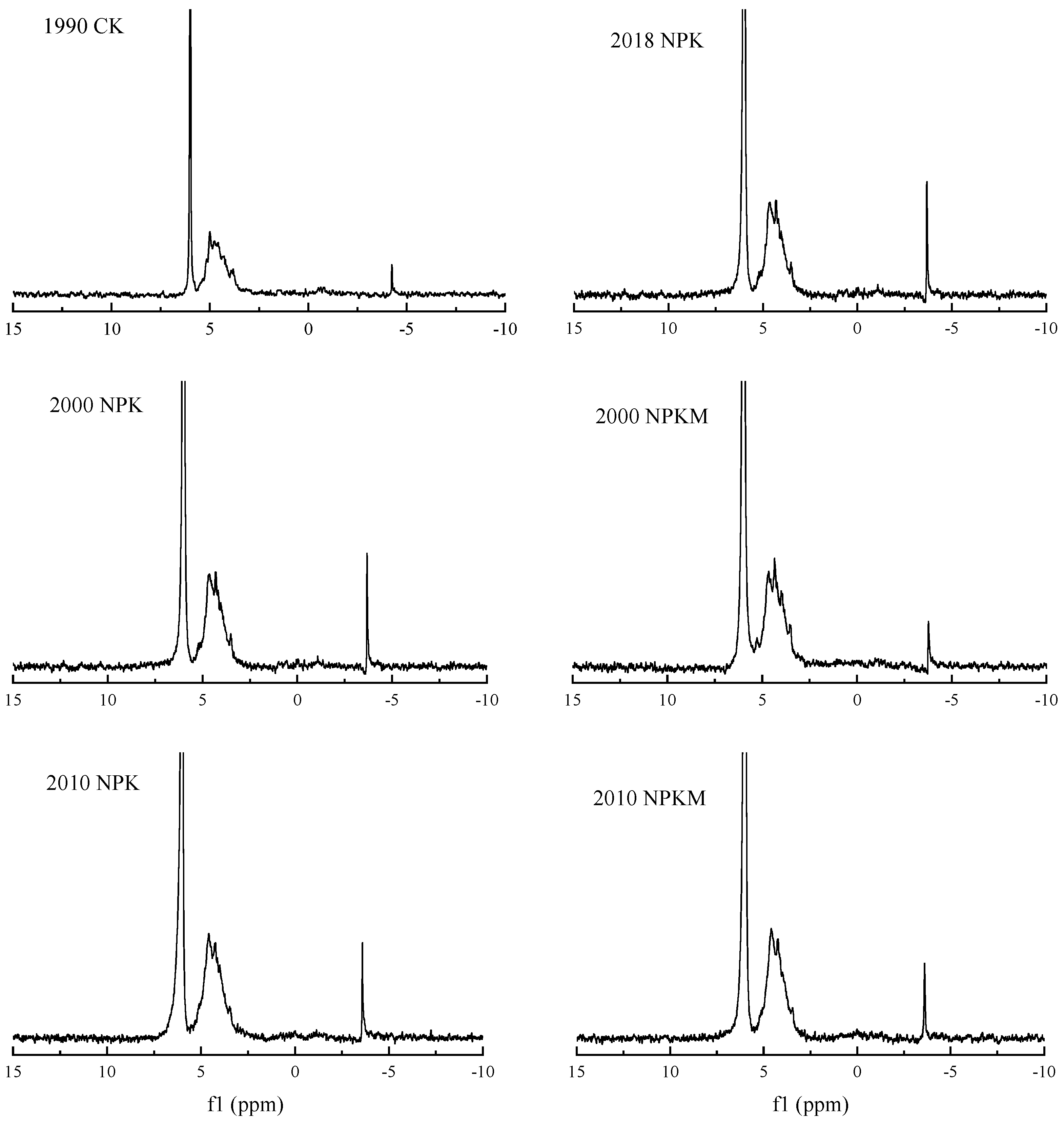
2.4. Solution 31P-NMR Spectroscopy
2.5. Statistical Analysis
3. Results
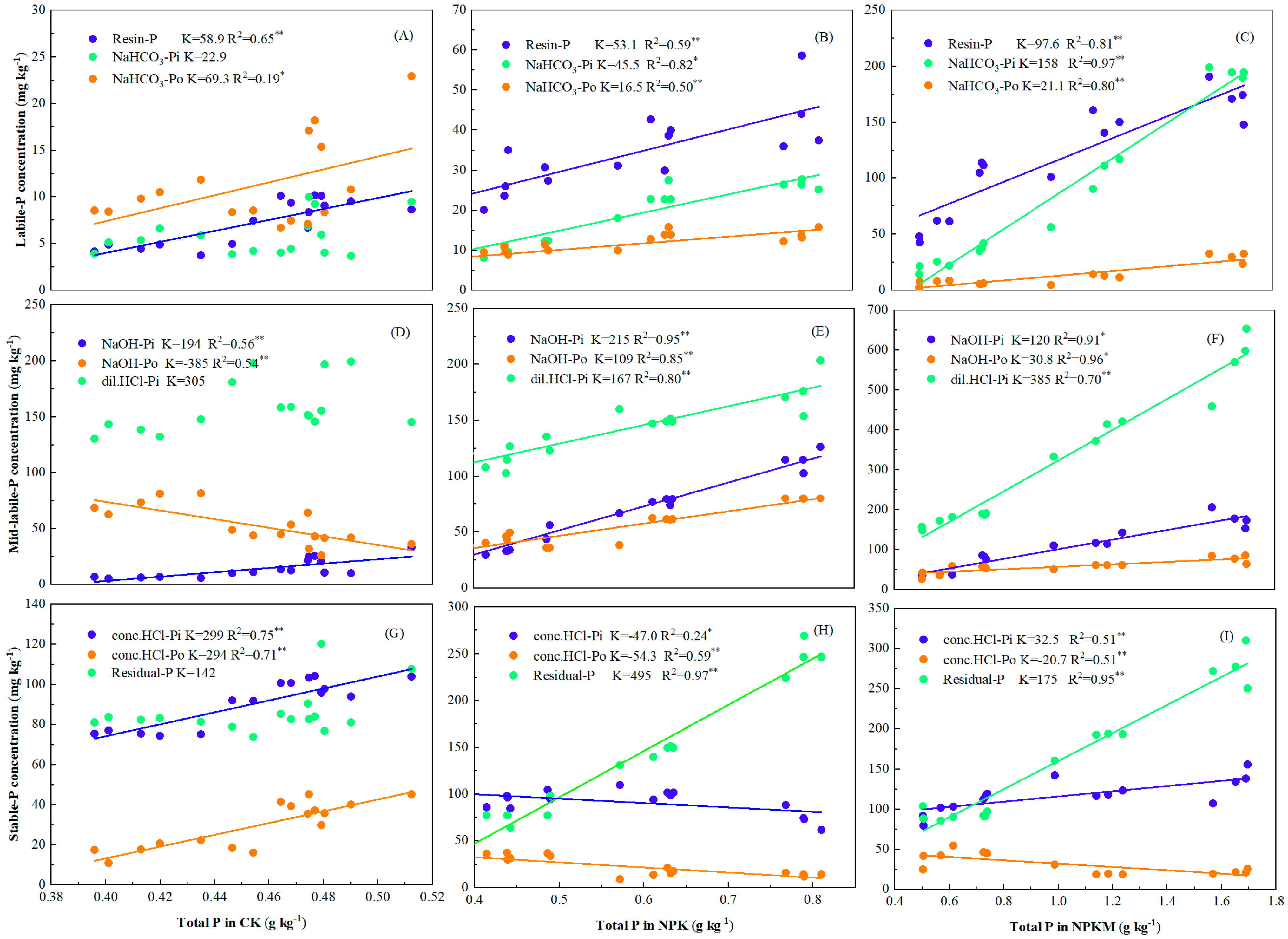
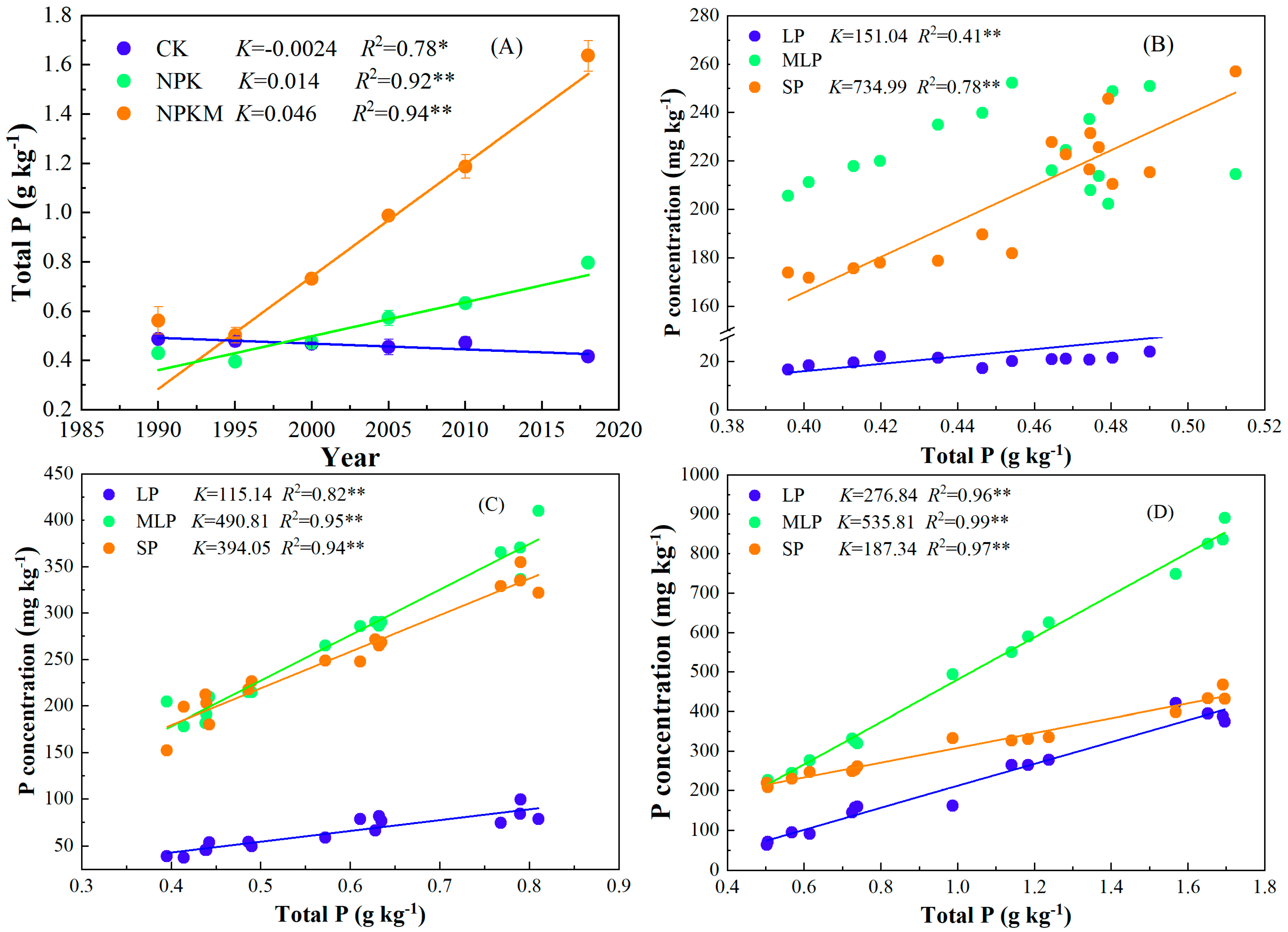
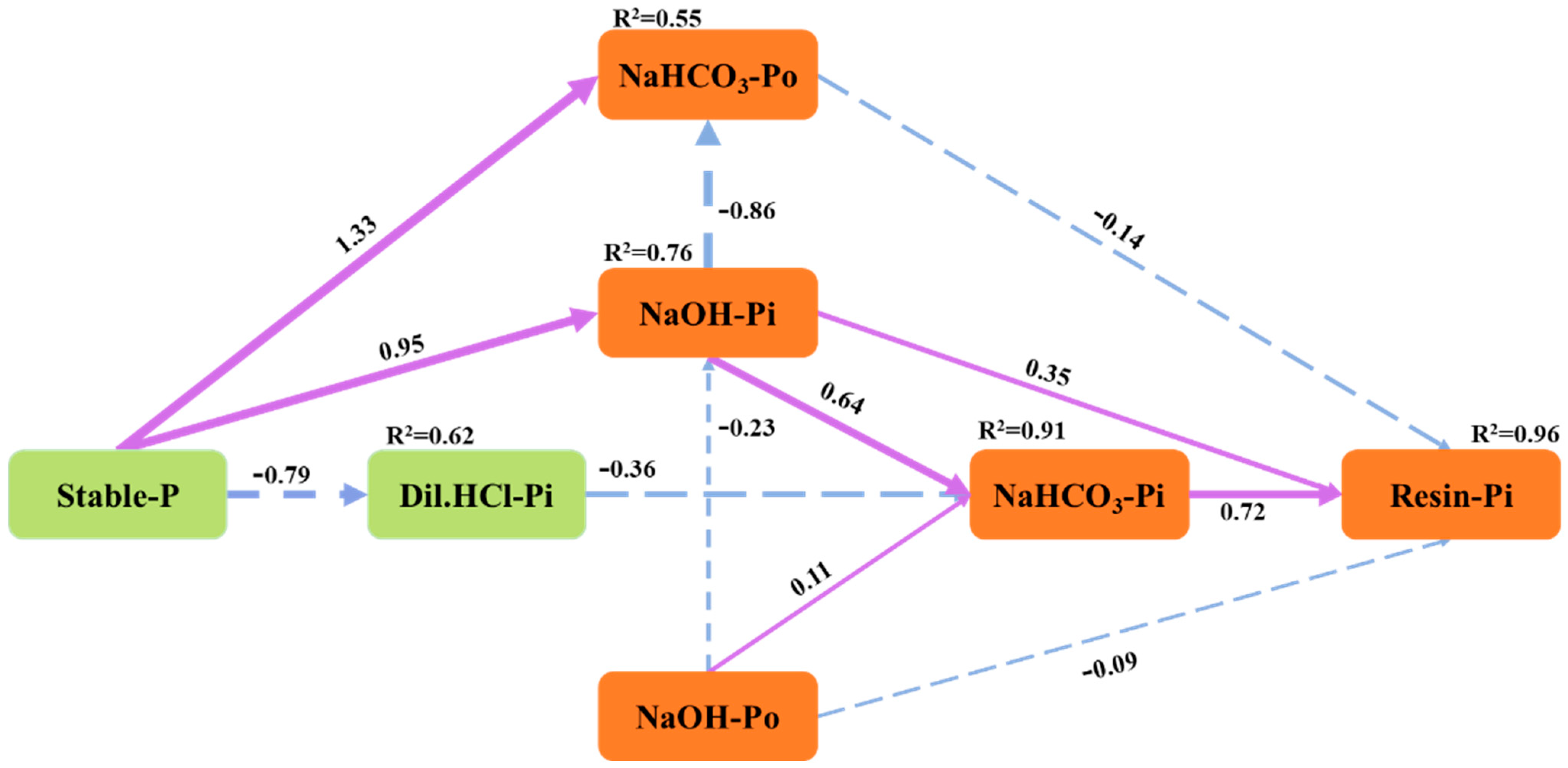
3.1. Soil Total P and Its Relationship with P Fraction Using Modified Hedley Sequential Method
3.2. P Fraction Using P Kedge-XANES
3.3. Soil P Fraction Using 31P-NMR
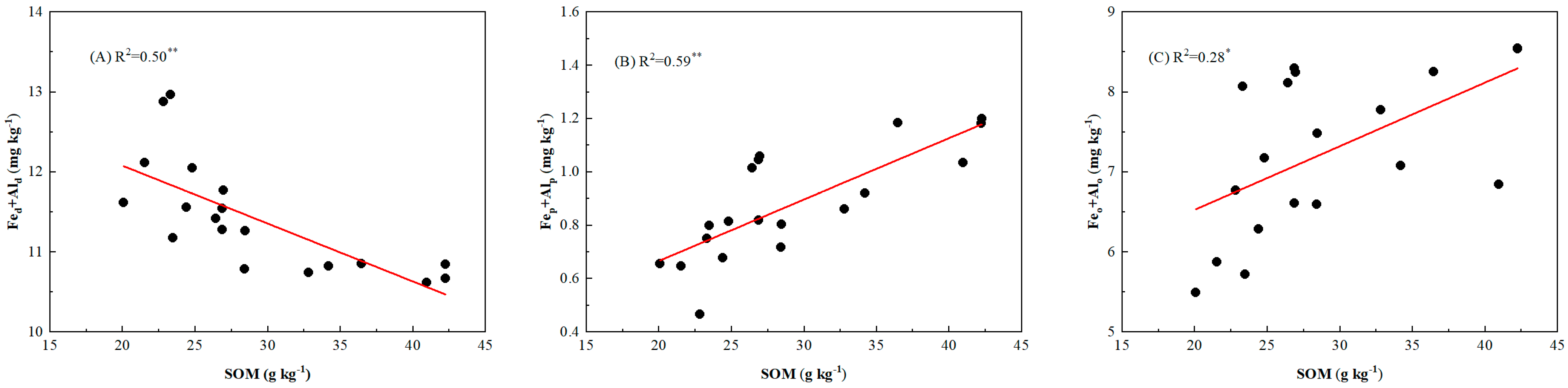
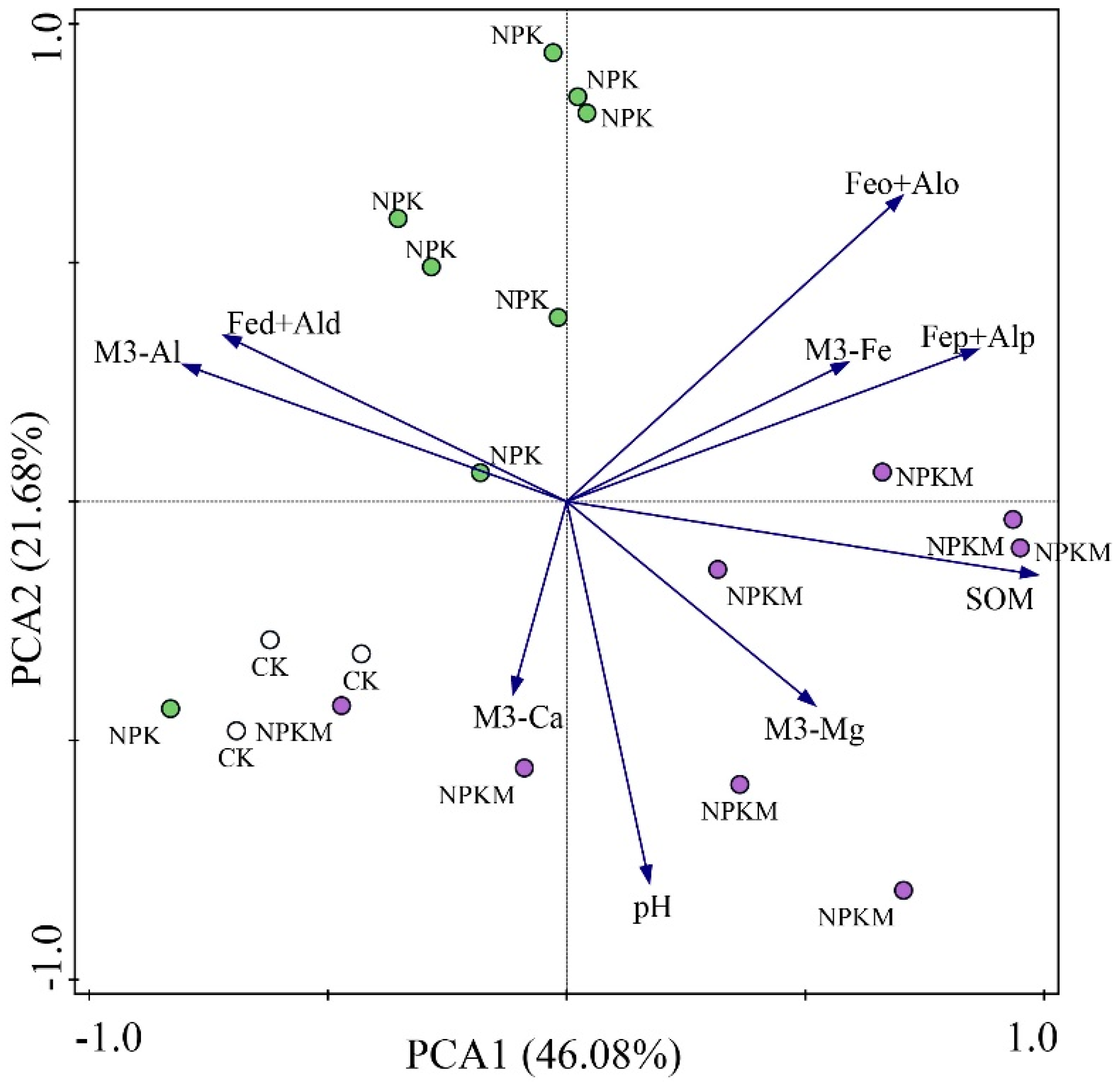
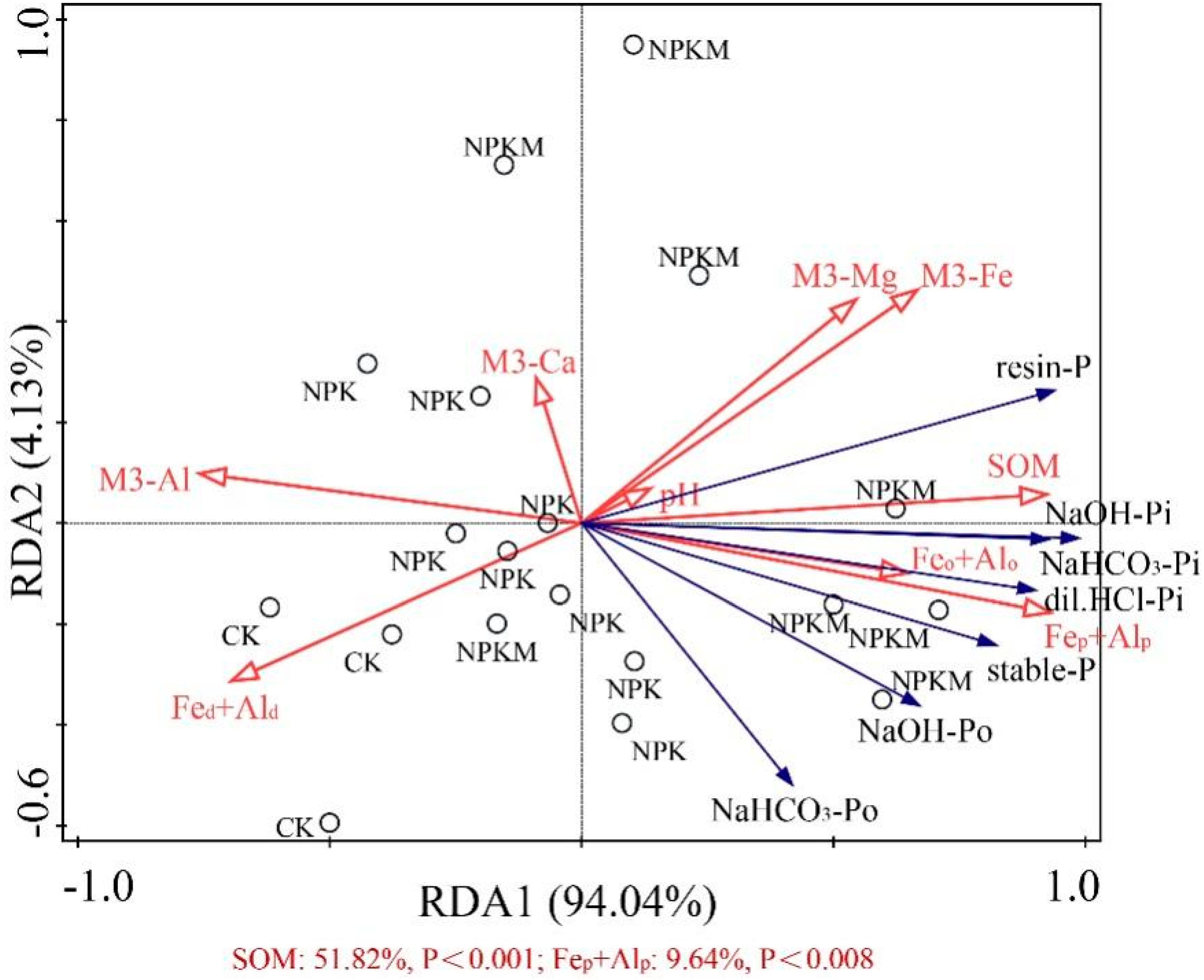
3.4. Soil Properties and Its Relationship with P Fraction
4. Discussion
4.1. Effect of Fertilization on the P Fractions with Multiple Techniques
4.2. Transformation of Soil P Fractions
4.3. Effects of Soil Property on Soil P Fractions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Treatment | Ortho | Pyro | Poly | Phos | scyllo-IHP | myo-IHP | neo-IHP | α-Glycb |
|---|---|---|---|---|---|---|---|---|---|
| 1990 | CK | 33.3 | 1.81 | 2.83 | 1.70 | 0.94 | 3.40 | 1.23 | 1.33 |
| 2000 | NPK | 47.2 | 0.92 | 0.01 | 3.73 | 0.76 | 4.66 | 1.03 | 3.31 |
| NPKM | 63.0 | 1.26 | 0.41 | 0.09 | 0.44 | 5.69 | 0.15 | 1.02 | |
| 2010 | NPK | 44.4 | 2.41 | 1.19 | 0.70 | 0.66 | 3.45 | 0.19 | 0.56 |
| NPKM | 65.4 | 1.54 | 0.77 | 0.55 | 0.43 | 1.83 | 0.93 | 0.20 | |
| 2018 | NPK | 60.1 | 2.73 | 0.08 | 1.05 | 0.49 | 2.46 | 1.37 | 0.61 |
| NPKM | 77.3 | 1.25 | 0.82 | 0.08 | 0.36 | 1.39 | 0.08 | 0.10 | |
| Pig manure | 44.5 | 0.41 | 0.41 | 7.19 | 14.8 | ||||
| Cow manure | 87.6 | 0.09 | 0.32 | 2.51 | 0.41 | ||||
| Year | Treatment | β-Glyc | Nucl | Pchol | G6P | Mono1 | Mono2 | Mono3 | OrthDi |
| 1990 | CK | 1.56 | 1.39 | 0.92 | 0.10 | 43.60 | 2.95 | 2.81 | |
| 2000 | NPK | 1.55 | 2.13 | 0.71 | 1.84 | 1.13 | 23.80 | 5.55 | 1.26 |
| NPKM | 1.20 | 0.91 | 0.35 | 0.25 | 0.78 | 19.88 | 3.53 | 1.06 | |
| 2010 | NPK | 1.75 | 0.74 | 0.98 | 0.45 | 1.88 | 33.54 | 5.04 | 2.08 |
| NPKM | 0.99 | 0.60 | 0.26 | 0.23 | 0.80 | 21.45 | 3.11 | 0.95 | |
| 2018 | NPK | 0.80 | 0.70 | 0.49 | 0.12 | 0.29 | 27.51 | 0.36 | 0.86 |
| NPKM | 0.49 | 0.44 | 0.21 | 0.22 | 0.73 | 15.26 | 0.72 | 0.51 | |
| Pig manure | 12.2 | 2.53 | 8.90 | 6.39 | 2.38 | ||||
| Cow manure | 1.89 | 0.30 | 3.58 | 1.63 | 0.19 | 1.53 |
| Resin-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | dil.HCl-Pi | Stable P | |
|---|---|---|---|---|---|---|---|
| SOM | 0.930 ** | 0.883 ** | 0.462 * | 0.825 ** | 0.507 * | 0.916 ** | 0.801 ** |
| pH | 0.353 | 0.357 | 0.214 | −0.003 | −0.190 | 0.404 | 0.117 |
| Fed + Ald | −0.703 ** | −0.559 * | −0.180 | −0.559 * | −0.410 | −0.564 * | −0.530 * |
| Fep + Alp | 0.790 ** | 0.788 ** | 0.537 * | 0.944 ** | 0.824 ** | 0.755 ** | 0.854 ** |
| Feo + Alo | 0.522 * | 0.542 * | 0.385 | 0.721 ** | 0.627 ** | 0.514 * | 0.641 ** |
| M3-Ca | −0.039 | −0.188 | −0.262 | −0.105 | −0.199 | −0.083 | −0.069 |
| M3-Mg | 0.645 ** | 0.563 * | 0.137 | 0.418 | −0.037 | 0.553 * | 0.249 |
| M3-Al | −0.772 ** | −0.748 ** | −0.458 * | −0.686 ** | −0.483 * | −0.826 ** | −0.754 ** |
| M3-Fe | 0.548 * | 0.341 | 0.063 | 0.616 ** | 0.429 | 0.284 | 0.465 * |
References
- Qin, X.; Guo, S.; Zhai, L.; Pan, J.; Khoshnevisan, B.; Wu, S.; Wang, H.; Yang, B.; Ji, J.; Liu, H. How long-term excessive manure application affects soil phosphorous species and risk of phosphorous loss in fluvo-aquic soil. Environ. Pollut. 2020, 266 Pt 2, 115304. [Google Scholar] [CrossRef] [PubMed]
- Medinski, T.; Freese, D.; Reitz, T. Changes in soil phosphorus balance and phosphorus-use efficiency under long-term fertilization conducted on agriculturally used Chernozem in Germany. Can. J. Soil Sci. 2018, 98, 650–662. [Google Scholar] [CrossRef]
- Zhang, F. Scientific understanding of the role of chemical fertilizer. China Agric. Technol. Ext. 2017, 33, 16–19. (In Chinese) [Google Scholar] [CrossRef]
- MacDonald, J.; Chantigny, M.; Royer, I.; Angers, D.; Rochette, P.; Gasser, M. Soil soluble carbon dynamics of manured and unmanured grasslands following chemical kill and ploughing. Geoderma 2011, 164, 64–72. [Google Scholar] [CrossRef]
- Cade-Menun, B. Characterizing phosphorus in animal waste with solution 31P NMR spectroscopy. In Environmental Chemistry of Animal Manure; Nova Science Publishers: New York, NY, USA, 2011; pp. 275–299. [Google Scholar]
- Wang, Q.; Zhan, X.; Zhang, S.; Peng, C.; Gao, H.; Zhang, X.; Zhu, P.; Colinet, G. Increment of soil phosphorus pool and activation coefficient through long-term combination of NPK fertilizers with manures in black soil. J. Plant Nutr. Fertil. 2018, 24, 1679–1688. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Z.; Zhang, W.; Chen, Y.; Zhu, P.; Peng, C.; Wang, L.; Zhang, S.; Colinet, G. Effect of long-term fertilization on phosphorus fractions in different soil layers and their quantitative relationships with soil properties. J. Integr. Agr. 2022, 21, 2–15. [Google Scholar] [CrossRef]
- Liu, J.; Han, C.; Zhao, Y.; Yang, J.; Cade-Menun, B.; Hu, Y.; Li, J.; Liu, H.; Sui, P.; Chen, Y.; et al. The chemical nature of soil phosphorus in response to long-term fertilization practices: Implications for sustainable phosphorus management. J. Clean. Prod. 2020, 272, 123093. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, S.; Zhu, P.; Huang, S.; Wang, B.; Zhao, L.; Xu, M. Characterizing differences in the phosphorus activation coefficient of three typical cropland soils and the influencing factors under long-term fertilization. PLoS ONE 2017, 12, e0176437. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Z.; Zhong, X.; Xu, C.; Lin, Y.; Fan, Y.; Wang, M.; Chen, G.; Yang, Y. Decreases in soil P availability are associated with soil organic P declines following forest conversion in subtropical China. Catena 2021, 205, 105459. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 293–306. [Google Scholar] [CrossRef]
- Hedley, M.; Stewart, J.; Chauhan, B. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Liu, J.; Sui, P.; Cade-Menun, B.; Hu, Y.; Yang, J.; Huang, S.; Ma, Y. Molecular-level understanding of phosphorus transformation with long-term phosphorus addition and depletion in an alkaline soil. Geoderma 2019, 353, 116–124. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Cade-Menun, B.; Liang, X.; Hu, Y.; Liu, C.; Zhao, Y.; Li, L.; Shi, J. Complementary Phosphorus Speciation in Agricultural Soils by Sequential Fractionation, Solution 31P Nuclear Magnetic Resonance, and Phosphorus K-edge X-ray Absorption Near-Edge Structure Spectroscopy. J. Environ. Qual. 2013, 42, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.; Yang, J.; Abdi, D.; Cade-Menun, B. Investigation of soil legacy phosphorus transformation in long-term agricultural fields using sequential fractionation, P K-edge XANES and solution P NMR spectroscopy. Environ. Sci. Technol. 2015, 49, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Rivard, C.; Tanabe, I.; Adam, C. Microspectroscopy—Promising techniques to characterize phosphorus in soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2088–2102. [Google Scholar] [CrossRef]
- Toor, G.; Hunger, S.; Peak, J.; Sims, J.; Sparks, D. Advances in the Characterization of phosphorus in organic wastes: Environmental and agronomic applications. Adv. Agron. 2006, 89, 1–72. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Hu, Y.; Li, J.; Zhang, X.; Ma, Y. Molecular speciation of phosphorus in an organically bound phosphorus fertilizer with high phytoavailability characterized by multiple spectroscopy. Spectrosc. Spect. Anal. 2018, 38, 958–962. [Google Scholar] [CrossRef]
- Weyers, E.; Strawn, D.; Peak, D.; Moore, A.; Baker, L.; Cade-Menum, B. Phosphorus speciation in dairy manure-amended calcareous soils. Soil Sci. Soc. Am. J. 2016, 80, 1531–1542. [Google Scholar] [CrossRef]
- Abdala, D.; da Silva, I.; Vergütz, L.; Sparks, D. Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils. Chemosphere 2015, 119, 504–514. [Google Scholar] [CrossRef]
- Kizewski, F.; Liu, Y.; Morris, A.; Hesterberg, D. Spectroscopic approaches for phosphorus speciation in soils and other environmental systems. J. Environ. Qual. 2011, 40, 751–766. [Google Scholar] [CrossRef]
- Beauchemin, S.; Hesterberg, D.; Chou, J.; Beauchemin, M.; Simard, R.; Sayers, D. Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J. Environ. Qual. 2003, 32, 1809–1819. [Google Scholar] [CrossRef]
- Liang, X.; Jin, Y.; He, M.; Liu, Y.; Hua, G.; Wang, S.; Tian, G. Composition of phosphorus species and phosphatase activities in a paddy soil treated with manure at varying rates. Agric. Ecosyst. Environ. 2017, 237, 173–180. [Google Scholar] [CrossRef]
- Hou, E.; Wen, D.; Kuang, Y.; Cong, J.; Chen, C.; He, X.; Heenan, M.; Lu, H.; Zhang, Y. Soil pH predominantly controls the forms of organic phosphorus in topsoils under natural broadleaved forests along a 2500 km latitudinal gradient. Geoderma 2018, 315, 65–74. [Google Scholar] [CrossRef]
- Hou, E.; Chen, C.; Kuang, Y.; Zhang, Y.; Heenan, M.; Wen, D. A structural equation model analysis of phosphorus transformations in global unfertilized and uncultivated soils. Glob. Biogeochem. Cycles 2016, 30, 1300–1309. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.; Cole, C. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Dari, B.; Sihi, D.; Chen, Q. Phosphorus transformation response to soil properties changes induced by manure application in a calcareous soil. Geoderma 2018, 322, 163–171. [Google Scholar] [CrossRef]
- MacDonald, G.; Bennett, E.; Taranu, Z. The influence of time, soil characteristics, and land-use history on soil phosphorus legacies: A global meta-analysis. Glob. Chang. Biol. 2012, 18, 1904–1917. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Phosphorus release kinetics and solubility capacity of phosphorus fractionation induced by organic acids from a black soil in northeast China. Can. J. Soil Sci. 2019, 99, 92–99. [Google Scholar] [CrossRef]
- van der Bom, F.; McLaren, T.; Doolette, A.; Magid, J.; Frossard, E.; Oberson, A.; Jensen, L. Influence of long-term phosphorus fertilisation history on the availability and chemical nature of soil phosphorus. Geoderma 2019, 355, 113909. [Google Scholar] [CrossRef]
- Poblete-Grant, P.; Suazo-Hernández, J.; Condron, L.; Rumpel, C.; Demanet, R.; Malone, S.; de La Luz Mora, M. Soil available P, soil organic carbon and aggregation as affected by long-term poultry manure application to Andisols under pastures in Southern Chile. Geoderma Reg. 2020, 21, e00271. [Google Scholar] [CrossRef]
- Redel, Y.; Cartes, P.; Demanet, R.; Velásquez, G.; Poblete-Grant, P.; Bol, R.; Mora, M. Assessment of phosphorus status influenced by Al and Fe compounds in volcanic grassland soils. J. Soil Sci. Plant Nutr. 2016, 16, 490–506. [Google Scholar] [CrossRef]
- Guppy, C.; Menzies, N.; Moody, P.; Blamey, F. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Wang, Q.; Zhan, X.; Zhang, S.; Peng, C.; Gao, H.; Zhang, X.; Zhu, P.; Colinet, G. Phosphorus adsorption and desorption characteristics and its response to soil properties of black soil under long-term different fertilization. Sci. Agric. Sin. 2019, 52, 3866–3877. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.; Xiong, J.; Hong, C.; Hu, H.; Violante, A. Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Chang, C.; Sommerfeldt, T.; Entz, T. Soil chemistry after eleven annual applications of cattle feedlot manure. J. Environ. Qual. 1991, 20, 475–480. [Google Scholar] [CrossRef]
- Nobile, M.; Bravin, M.; Becquer, T.; Paillat, J. Phosphorus sorption and availability in an andosol after a decade of organic or mineral fertilizer applications: Importance of pH and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239, 124709. [Google Scholar] [CrossRef] [PubMed]
- Kar, G.; Schoenau, J.; Hilger, D.; Peak, D. Direct chemical speciation of soil phosphorus in a Saskatchewan Chernozem after long- and short-term manure amendments. Can. J. Soil Sci. 2017, 97, 626–636. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, US Government Printing Office: Washington, DC, USA, 2014; pp. 211–212.
- Liu, J.; Yang, J.; Cade-Menun, B.; Hu, Y.; Li, J.; Peng, C.; Ma, Y. Molecular speciation and transformation of soil legacy phosphorus with and without long-term phosphorus fertilization: Insights from bulk and microprobe spectroscopy. Sci. Rep. 2017, 7, 15354. [Google Scholar] [CrossRef]
- Skjemstad, J.; Baldock, J. Total and organic carbon. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC: Boca Raton, FL, USA, 2007; pp. 225–237. [Google Scholar]
- Lu, R. Soil and Agro-Chemistry Analytical Method; China Agricultural Science and Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Mehra, O.; Jackson, M. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clay. Clay Miner. 2013, 7, 317–327. [Google Scholar] [CrossRef]
- Mckeague, J.; Brydon, J.; Miles, N. Differentiation of forms of extractable Iron and Aluminum in soils. Soil. Sci. Soc. Am. J. 1971, 35, 33–38. [Google Scholar] [CrossRef]
- Loveland, P.; Digby, P. The extraction of Fe and Al by 0.1 M pyrophosphate solutions: A comparison of some techniques. Eur. J. Soil. Sci. 1984, 35, 243–250. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil. Sci. Plan. 2008, 15, 1409–1416. [Google Scholar] [CrossRef]
- Yang, X.; Post, W. Phosphorus transformations as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef]
- Cross, A.; Schlesinger, W. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Bowman, R.; Cole, C. Transformations of organic phosphorus substances in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Condron, L.; Newman, S. Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediments 2011, 11, 830–840. [Google Scholar] [CrossRef]
- Hedley, M.; Nye, P.; White, R. Plant-Induced Changes in the Rhizosphere of Rape (Brassica napus Var. Emerald) Seedlings. N. Phytol. 1983, 95, 69–82. [Google Scholar] [CrossRef]
- Schmieder, F.; Bergström, L.; Riddle, M.; Gustafsson, J.; Klysubun, W.; Zehetner, F.; Condrond, L.; Kirchmanna, H. Phosphorus speciation in a long-term manure-amended soil profile–evidence from wet chemical extraction,31P-NMR and P K-edge XANES spectroscopy. Geoderma 2018, 322, 19–27. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Zhang, H.; Tian, G. The influence of no-till coupled with straw return on soil phosphorus speciation in a two-year rice-fallow practice. Soil Tillage Res. 2019, 195, 104389. [Google Scholar] [CrossRef]
- Zuo, M.; Renman, G.; Gustafsson, J.; Renman, A. Phosphorus removal performance and speciation in virgin and modified argon oxygen decarburisation slag designed for wastewater treatment. Water Res. 2015, 87, 271–281. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Cade-Menun, B. Improved peak identification in 31P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 2015, 257–258, 102–114. [Google Scholar] [CrossRef]
- Schneider, K.; Cade-Menun, B.; Lynch, D.; Voroney, R. Soil Phosphorus Forms from Organic and Conventional Forage Fields. Soil Biol. Biochem. 2016, 80, 328–340. [Google Scholar] [CrossRef]
- Young, E.; Ross, D.; Cade-Menun, B.; Liu, C. Phosphorus Speciation in Riparian Soils: A Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy and Enzyme Hydrolysis Study. Soil Sci. Soc. Am. J. 2013, 77, 1636–1647. [Google Scholar] [CrossRef]
- Grace, J.; Anderson, T.; Olff, H.; Scheiner, S. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010, 80, 67–87. [Google Scholar] [CrossRef]
- Rosseel, Y. Iavaan: An R package for structural equation modeling and more Version 0.5-12 (BETA). J. Stat. Softw. 2010, 48, 1–36. [Google Scholar]
- Koch, M.; Kruse, J.; Eichler-Lobermann, B.; Zimmer, D.; Willbold, S.; Leinweber, P.; Siebers, N. Phosphorus stocks and speciation in soil profiles of a longterm fertilizer experiment: Evidence from sequential fractionation, P K-edge XANES, and 31P NMR spectroscopy. Geoderma 2018, 316, 115–126. [Google Scholar] [CrossRef]
- Sato, S.; Solomon, D.; Hyland, C.; Ketterings, Q.; Lehmann, J. Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ. Sci. Technol. 2005, 39, 7485–7491. [Google Scholar] [CrossRef]
- Güngör, K.; Jürgensen, A.; Karthikeyan, K. Determination of phosphorus speciation in dairy manure using XRD and XANES spectroscopy. J. Environ. Qual. 2007, 36, 1856–1863. [Google Scholar] [CrossRef]
- Sharpley, A.; Moyer, B. Phosphorus forms in manure and compost and their release during simulated rainfall. J. Environ. Qual. 2000, 29, 1462–1469. [Google Scholar] [CrossRef]
- Ge, X.; Wang, L.; Zhang, W.; Putnis, C. Molecular understanding of humic acid-limited phosphate precipitation and transformation. Environ. Sci. Technol. 2020, 54, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Graetz, D.; Dooley, D. Phosphorus release characteristics of manure and manure-impacted soils. J. Food Agric. Environ. 2003, 1, 217–223. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yan, X.; Zhao, M.; Zheng, S.; Wang, X. Exudation of organic acids in common bean as related to mobilization of aluminum- and iron-bound phosphates. Environ. Exp. Bot. 2002, 48, 1–9. [Google Scholar] [CrossRef]
- Prietzel, J.; Dumig, A.; Wu, Y.; Zhou, J.; Klysubun, W. Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim. Cosmochim. Acta 2013, 108, 154–171. [Google Scholar] [CrossRef]
- Fan, Y.; Zhong, X.; Lin, F.; Liu, C.; Yang, L.; Wang, M.; Chen, G.; Chen, Y.; Yang, Y. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots. Geoderma 2019, 337, 246–255. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Yan, Z.; Peng, Y.; Chen, Q. Differences in main processes to transform phosphorus influenced by ammonium nitrogen in flooded intensive agricultural and steppe soils. Chemosphere 2019, 226, 192–200. [Google Scholar] [CrossRef]
- Fink, J.; Inda, A.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. Agrotec. 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Bünemann, E.; Smernik, R.; Marschner, P.; McNeill, A. Microbial synthesis of organic and condensed forms of phosphorus in acid and calcareous soils. Soil Biol. Biochem. 2008, 40, 932–946. [Google Scholar] [CrossRef]
- Spohn, M. Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Glob. Chang. Biol. 2020, 26, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, H.; Luo, M.; He, G. The morphological change of Fe/Al-oxide minerals in red soils in the process of acidification and its environmental significance. Acta Petrol. Mineral. 2007, 26, 515–521. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Zhang, N.; Qin, Z.; Jin, Y.; Zhu, P.; Peng, C.; Colinet, G.; Zhang, S. Phosphorus adsorption and desorption characteristics as affected by long-term phosphorus application in black soil. J. Plant Nutr. Fertil. 2022, 28, 1–13. (In Chinese) [Google Scholar] [CrossRef]
- Kang Kang, J.; Hesterberg, D.; Osmond, D. Soil organic matter effects on phosphorus sorption: A path analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Ma, J.; Ma, Y.; Wei, R.; Chen, Y.; Weng, L.; Ouyang, X.; Li, Y. Phosphorus transport in different soil types and the contribution of control factors to phosphorus retardation. Chemosphere 2021, 276, 130012. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Phosphate adsorption on synthetic goethite and akaganeite. J. Colloid Interface Sci. 2006, 298, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Luengo, C.; Brigante, M.; Antelo, J.; Avena, M. Kinetics of phosphate adsorption on goethite: Comparing batch adsorption and A TR-IR measurements. J. Colloid Interface Sci. 2006, 300, 511–518. [Google Scholar] [CrossRef]

| Soil Depth | pH | Bulk Density | Soil Organic Matter | Total N | Total P | Total K | Olsen-P | Available K |
|---|---|---|---|---|---|---|---|---|
| cm | g cm−3 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | |
| 0–20 | 7.6 | 1.19 | 20 | 1.34 | 0.61 | 16.36 | 11.8 | 190 |
| Linear Combination Fitting | Goodness of Fit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HAP | Al-P | IHP | MCP | TCP | Fe-P | Ca-P | |||
| Proportion (%) | R Factor | ||||||||
| 1990 | CK | 42.1 ± 0.2 a | 10.3 ± 1.4 c | 42.4 ± 1.3 | 42.1 | 0.0125 | |||
| 2000 | NPK | 21.5 ± 2.2 b | 21.1 ± 0.7 bc | 19.9 ± 0.9 a | 36.6 ± 0.2 b | 58.1 | 0.0143 | ||
| NPKM | 17.9 ± 1.1 bc | 23.6 ± 2.0 bc | 14.8 ± 1.4 b | 27.7 ± 1.4 d | 25.8 ± 1.0 b | 71.4 | 0.0055 | ||
| 2010 | NPK | 18.7 ± 0.5 bc | 28.9 ± 1.1 a | 20.8 ± 1.8 a | 35.9 ± 0.3 b | 54.6 | 0.007 | ||
| NPKM | 15.9 ± 0.1 c | 19.0 ± 0.4 c | 10.8 ± 0.6 c | 33.4 ± 0.1 c | 25.7 ± 0.2 b | 75.0 | 0.0061 | ||
| 2018 | NPK | 5.4 ± 0.8 d | 24.7 ± 0.5 ab | 18.8 ± 0.2 a | 39.4 ± 0.1 a | 14.0 ± 1.1 c | 58.8 | 0.0073 | |
| NPKM | 24.2 ± 0.7 ab | 7.2 ± 0.6 c | 33.8 ± 0.6 c | 43.0 ± 0.6 a | 76.8 | 0.0038 | |||
| Year | Treatment | NaOH-EDTA Extraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | Pi | Po | Total IHP | Mono | Di | D:M | cMono | cDi | cD:cM | ||
| 1990 | CK | 40.1 | 37.9 | 62.1 | 5.57 | 57.6 | 2.81 | 0.05 | 53.3 | 7.09 | 0.13 |
| 2000 | NPK | 33.7 | 48.1 | 51.9 | 6.44 | 46.9 | 1.26 | 0.03 | 39.9 | 8.25 | 0.21 |
| NPKM | 27.6 | 64.7 | 35.3 | 6.28 | 34.2 | 1.06 | 0.03 | 31.0 | 4.17 | 0.13 | |
| 2010 | NPK | 46.9 | 48.0 | 52.0 | 4.29 | 49.2 | 2.08 | 0.04 | 46.2 | 5.13 | 0.11 |
| NPKM | 26.7 | 67.7 | 32.3 | 3.19 | 30.8 | 0.95 | 0.03 | 29.0 | 2.73 | 0.09 | |
| 2018 | NPK | 62.7 | 62.9 | 37.1 | 4.31 | 35.2 | 0.86 | 0.02 | 33.1 | 2.96 | 0.09 |
| NPKM | 54.5 | 79.4 | 20.6 | 1.83 | 20.0 | 0.51 | 0.03 | 19.0 | 1.55 | 0.08 | |
| pH | SOM | M3-Ca | M3-Mg | M3-Al | M3-Fe | Fed + Ald | Fep + Alp | Feo + Alo | ||
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | g kg−1 | g kg−1 | g·kg−1 | g kg−1 | g kg−1 | |||
| 1990 | CK | 7.40 ± 0.3 a | 21.5 ± 0.2 d | 4.55 ± 0.04 b | 0.35 ± 0.00 e | 1.20 ± 0.01 b | 0.22 ± 0.01 b | 12.12 ± 0.04 a | 0.65 ± 0.01 e | 5.88 ± 0.04 g |
| 2000 | NPK | 6.03 ± 0.1 c | 28.5 ± 1.8 c | 3.82 ± 0.05 d | 0.41 ± 0.01 d | 1.30 ± 0.02 a | 0.25 ± 0.00 a | 11.26 ± 0.09 c | 0.80 ± 0.03 d | 7.49 ± 0.02 d |
| NPKM | 7.10 ± 0.1 a | 32.8 ± 2 b | 4.62 ± 0.04 a | 0.45 ± 0.01 c | 1.15 ± 0.02 b | 0.26 ± 0.01 a | 11.28 ± 0.08 c | 0.82 ± 0.02 cd | 6.61 ± 0.01 f | |
| 2010 | NPK | 6.73 ± 0.1 b | 26.9 ± 0.4 c | 3.83 ± 0.04 d | 0.41 ± 0.01 d | 1.35 ± 0.02 a | 0.25 ± 0.01 a | 11.58 ± 0.10 b | 1.04 ± 0.02 b | 8.22 ± 0.06 b |
| NPKM | 7.21 ± 0.02 a | 41.0 ± 2 a | 4.57 ± 0.18 b | 0.54 ± 0.00 a | 1.04 ± 0.02 c | 0.24 ± 0.01 a | 10.74 ± 0.05 d | 0.86 ± 0.02 c | 7.78 ± 0.03 c | |
| 2018 | NPK | 6.11 ± 0.2 c | 26.7 ± 0.1 c | 4.17 ± 0.01 c | 0.28 ± 0.01 f | 1.18 ± 0.03 b | 0.25 ± 0.00 a | 10.62 ± 0.09 d | 1.04 ± 0.02 b | 6.84 ± 0.01 e |
| NPKM | 7.36 ± 0.04 a | 40.3 ± 1.9 a | 3.89 ± 0.01 d | 0.47 ± 0.00 b | 1.04 ± 0.03 c | 0.24 ± 0.01 a | 10.78 ± 0.11 d | 1.19 ± 0.01 a | 8.45 ± 0.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, N.; Chen, Y.; Qin, Z.; Jin, Y.; Zhu, P.; Peng, C.; Colinet, G.; Zhang, S.; Liu, J. The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes. Agronomy 2022, 12, 2364. https://doi.org/10.3390/agronomy12102364
Wang Q, Zhang N, Chen Y, Qin Z, Jin Y, Zhu P, Peng C, Colinet G, Zhang S, Liu J. The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes. Agronomy. 2022; 12(10):2364. https://doi.org/10.3390/agronomy12102364
Chicago/Turabian StyleWang, Qiong, Naiyu Zhang, Yanhua Chen, Zhenhan Qin, Yuwen Jin, Ping Zhu, Chang Peng, Gilles Colinet, Shuxiang Zhang, and Jin Liu. 2022. "The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes" Agronomy 12, no. 10: 2364. https://doi.org/10.3390/agronomy12102364
APA StyleWang, Q., Zhang, N., Chen, Y., Qin, Z., Jin, Y., Zhu, P., Peng, C., Colinet, G., Zhang, S., & Liu, J. (2022). The Phosphorus Availability in Mollisol Is Determined by Inorganic Phosphorus Fraction under Long-Term Different Phosphorus Fertilization Regimes. Agronomy, 12(10), 2364. https://doi.org/10.3390/agronomy12102364






