Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Development
2.2. Treatments
2.3. Agronomic Variables and Plant Material Sampling
2.4. Arsenic, Silicon and Macro-Micronutrient
2.5. Biochemical Analysis
2.5.1. Chlorophyll Content
2.5.2. Antioxidant Enzymes
2.5.3. Non-Enzymatic Compounds
2.5.4. H2O2 Concentration and ABTS Radical
2.6. Fruit Quality and Antioxidants
2.7. Statistical Analysis
3. Results
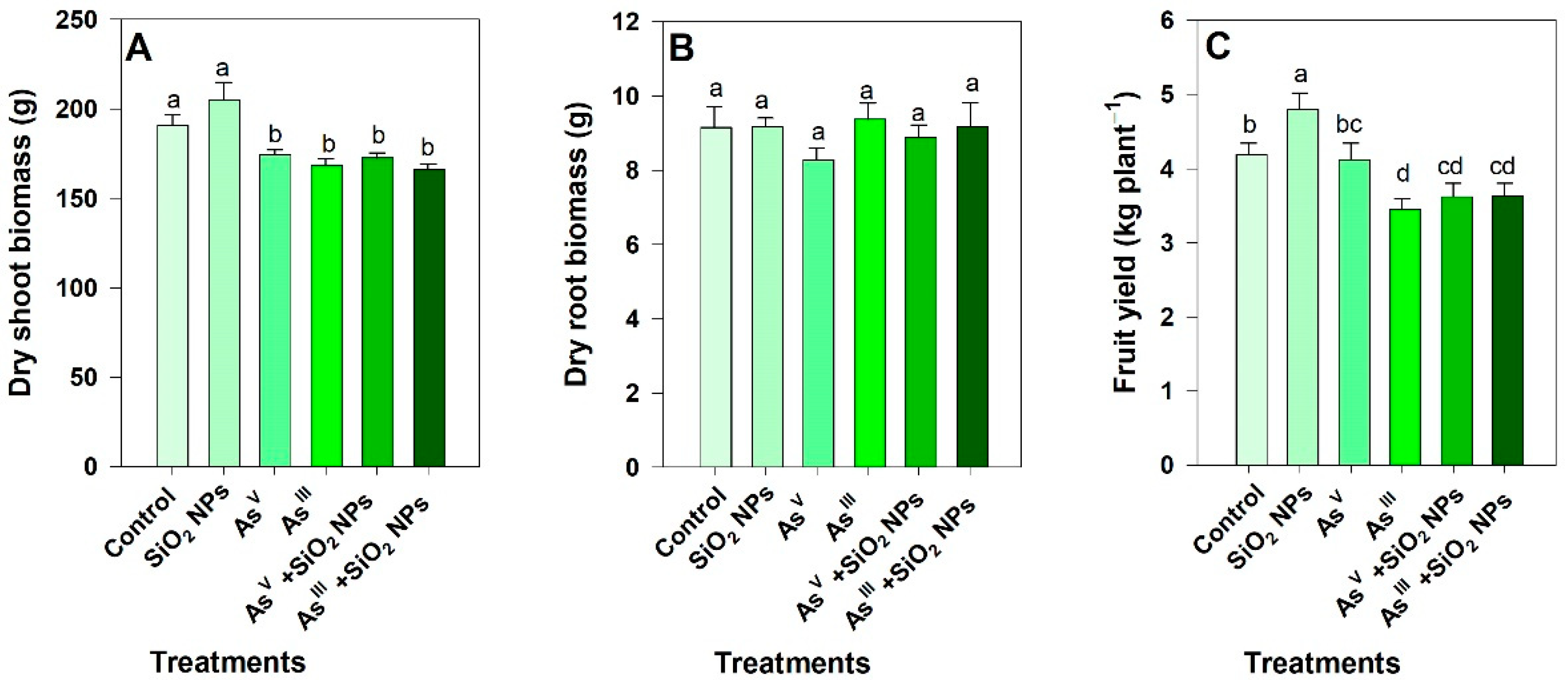
3.1. Effect of SiO2 NPs and Arsenic on Tomato Plants
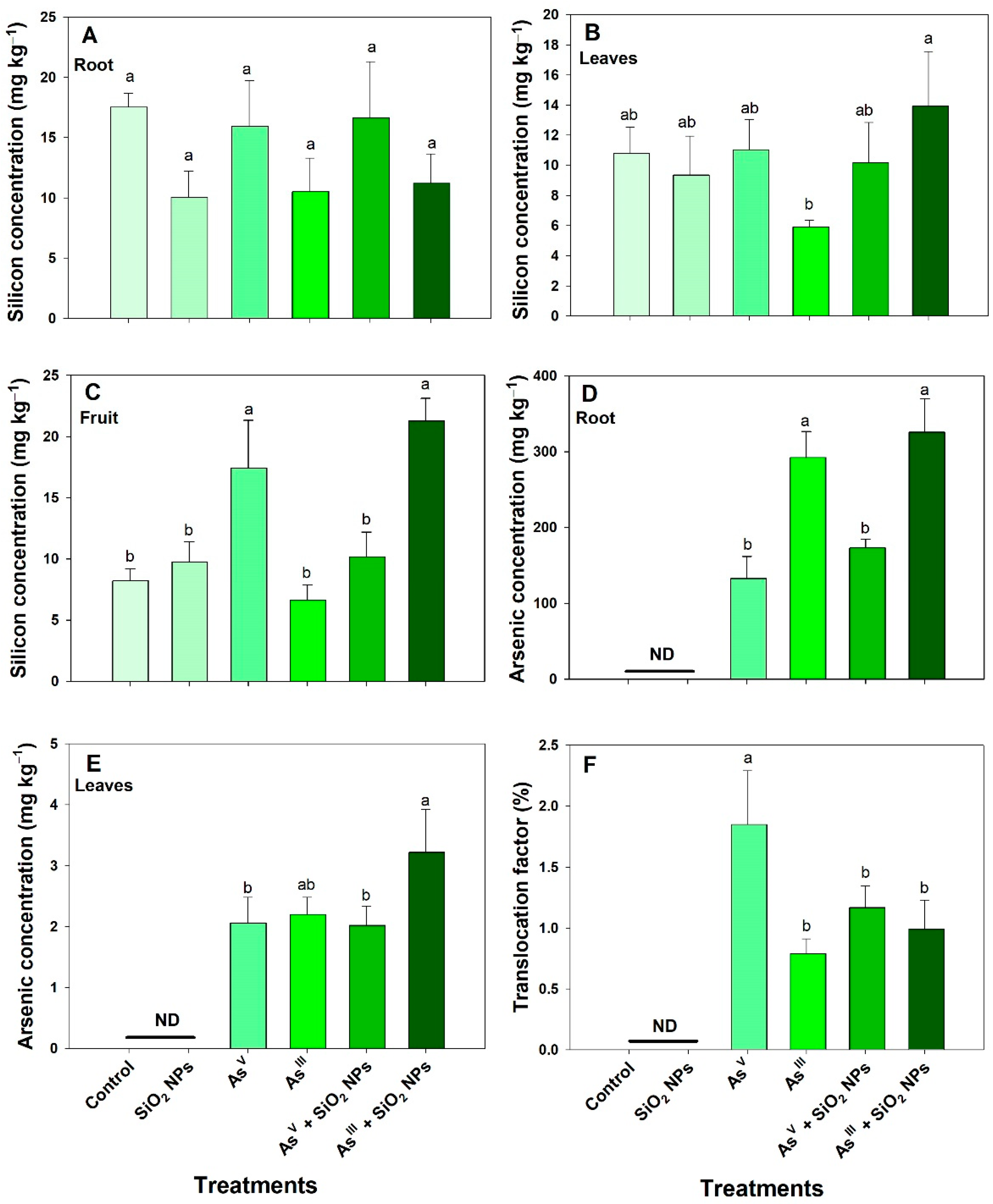
3.2. Silicon and Arsenic Concentration, and Translocation Factor
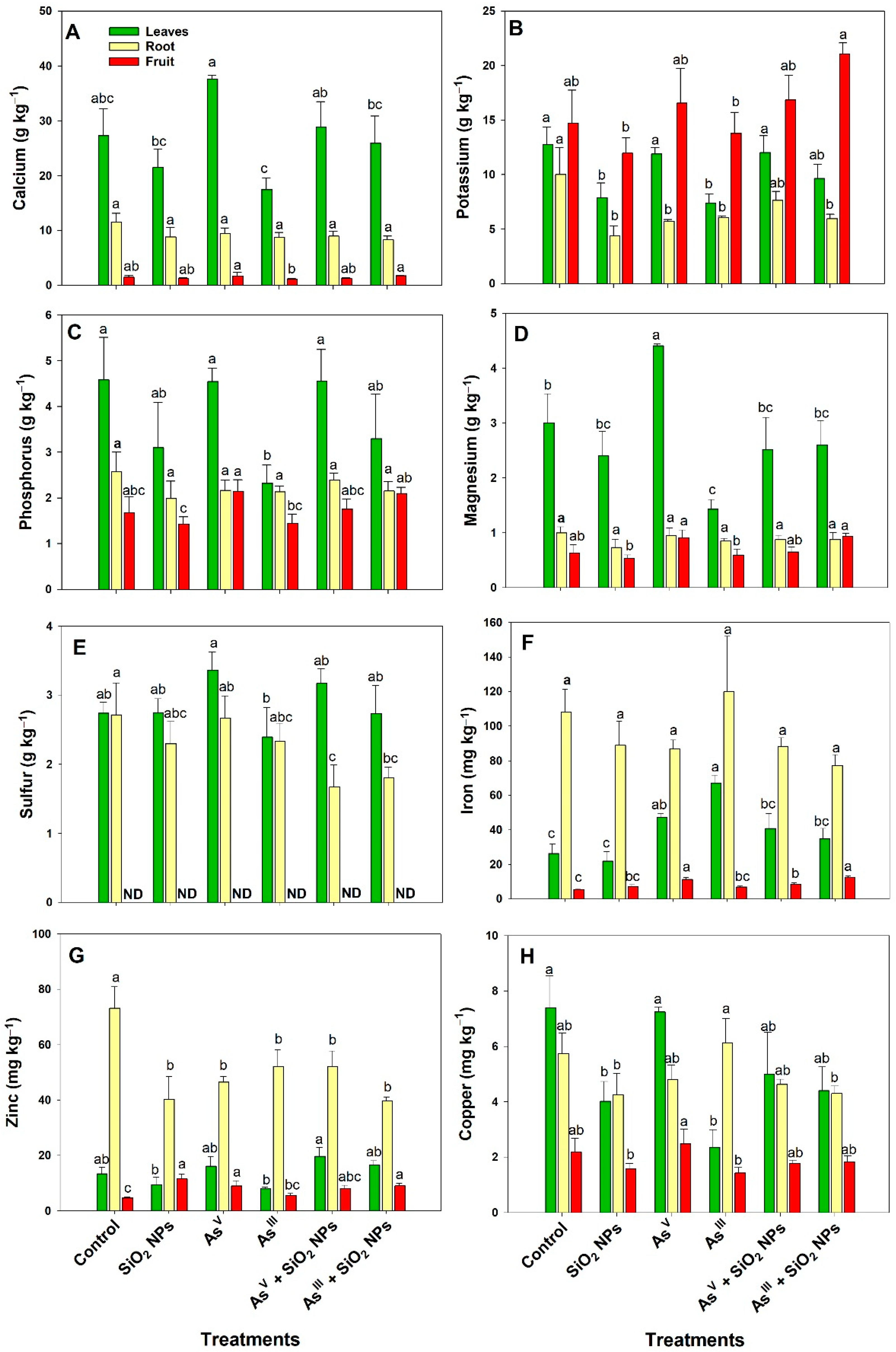
3.3. Concentration of Macro and Micronutrients in Tissues
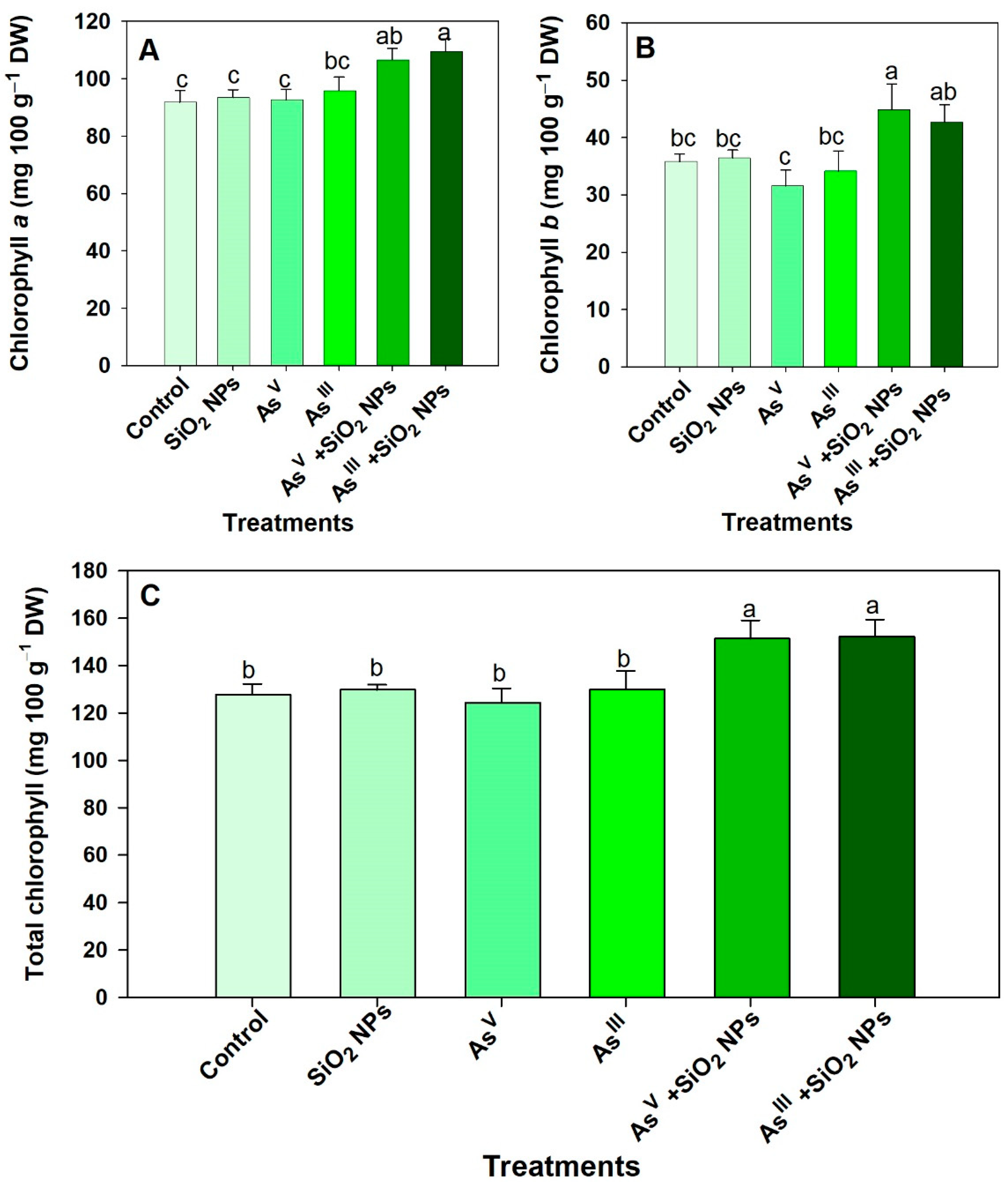
3.4. Impact on Chlorophyll Content
3.5. Antioxidant Enzymes
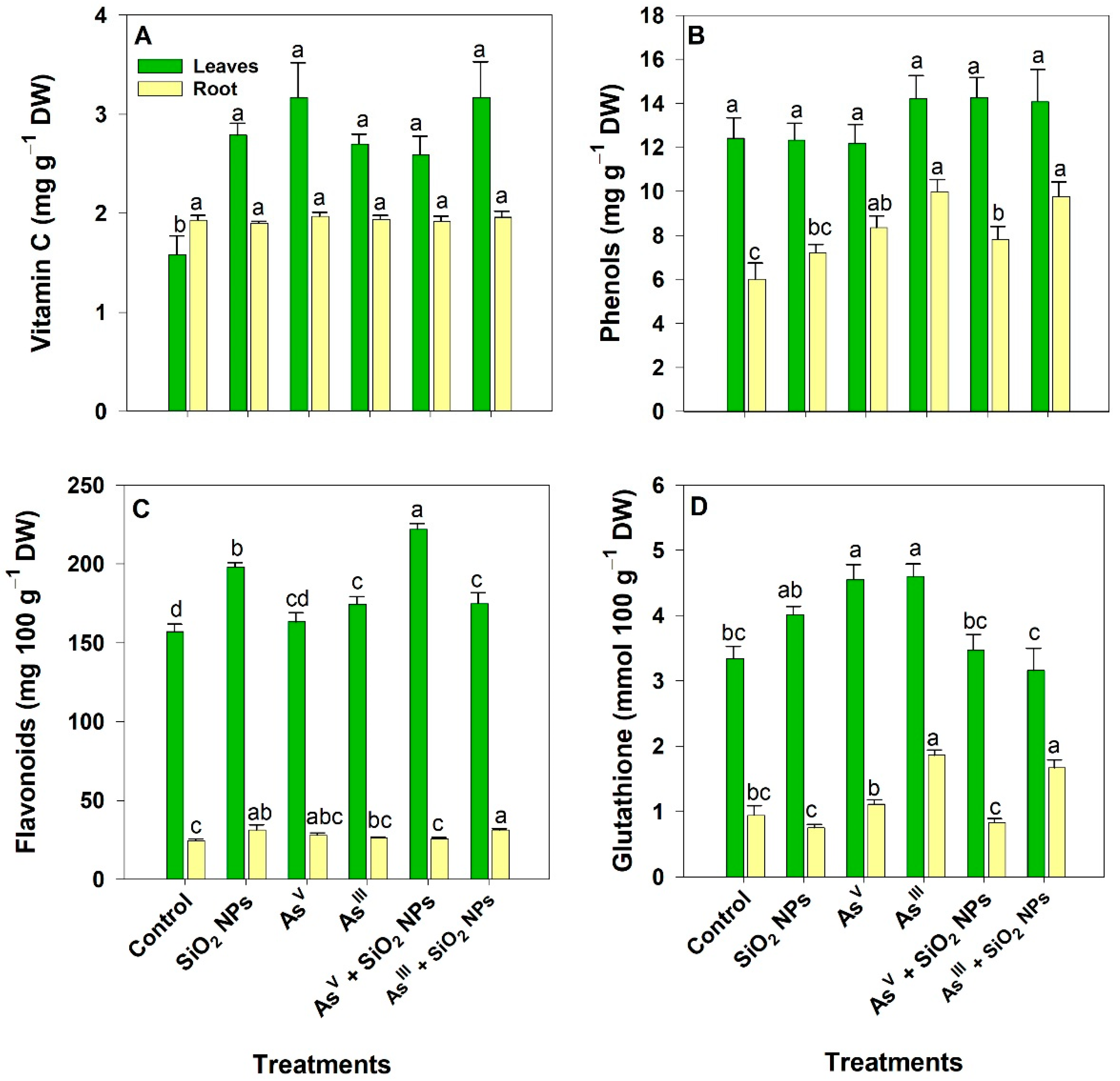
3.6. Non-Enzymatic Antioxidants
3.7. Hydrogen Peroxide and Antioxidant Capacity
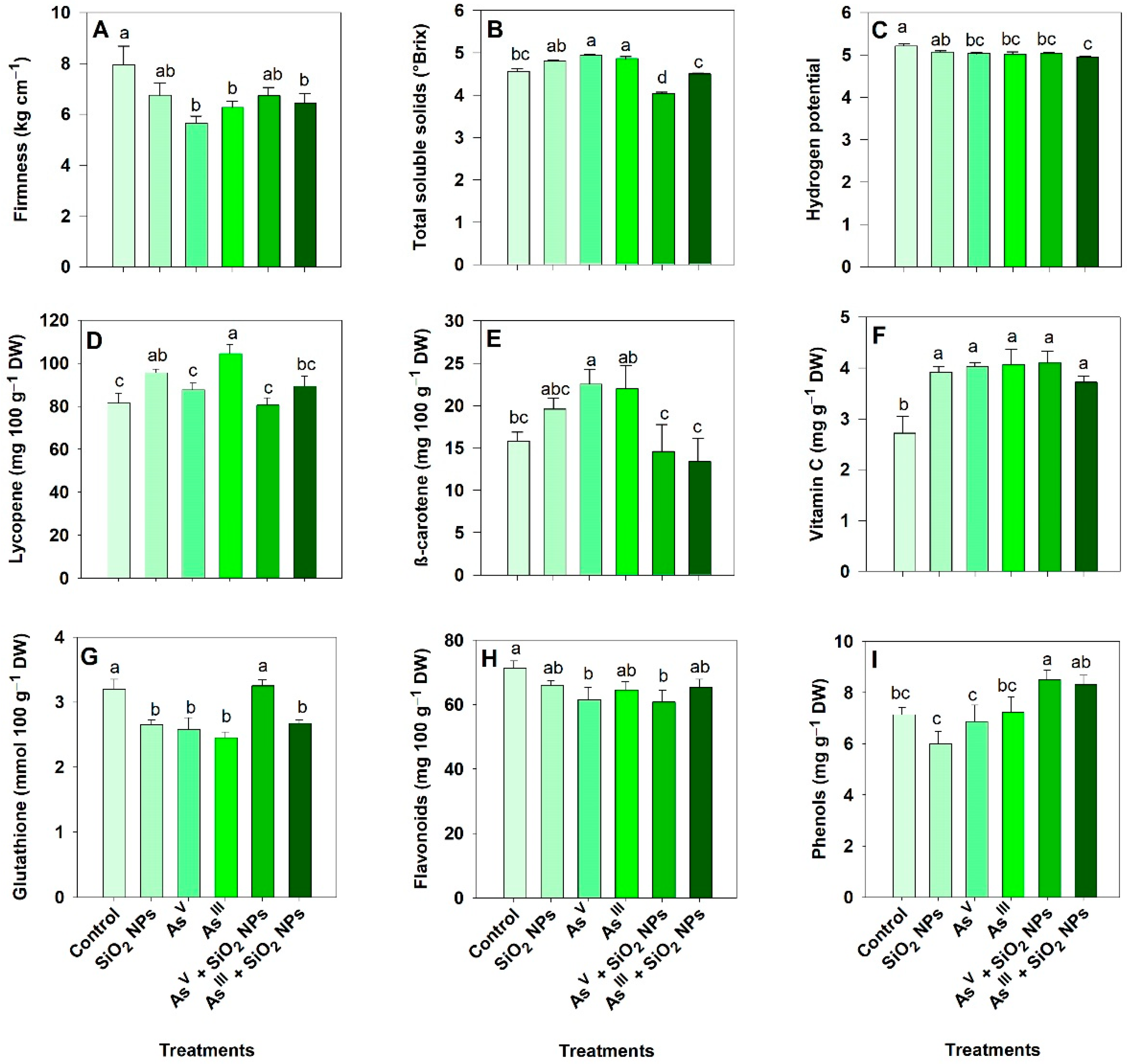
3.8. Effect of As and SiO2 NPs on the Quality of Tomato Fruits
4. Discussion
4.1. Impact of As and SiO2 NPs on Biomass and Fruit Yield
4.2. Concentration of Si, As and Nutrients
4.3. Impact of As and SiO2 NPs on Plant Photosynthetic Pigments
4.4. Impact of As and SiO2 NPs on Antioxidant System
4.5. Impact of As and SiO2 NPs on the Quality of Ripe Tomato Fruits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, D.E.B.; Crook, S.L.; Evans, C.T.; Nader, M.N.; Atia, M.; Hicks, J.M.T.; Sweeney, E.; McFarlane, C.R.; Kim, J.S.; Keltie, E.; et al. Assessing arsenic in human toenail clippings using portable X-Ray fluorescence. Appl. Radiat. Isot. 2020, 167, 109491. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, N.; Shaharuddin, N.A.; Ahmad, S.A.; Nulit, R.; Abiri, R. Assessment of water mimosa (Neptunia oleracea Lour.) morphological, physiological, and removal efficiency for phytoremediation of arsenic-polluted water. Plants 2020, 9, 1500. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xiong, Y.; Wang, L.; Sun, W.; Liu, R.; Yue, T. Arsenic (III) removal from a high-concentration arsenic (III) solution by forming ferric arsenite on red mud surface. Minerals 2020, 10, 583. [Google Scholar] [CrossRef]
- Xu, W.; Dai, W.; Yan, H.; Li, S.; Shen, H.; Chen, Y.; Xu, H.; Sun, Y.; He, Z.; Ma, M. Arabidopsis NIP3;1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol. Plant 2015, 8, 722–733. [Google Scholar] [CrossRef]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2016, 71, 367–377. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Ruíz-Huerta, E.A.; de la Garza Varela, A.; Gómez-Bernal, J.M.; Castillo, F.; Avalos-Borja, M.; SenGupta, B.; Martínez-Villegas, N. Arsenic contamination in irrigation water, agricultural soil and maize crop from an abandoned smelter site in Matehuala, Mexico. J. Hazard. Mater. 2017, 339, 330–339. [Google Scholar] [CrossRef]
- González-Moscoso, M.; González-García, Y.; Martínez-Villegas, N.V.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Nitric oxide modified growth, nutrient uptake and the antioxidant defense system in tomato seedlings stressed with arsenic. Theor. Exp. Plant Physiol. 2021, 33, 205–223. [Google Scholar] [CrossRef]
- Madeira, A.C.; de Varennes, A.; Abreu, M.M.; Esteves, C.; Magalhães, M.C.F. Tomato and parsley growth, arsenic uptake and translocation in a contaminated amended soil. J. Geochem. Explor. 2012, 123, 114–121. [Google Scholar] [CrossRef]
- Singh, V.K.; Upadhyay, R.S. Effects of arsenic on reactive oxygen species and antioxidant defense system in tomato plants. Toxicol. Environ. Chem. 2014, 96, 1374–1383. [Google Scholar] [CrossRef]
- Marmiroli, M.; Mussi, F.; Imperiale, D.; Lencioni, G.; Marmiroli, N. Abiotic stress response to As and As+Si, composite reprogramming of fruit metabolites in tomato cultivars. Front. Plant Sci. 2017, 8, 2201. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in us agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Deng, Q.; Yu, T.; Zeng, Z.; Ashraf, U.; Shi, Q.; Huang, S.; Lian, T.; Chen, J.; Muzaffar, W.; Shen, W. Silicon application modulates the growth, rhizosphere soil characteristics, and bacterial community structure in sugarcane. Front. Plant Sci. 2021, 12, 710139. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. (Amst.) 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Baggio, G.; Dupas, E.; Galindo, F.S.; Megda, M.M.; Pereira, N.C.M.; Luchetta, M.O.; Tritapepe, C.A.; da Silva, M.R.; Jalal, A.; Teixeira Filho, M.C.M. Silicon application induced alleviation of aluminum toxicity in xaraés palisadegrass. Agronomy 2021, 11, 1938. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact 2020, 19, 100232. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortiz, H.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-de la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef]
- Kandhol, N.; Aggarwal, B.; Bansal, R.; Parveen, N.; Singh, V.P.; Chauhan, D.K.; Sonah, H.; Sahi, S.; Grillo, R.; Peralta-Videa, J.; et al. Nanoparticles as a potential protective agent for arsenic toxicity alleviation in plants. Environ. Pollut. 2022, 300, 118887. [Google Scholar] [CrossRef]
- Sharma, A.; Vishwakarma, K.; Singh, N.K.; Prakash, V.; Ramawat, N.; Prasad, R.; Sahi, S.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Synergistic action of silicon nanoparticles and indole acetic acid in alleviation of chromium (CrVI) toxicity in Oryza sativa seedlings. J. Biotechnol. 2021; in press. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z.; Xie, Y. Determination of heavy metal tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob. Ecol. Conserv. 2020, 24, e01306. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Zia ur Rehman, M.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum Aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh Memari-Tabrizi, E.; Yousefpour-Dokhanieh, A.; Babashpour-Asl, M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.). Plant Physiol. Biochem. 2021, 165, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Mohammad, F. Rol of nanoparticles in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Cham, Switzerland, 2015; pp. 19–35. ISBN 9783319145020. [Google Scholar]
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells: Via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2019, 7, 162–171. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; Meza-Figueroa, D.; SenGupta, B.; Martínez-Villegas, N. Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Steiner, A.A. A Universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- DOF Norma Oficial Mexicana NOM-001-ECOL-1996, Que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. D. Of. Fed. 1997, 1–14. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4863829&fecha=06/01/1997#gsc.tab=0 (accessed on 7 April 2022).
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, Transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and Growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Hung, C.Y.; Yen, G.C. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Cumplido-Nájera, C.F.; González-Morales, S.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. (Amst.) 2019, 245, 82–89. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.V.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Del Carmen Rivera-Cruz, M.; González-Morales, S.; Juárez-Maldonado, A. Impact of silicon nanoparticles on the antioxidant compounds of tomato fruits stressed by arsenic. Foods 2019, 8, 612. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A. Antioxidant Activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- USDA. United States Standards for Grades of Fresh Tomatoes. United States Department of Agriculture. 1977; pp. 1–13. Available online: https://www.ams.usda.gov/grades-standards/tomato-grades-and-standards (accessed on 7 April 2022).
- López-Vargas, E.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Wang, H.B.; He, H.B.; Yang, G.D.; Ye, C.Y.; Niu, B.H.; Lin, W.X. Effects of two species of inorganic arsenic on the nutrient physiology of rice seedlings. Acta Physiol. Plant. 2010, 32, 245–251. [Google Scholar] [CrossRef]
- Vromman, D.; Martínez, J.P.; Kumar, M.; Šlejkovec, Z.; Lutts, S. Comparative effects of arsenite (As(III)) and arsenate (As(V)) on whole plants and cell lines of the arsenic-resistant halophyte plant species Atriplex atacamensis. Environ. Sci. Pollut. Res. 2018, 25, 34473–34486. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Gusman, G.S.; Oliveira, J.A.; Farnese, F.S.; Cambraia, J. Mineral nutrition and enzymatic adaptation induced by arsenate and arsenite exposure in lettuce plants. Plant Physiol. Biochem. 2013, 71, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.; Wood, M.; Ripton, A. Integrated tolerance mechanisnfis: Constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant Cell Environ. 1994, 17, 989–993. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic Toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef]
- Landa, P. Positive effects of metallic nanoparticles on plants: Overview of involved mechanisms. Plant Physiol. Biochem. 2021, 161, 12–24. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Gharib, F.A.E.-L.; Ghazi, S.M.; Hegazi, A.Z.; Hafz, A.G.M.A. Physiological responses of two varieties of common bean (Phaseolus vulgaris L.) to foliar application of silver nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, H.; Zhang, X.; Xiao, Z.; Fan, X.; Yin, C.; Tang, X.; Han, F.X.; Liang, Y. Silicon enhances abundances of reducing microbes in rhizoplane and decreases arsenite uptake by rice (Oryza sativa L.). Environ. Pollut. 2022, 306, 119405. [Google Scholar] [CrossRef] [PubMed]
- Ahire, M.L.; Mundada, P.S.; Nikam, T.D.; Bapat, V.A.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef]
- Mehrabanjoubani, P.; Abdolzadeh, A.; Sadeghipour, H.R.; Aghdasi, M. Impacts of silicon nutrition on growth and nutrient status of rice plants grown under varying zinc regimes. Theor. Exp. Plant Physiol. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Huerta, A.J. Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 2008, 311, 73–86. [Google Scholar] [CrossRef]
- Karimian, N.; Nazari, F.; Samadi, S. Morphological and biochemical properties, leaf nutrient content, and vase life of tuberose (Polianthes tuberosa L.) affected by root or foliar applications of silicon (Si) and silicon nanoparticles (SiNPs). J. Plant Growth Regul. 2021, 40, 2221–2235. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Dou, F.; Sun, W.; Ma, X. Simultaneous mitigation of arsenic and cadmium accumulation in rice (Oryza sativa L.) seedlings by silicon oxide nanoparticles under different water management schemes. Paddy Water Environ. 2021, 19, 569–584. [Google Scholar] [CrossRef]
- Garbinski, L.D.; Rosen, B.P.; Chen, J. Pathways of arsenic uptake and efflux. Environ. Int. 2019, 126, 585–597. [Google Scholar] [CrossRef]
- Sandil, S.; Óvári, M.; Dobosy, P.; Vetési, V.; Endrédi, A.; Takács, A.; Füzy, A.; Záray, G. Effect of arsenic-contaminated irrigation water on growth and elemental composition of tomato and cabbage cultivated in three different soils, and related health risk assessment. Environ. Res. 2021, 197, 111098. [Google Scholar] [CrossRef]
- Ghorbani, A.; Pishkar, L.; Roodbari, N.; Pehlivan, N.; Wu, C. Nitric oxide could allay arsenic phytotoxicity in tomato (Solanum lycopersicum L.) by modulating photosynthetic pigments, phytochelatin metabolism, molecular redox status and arsenic sequestration. Plant Physiol. Biochem. 2021, 167, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Stazi, S.R.; Cassaniti, C.; Marabottini, R.; Giuffrida, F.; Leonardi, C. Arsenic uptake and partitioning in grafted tomato plants. Hortic. Environ. Biotechnol. 2016, 57, 241–247. [Google Scholar] [CrossRef]
- Tlustoš, P.; Száková, J.; Pavlíková, D.; Balík, J. The response of tomato (Lycopersicon esculentum) to different concentrations of inorganic and organic compounds of arsenic. Biologia 2006, 61, 91–96. [Google Scholar] [CrossRef]
- Carbonell-Barrachina, A.A.; Burló, F.; Burgos-Hernández, A.; López, E.; Mataix, J. The influence of arsenite concentration on arsenic accumulation in tomato and bean plants. Sci. Hortic. (Amst.) 1997, 71, 167–176. [Google Scholar] [CrossRef]
- Jia-Yi, Y.; Meng-Qiang, S.; Zhi-Liang, C.; Yu-Tang, X.; Hang, W.; Jian-Qiang, Z.; Ling, H.; Qi, Z. Effect of foliage applied chitosan-based silicon nanoparticles on arsenic uptake and translocation in rice (Oryza sativa L.). J. Hazard. Mater. 2022, 433, 128781. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Praveen, A.; Mehrotra, S.; Singh, N.; Pandey, V. Nutrient constraints in arsenic phytoremediation. Russ. J. Plant Physiol. 2018, 65, 15–22. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Song, G.; Zhang, X. Correlations of arsenic and nutrient elements in different tissues of perennial ryegrass under arsenic stress. J. Soil Sci. Plant Nutr. 2021, 21, 1542–1551. [Google Scholar] [CrossRef]
- Garg, N.; Kashyap, L. Silicon and rhizophagus irregularis: Potential candidates for ameliorating negative impacts of arsenate and arsenite stress on growth, nutrient acquisition and productivity in Cajanus cajan (L.) Millsp. genotypes. Environ. Sci. Pollut. Res. 2017, 24, 18520–18535. [Google Scholar] [CrossRef]
- Sanchez-Navarro, J.F.; Gonzalez-García, Y.; Benavides-mendoza, A.; Morales-Días, A.B.; Gonzalez-Morales, S.; Cadenas-pliego, G.; Garcia-Guillermo, M. del S.; Juárez-Maldonado, A. Silicon nanoparticles improve the shelf life and antioxidant status of lilium. Plants 2021, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.V.; Meza-Figueroa, D.; Rivera-Cruz, M. del C.; Cadenas-Pliego, G.; Juárez-Maldonado, A. SiO2 nanoparticles improve nutrient uptake in tomato plants developed in the presence of arsenic. Rev. Bio Cienc. 2021, 8, e1084. [Google Scholar] [CrossRef]
- de Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef]
- González de las Torres, A.I.; Giráldez, I.; Martínez, F.; Palencia, P.; Corns, W.T.; Sánchez-Rodas, D. Arsenic accumulation and speciation in strawberry plants exposed to inorganic arsenic enriched irrigation. Food Chem. 2020, 315, 126215. [Google Scholar] [CrossRef] [PubMed]
- Sghaier, D.B.; Duarte, B.; Bankaji, I.; Caçador, I.; Sleimi, N. Growth, chlorophyll fluorescence and mineral nutrition in the halophyte Tamarix gallica cultivated in combined stress conditions: Arsenic and NaCl. J. Photochem. Photobiol. B Biol. 2015, 149, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhao, C.Y.; Liu, J.; Zhang, J.; Du, Y.X.; Li, J.Z.; Sun, H.Z.; Zhao, H.B.; Zhao, Q.Z. Effect of sulphate nutrition on arsenic translocation and photosynthesis of rice seedlings. Acta Physiol. Plant. 2013, 35, 3237–3243. [Google Scholar] [CrossRef]
- Songy, A.; Vallet, J.; Gantet, M.; Boos, A.; Ronot, P.; Tarnus, C.; Clément, C.; Larignon, P.; Goddard, M.L.; Fontaine, F. Sodium arsenite effect on Vitis vinifera L. Physiology. J. Plant Physiol. 2019, 238, 72–79. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plants 2020, 9, 129. [Google Scholar] [CrossRef]
- Sun, S.K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.J. Decreasing arsenic accumulation in rice by overexpressing OsNIP1;1 and OsNIP3;3 through disrupting arsenite radial transport in roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef]
- Praveen, A.; Khan, E.; Ngiimei, S.; Perwez, M.; Sardar, M.; Gupta, M. Iron oxide nanoparticles as nano-adsorbents: A possible way to reduce arsenic phytotoxicity in indian mustard plant (Brassica juncea L.). J. Plant Growth Regul. 2018, 37, 612–624. [Google Scholar] [CrossRef]
- Kataria, S.; Jain, M.; Rastogi, A.; Živčák, M.; Brestic, M.; Liu, S.; Tripathi, D.K. Role of nanoparticles on photosynthesis: Avenues and applications. In Nanomaterials in Plants, Algae and Microorganisms: Concepts and Controversies: Volume 2; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 103–127. ISBN 9780128114889. [Google Scholar]
- Mukarram, M.; Khan, M.M.A.; Corpas, F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (steud.) wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021, 412, 125254. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Petrik, P.; Mushtaq, Z.; Khan, M.M.A.; Gulfishan, M.; Lux, A. Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ. Pollut. 2022, 310, 119855. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Tan, W.S.; Wong, K.W.; Ong, G.H.; Cheng, W.H.; Nulit, R.; Ibrahim, M.H.; Chew, W.; Berandah Edward, F.; Okamura, H.; et al. Antioxidant enzyme activities as biomarkers of Cu and Pb stress in Centella asiatica. Stresses 2021, 1, 253–265. [Google Scholar] [CrossRef]
- Souza, L.A.; Piotto, F.A.; Dourado, M.N.; Schmidt, D.; Franco, M.R.; Boaretto, L.F.; Tezotto, T.; Ferreira, R.R.; Azevedo, R.A. Physiological and biochemical responses of Dolichos Lablab L. to cadmium support its potential as a cadmium phytoremediator. J. Soils Sediments 2015, 17, 1413–1426. [Google Scholar] [CrossRef]
- Duquesnoy, I.; Champeau, G.M.; Evray, G.; Ledoigt, G.; Piquet-Pissaloux, A. Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. Comptes Rendus Biol. 2010, 333, 814–824. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, S.; Tripathi, R.D.; Dwivedi, S.; Trivedi, P.K.; Tandon, P.K. Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ. Sci. Technol. 2007, 41, 2930–2936. [Google Scholar] [CrossRef]
- Sharma, I. Arsenic induced oxidative stress in plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front. Environ. Sci. 2016, 4, 46. [Google Scholar] [CrossRef]
- Shabnam, N.; Kim, M.; Kim, H. Iron (III) oxide nanoparticles alleviate arsenic induced stunting in Vigna radiata. Ecotoxicol. Environ. Saf. 2019, 183, 109496. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Mendoza, A.; Gonzalez-Moscoso, M.; Ojeda-Barrios, D.L.; Fuentes-lara, L.O. Biostimulation and toxicity: Two levels of action of nanomaterials in plants. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 283–303. [Google Scholar]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Requejo, R.; Tena, M. Influence of glutathione chemical effectors in the response of maize to arsenic exposure. J. Plant Physiol. 2012, 169, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, X.; Williams, P.N.; Gao, X.; Zhang, L.; Zhang, J.; Shan, H.; Su, S. Arsenic resistance in fungi conferred by extracellular bonding and vacuole-septa compartmentalization. J. Hazard. Mater. 2021, 401, 123370. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wong, M.H.; Lan, C.Y.; Qin, Y.R.; Shu, W.S.; Qiu, R.L.; Ye, Z.H. Effect of arsenic on flavonoid contents in pteris species. Biochem. Syst. Ecol. 2010, 38, 529–537. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Gąsecka, M.; Rutkowski, P.; Magdziak, Z.; Goliński, P.; Mleczek, M. Arsenic forms and their combinations induce differences in phenolic accumulation in Ulmus laevis Pall. J. Plant Physiol. 2018, 220, 34–42. [Google Scholar] [CrossRef]
- Woźniak, A.; Drzewiecka, K.; Kȩsy, J.; Marczak, Ł.; Narozna, D.; Grobela, M.; Motała, R.; Bocianowski, J.; Morkunas, I. The influence of lead on generation of signalling molecules and accumulation of flavonoids in pea seedlings in response to pea aphid infestation. Molecules 2017, 22, 1404. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. (Amst.) 2020, 272, 109537. [Google Scholar] [CrossRef]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Abdollahi Mandoulakani, B. Iron oxide nanoparticles: A novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J. Sci. Food Agric. 2019, 99, 6418–6430. [Google Scholar] [CrossRef] [PubMed]
- Jurkow, R.; Agnieszka, S.; Pokluda, R.; Smole, S.; Kalisz, A. Biochemical Response of Oakleaf Lettuce Seedlings to Different Concentrations of Some Metal(oid) Oxide Nanoparticles. Agronomy 2020, 10, 997. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014, 21, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yang, S.; Han, D.; Zhou, Z.; Li, X.; Liu, Y.; Zhang, B. Silicon alleviates cadmium toxicity in wheat seedlings (Triticum aestivum L.) by reducing cadmium ion uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. 2018, 25, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M. Antioxidant properties of BHT estimated by ABTS assay in systems differing in pH or metal ion or water concentration. Eur. Food Res. Technol. 2011, 232, 837–842. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Engineered gold nanoparticles and plant adaptation potential. Nanoscale Res. Lett. 2016, 11, 400. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; Matros, A.; Scheibe, R.; Mock, H.P.; Dietz, K.J. The hydrogen peroxide-sensitive proteome of the chloroplast in vitro and in vivo. Front. Plant Sci. 2013, 4, 54. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bioactive compounds, antioxidant activity and color of hydroponic tomato fruits at different stages of ripening. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 404–412. [Google Scholar] [CrossRef]
- Marmiroli, M.; Mussi, F.; Imperiale, D.; Marmiroli, N. Target proteins reprogrammed by As and As+Si treatments in Solanum lycopersicum L. Fruit. BMC Plant Biol. 2017, 17, 210. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pigoni, V.; Savo-Sardaro, M.L.; Marmiroli, N. The effect of silicon on the uptake and translocation of arsenic in tomato (Solanum lycopersicum L.). Environ. Exp. Bot. 2014, 99, 9–17. [Google Scholar] [CrossRef]
- Marmiroli, M.; Mussi, F.; Imperiale, D.; Marmiroli, N. Arsenic and Silicon in tomato fruit: An “omics” analysis for food safety. In Proceedings of the IPC2019 CHANGSHA The 16th International Phytotechnology Conference—Phytotechnologies for Food Safety & Environmental Health, Changsha, China, 23–27 September 2019; p. 178. [Google Scholar]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, C.; Neocleous, D.; Dalias, P.; Ioannou, A.; Fotopoulos, V. Uptake of hexavalent chromium by tomato (Solanum lycopersicum L.) plants and mediated effects on their physiology and productivity, along with fruit quality and safety. Environ. Exp. Bot. 2021, 189, 104564. [Google Scholar] [CrossRef]
- Dai, Y.; Li, T.; Wang, Z.; Xing, B. Physiological and proteomic analyses reveal the effect of CeO2 Nanoparticles on strawberry reproductive system and fruit quality. Sci. Total Environ. 2022, 814, 152494. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef]
- Ogunkunle, C.O.; Adegboye, E.F.; Okoro, H.K.; Vishwakarma, V.; Alagarsamy, K.; Fatoba, P.O. Effect of nanosized anatase TiO2 on germination, stress defense enzymes, and fruit nutritional quality of Abelmoschus esculentus (L.) Moench (Okra). Arab. J. Geosci. 2020, 13, 120. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Skodra, C.; Molassiotis, A.; Tanou, G. Silicon influenced ripening metabolism and improved fruit quality traits in apples. Plant Physiol. Biochem. 2021, 166, 270–277. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy 2022, 12, 2366. https://doi.org/10.3390/agronomy12102366
González-Moscoso M, Martínez-Villegas N, Cadenas-Pliego G, Juárez-Maldonado A. Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic. Agronomy. 2022; 12(10):2366. https://doi.org/10.3390/agronomy12102366
Chicago/Turabian StyleGonzález-Moscoso, Magín, Nadia Martínez-Villegas, Gregorio Cadenas-Pliego, and Antonio Juárez-Maldonado. 2022. "Effect of Silicon Nanoparticles on Tomato Plants Exposed to Two Forms of Inorganic Arsenic" Agronomy 12, no. 10: 2366. https://doi.org/10.3390/agronomy12102366





