Effects of 10 Years of the Return of Corn Straw on Soil Aggregates and the Distribution of Organic Carbon in a Mollisol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Description
2.2. Experimental Design
2.3. Soils Sampling
2.4. Separation of Aggregates by Size
2.5. Density Fractionation in SOC
2.6. Statistical Analysis
3. Results
3.1. SOC Content in Bulk Soil and Density Fractions
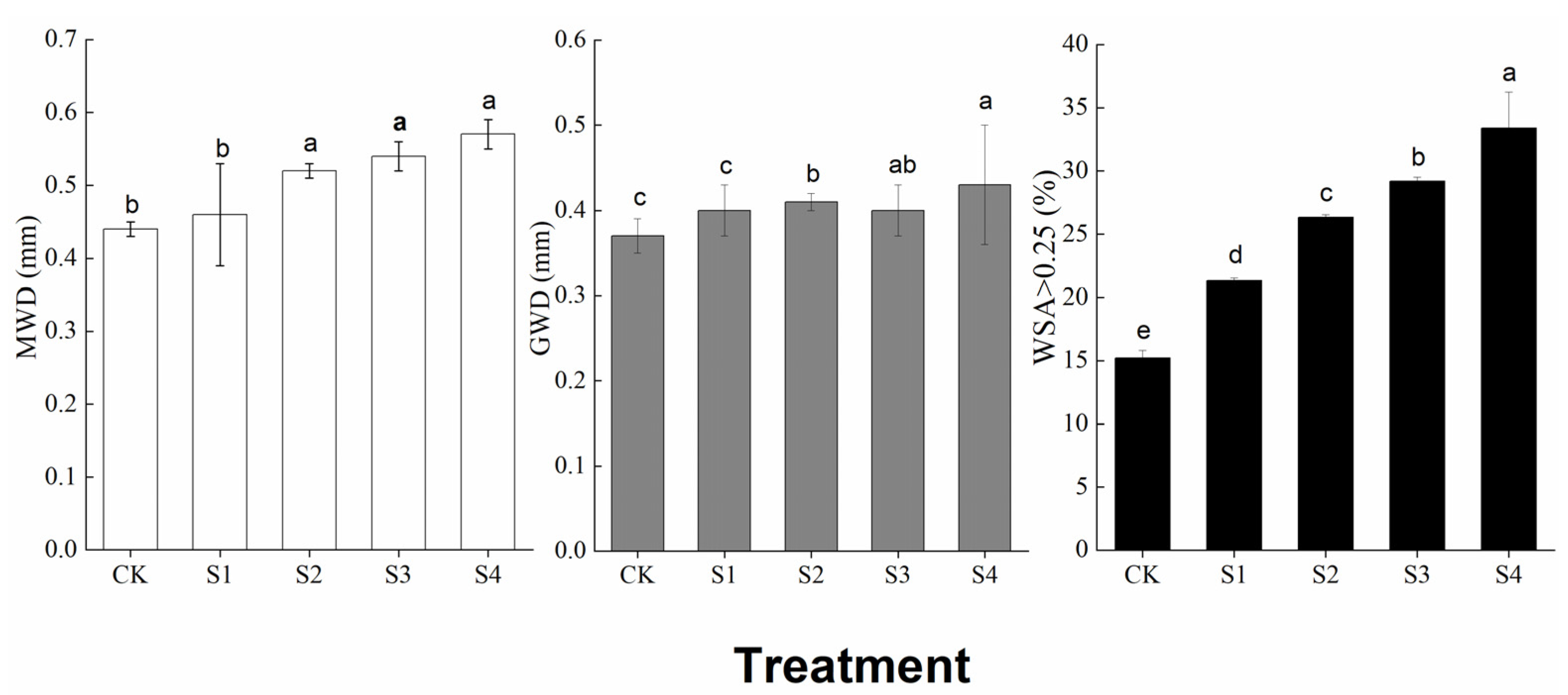
3.2. Aggregate Distribution and Stability
3.3. SOC Content in the Aggregate Size Classes
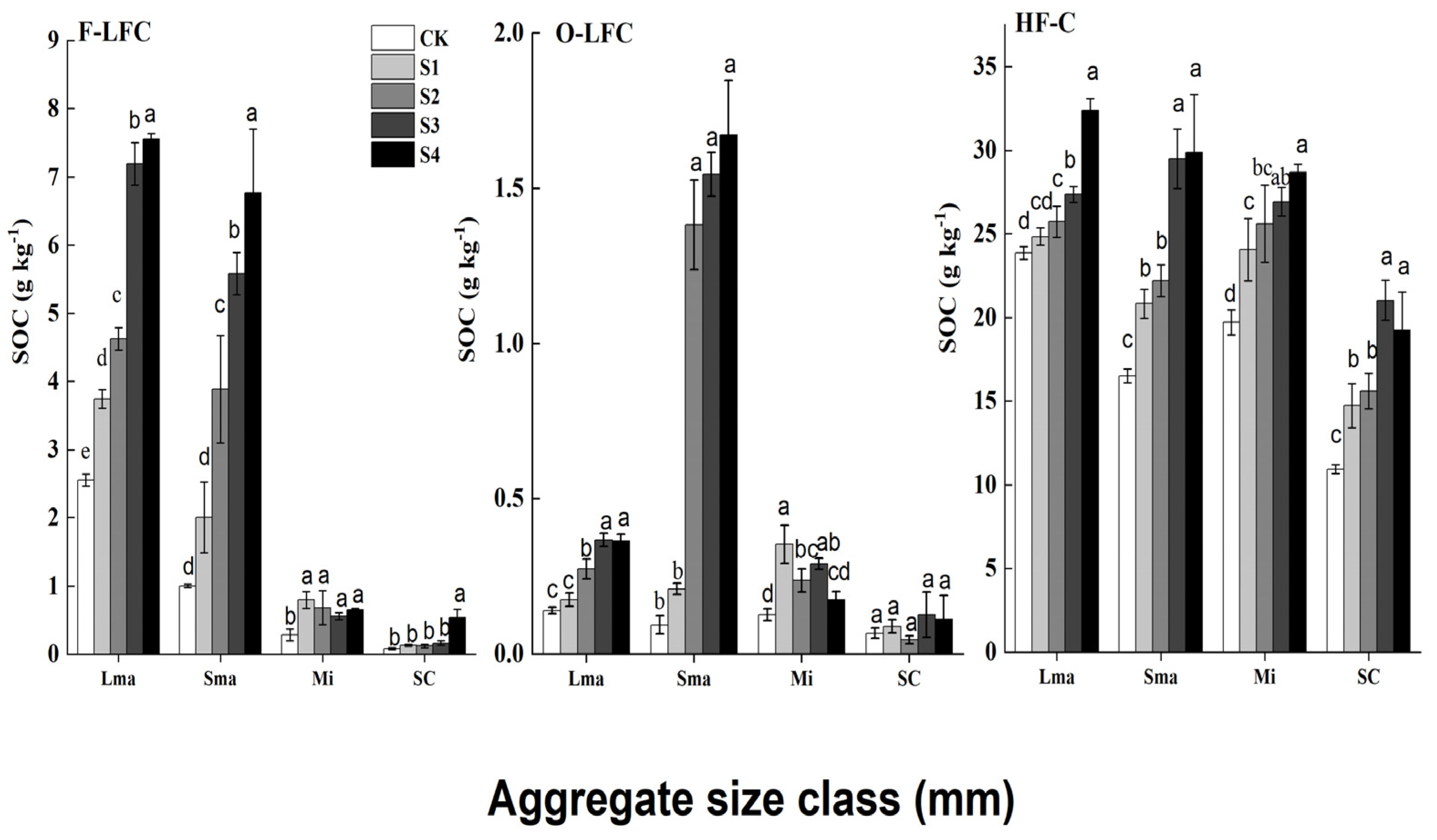
3.4. Density Fractions of SOC in the Aggregate Size Classes
3.5. Relationships among Straw Return, SOC Indicators, and Aggregate Indicators
4. Discussion
4.1. Effects on SOC Content after 10 Years of Returning Corn Straw
4.2. Effects of Straw Return on Aggregate Distribution and Stability
4.3. Effects of Straw Return on the SOC Density Fractions in the Aggregate Sizes
4.4. Relationship between the Aggregates and SOC with Straw Return
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wu, P.; Mei, F.; Ling, Y.; Qiao, Y.; Liu, C.; Leghari, S.J.; Guan, X.; Wang, T. Does continuous straw returning keep China farmland soil organic carbon continued increase? A meta-analysis. J. Environ. Manag. 2021, 288, 112391. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pei, J.; Xu, Y.; Wang, J. Soil organic carbon depletion in global Mollisols regions and restoration by management practices: A review. J. Soils Sediment. 2020, 20, 1173–1181. [Google Scholar] [CrossRef]
- Knorr, W.; Prentice, I.C.; House, J.I.; Holland, E.A. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Li, Y.; Wang, Q.; Zhang, C.; Ma, D.; Li, J.; Zhang, J. N, P and straw return influence the accrual of organic carbon fractions and microbial traits in a Mollisol. Geoderma 2021, 403, 115373. [Google Scholar] [CrossRef]
- Hao, X.; Han, X.; Wang, S.; Li, L.-J. Dynamics and composition of soil organic carbon in response to 15 years of straw return in a Mollisol. Soil Tillage Res. 2022, 215, 105221. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission from soil. Sci. Total Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef]
- Lal, R. Soil quality impacts of residue removal for bioethanol production. Soil Tillage Res. 2009, 102, 233–241. [Google Scholar] [CrossRef]
- Niu, X.; Ju, X. Organic fertilizer resources and utilization in China. J. Plant Nutr. Fert. 2017, 23, 1462–1479. [Google Scholar]
- Chabbi, A.; Lehmann, J.; Ciais, P.; Loescher, H.W.; Cotrufo, M.F.; Don, A.; SanClements, M.; Schipper, L.; Six, J.; Smith, P.; et al. Aligning agriculture and climate policy. Nat. Clim. Chang. 2017, 7, 307–309. [Google Scholar] [CrossRef]
- Oliveira, M.; Barre, P.; Trindade, H.; Virto, I. Different efficiencies of grain legumes in crop rotations to improve soil aggregation and organic carbon in the short-term in a sandy Cambisol. Soil Tillage Res. 2019, 186, 23–35. [Google Scholar] [CrossRef]
- Ma, E.; Zhang, G.; Ma, J.; Xu, H.; Cai, Z.; Yagi, K. Effects of rice straw returning methods on N2O emission during wheat-growing season. Nutr. Cycling Agroecosyst. 2010, 88, 463–469. [Google Scholar] [CrossRef]
- Bai, Y.-l.; Wang, L.; Lu, Y.-l.; Yang, L.-p.; Zhou, L.-p.; Ni, L.; Cheng, M.-f. Effects of long-term full straw return on yield and potassium response in wheat-maize rotation. J. Integr. Agric. 2015, 14, 2467–2476. [Google Scholar] [CrossRef]
- Ahmed, W.; Qaswar, M.; Jing, H.; Wenjun, D.; Geng, S.; Kailou, L.; Ying, M.; Ao, T.; Mei, S.; Chao, L.; et al. Tillage practices improve rice yield and soil phosphorus fractions in two typical paddy soils. J. Soils Sediments 2020, 20, 850–861. [Google Scholar] [CrossRef]
- Bossuyt, H.; Denef, K.; Six, J.; Frey, S.D.; Merckx, R.; Paustian, K. Influence of microbial populations and residue quality on aggregate stability. Appl. Soil Ecol. 2001, 16, 195–208. [Google Scholar] [CrossRef]
- Soon, Y.K.; Lupwayi, N.Z. Straw management in a cold semi-arid region: Impact on soil quality and crop productivity. Field Crops Res. 2012, 139, 39–46. [Google Scholar] [CrossRef]
- Zhao, H.; Shar, A.G.; Li, S.; Chen, Y.; Shi, J.; Zhang, X.; Tian, X. Effect of straw return mode on soil aggregation and aggregate carbon content in an annual maize-wheat double cropping system. Soil Tillage Res. 2018, 175, 178–186. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Soil-Structure and Carbon Cycling. Aust. J. Soil Res. 1994, 32, 1043–1068. [Google Scholar] [CrossRef]
- Ndzelu, B.S.; Dou, S.; Zhang, X. Corn straw return can increase labile soil organic carbon fractions and improve water-stable aggregates in Haplic Cambisol. J. Arid Land 2020, 12, 1018–1030. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter as an indicator of organic matter quality in arable and pastoral soils in New Zealand. Soil Biol. Biochem. 2000, 32, 211–219. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Chen, H.Q.; Hou, R.X.; Gong, Y.S.; Li, H.W.; Fan, M.S.; Kuzyakov, Y. Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Tillage Res. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Golchin, A.; Clarke, P.; Baldock, J.A.; Higashi, T.; Skjemstad, J.O.; Oades, J.M. The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by C-13 NMR spectroscopy. 1. Whole soil and humic acid fraction. Geoderma 1997, 76, 155–174. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Beare, M.H.; McKim, U.F.; Skjemstad, J.O. Chemical and biological characteristics of physically uncomplexed organic matter. Soil Sci. Soc. Am. J. 2006, 70, 975–985. [Google Scholar] [CrossRef]
- Roscoe, R.; Buurman, P. Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol. Soil Tillage Res. 2003, 70, 107–119. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.H.; Zhang, Z.H. Influences of intensive tillage on water-stable aggregate distribution on a steep hillslope. Soil Tillage Res. 2015, 151, 82–92. [Google Scholar] [CrossRef]
- Wang, X.; Cammeraat, E.L.H.; Cerli, C.; Kalbitz, K. Soil aggregation and the stabilization of organic carbon as affected by erosion and deposition. Soil Biol. Biochem. 2014, 72, 55–65. [Google Scholar] [CrossRef]
- Huang, R.; Lan, M.; Liu, J.; Gao, M. Soil aggregate and organic carbon distribution at dry land soil and paddy soil: The role of different straws returning. Sci. Total Environ. 2017, 24, 27942–27952. [Google Scholar] [CrossRef]
- Naser, H.M.; Nagata, O.; Tamura, S.; Hatano, R. Methane emissions from five paddy fields with different amounts of rice straw application in central Hokkaido, Japan. Soil Sci. Plant Nutr. 2007, 53, 95–101. [Google Scholar] [CrossRef]
- Sun, H.; Ji, Q.; Wang, Y.; Wang, X. The Distribution of Water-stable Aggregate-associated Organic Carbon and Its Oxidation Stability Under Different Straw Returning Modes. J. Agr. Environ. Sci. 2012, 31, 369–376. [Google Scholar]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 4, 10108–10120. [Google Scholar] [CrossRef]
- Tian, P.; Lian, H.; Wang, Z.; Jiang, Y.; Li, C.; Sui, P.; Qi, H. Effects of Deep and Shallow Tillage with Straw Incorporation on Soil Organic Carbon, Total Nitrogen and Enzyme Activities in Northeast China. Sustainability 2020, 12, 8679. [Google Scholar] [CrossRef]
- Zhou, M.; Xiao, Y.; Li, Y.; Zhang, X.; Wang, G.; Jin, J.; Ding, G.; Liu, X. Soil quality index evaluation model in responses to six-year fertilization practices in Mollisols. Arch. Agron. Soil Sci. 2022, 68, 180–194. [Google Scholar] [CrossRef]
- Staff, S.S. Keys to Soil Taxonomy; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2010.
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Puget, P.; Angers, D.A.; Chenu, C. Nature of carbohydrates associated with water-stable aggregates of two cultivated soils. Soil Biol. Biochem. 1999, 31, 55–63. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA: Madison, WI, USA, 1986; pp. 425–440. [Google Scholar]
- Wang, X.; Jia, Z.; Liang, L.; Han, Q.; Ding, R.; Yang, B.; Cui, R. Effects of organic manure application on dry land soil organic matter andwater stable aggregates. Chin. J. Appl. Ecol. 2012, 23, 159–165. [Google Scholar]
- Spycher, G.; Sollins, P.; Rose, S. Carbon and nitrogen in the light fraction of a forest Soil-vertical-distribution and seasnal patterns. Soil Sci. 1983, 135, 79–87. [Google Scholar] [CrossRef]
- John, B.; Yamashita, T.; Ludwig, B.; Flessa, H. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 2005, 128, 63–79. [Google Scholar] [CrossRef]
- Quiroga, A.R.; Buschiazzo, D.E.; Peinemann, N. Soil organic matter particle size fractions in soils of the semiarid Argentinian pampas. Soil Sci. 1996, 161, 104–108. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Prakash, V.; Bhatt, J.C. Long term fertilization effects on soil organic carbon pools in a sandy loam soil of the Indian sub-Himalayas. Plant Soil 2011, 341, 109–124. [Google Scholar] [CrossRef]
- Mitchell, E.; Scheer, C.; Rowlings, D.; Conant, R.T.; Cotrufo, M.F.; Grace, P. Amount and incorporation of plant residue inputs modify residue stabilisation dynamics in soil organic matter fractions. Agric. Ecosyst. Environ. 2018, 256, 82–91. [Google Scholar] [CrossRef]
- Huang, T.; Yang, N.; Lu, C.; Qin, X.; Siddique, K.H.M. Soil organic carbon, total nitrogen, available nutrients, and yield under different straw returning methods. Soil Tillage Res. 2021, 214, 105171. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Liang, L.; Yang, B.; Ding, R.; Nie, J.; Wang, J. Maize straw effects on soil aggregation and other properties in arid land. Soil Tillage Res. 2015, 153, 131–136. [Google Scholar] [CrossRef]
- Monreal, C.M.; Schnitzer, M.; Schulten, H.R.; Campbell, C.A.; Anderson, D.W. Soil Organic Structures in Macro and Microaggregates of a Cultivated Brown Chernozem. Soil Biol. Biochem. 1995, 27, 845–853. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Yin, Y.; Liang, C.-H.; Pei, Z.-J. Effect of greenhouse soil management on soil aggregation and organic matter in northeast China. Catena 2015, 133, 412–419. [Google Scholar] [CrossRef]
- Manna, M.C.; Swarup, A.; Wanjari, R.H.; Mishra, B.; Shahi, D.K. Long-term fertilization, manure and liming effects on soil organic matter and crop yields. Soil Tillage Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize-wheat-cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Banger, K.; Toor, G.S.; Biswas, A.; Sidhu, S.S.; Sudhir, K. Soil organic carbon fractions after 16-years of applications of fertilizers and organic manure in a Typic Rhodalfs in semi-arid tropics. Nutr. Cycl. Agroecosyst. 2010, 86, 391–399. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Meier, E.A.; Collins, K.; Robertson, F.A. Changes in soil carbon sequestration, fractionation and soil fertility in response to sugarcane residue retention are site-specific. Soil Tillage Res. 2012, 120, 99–111. [Google Scholar] [CrossRef]
- Oades, J.M. Soil Organic-Matter and Structural Stability-Mechanisms and Implications for Management. Plant Soil. 1984, 76, 319–337. [Google Scholar] [CrossRef]
- Angers, D.A.; Recous, S.; Aita, C. Fate of carbon and nitrogen in water-stable aggregates during decomposition of (CN)-C-13-N-15-labelled wheat straw in situ. Eur. J. Soil Sci. 1997, 48, 295–300. [Google Scholar] [CrossRef]
- Sheng, H.; Zhou, P.; Zhang, Y.; Kuzyakov, Y.; Zhou, Q.; Ge, T.; Wang, C. Loss of labile organic carbon from subsoil due to land-use changes in subtropical China. Soil Biol. Biochem. 2015, 88, 148–157. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Martinez, J.M.; Limbozzi, F. Labile soil organic carbon for assessing soil quality: Influence of management practices and edaphic conditions. Catena 2018, 171, 316–326. [Google Scholar] [CrossRef]
- Samahadthai, P.; Vityakon, P.; Saenjan, P. Effects of different quality plant residues on soil carbon accumulation and aggregate formation in a tropiacl sandy soil in northeast thailand as revealed by a 10-year field experiment. Land Degrad. Dev. 2010, 21, 463–473. [Google Scholar] [CrossRef]
- Martin, J.P.; Waksman, S.A. Influence of microorganisms on soil aggregation and erosion. Soil Sci. 1940, 50, 29–47. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic-matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Carbon and Nitrogen Distribution in Aggregates from Culltivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef]
- Li, X.-G.; Wang, Z.-F.; Ma, Q.-F.; Li, F.-M. Crop cultivation and intensive grazing affect organic C pools and aggregate stability in and grassland soil. Soil Tillage Res. 2007, 95, 172–181. [Google Scholar] [CrossRef]



| Treatment | Bulk Soil (g kg−1) | Density Fraction (g kg−1) | ||
|---|---|---|---|---|
| F-LF | O-LF | HF | ||
| CK | 21.89 ± 0.69 b | 0.33 ± 0.01 c | 0.08 ± 0.009 c | 16.42 ± 0.666 d |
| S1 | 22.76 ± 0.71 b | 0.69 ± 0.05 c | 0.29 ± 0.070 b | 19.95 ± 0.356 c |
| S2 | 24.75 ± 0.84 b | 1.30 ± 0.39 bc | 0.29 ± 0.106 ab | 21.92 ± 1.277 b |
| S3 | 24.82 ± 0.69 b | 2.05 ± 0.09 ab | 0.48 ± 0.018 b | 22.34 ± 0.520 b |
| S4 | 27.80 ± 0.64 a | 2.49 ± 1.25 a | 0.66 ± 0.183 a | 27.79 ± 1.423 a |
| Treatments | Lma | Sma | Mi | SC |
|---|---|---|---|---|
| CK | 23.33 ± 2.34 b | 23.68 ± 0.64 a | 26.96 ± 1.65 a | 13.97 ± 0.78 a |
| S1 | 24.83 ± 2.09 ab | 24.46 ± 0.52 a | 29.07 ± 1.23 a | 14.24 ± 0.52 a |
| S2 | 25.44 ± 0.55 ab | 25.49 ± 0.56 a | 27.80 ± 0.45 a | 14.15 ± 0.18 a |
| S3 | 26.86 ± 0.47 ab | 26.79 ± 0.81 a | 29.34 ± 0.67 a | 14.55 ± 0.40 a |
| S4 | 27.11 ± 1.31 a | 25.98 ± 0.73 a | 27.41 ± 1.80 a | 14.44 ± 1.30 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Qiu, C.; Han, X.; Kwaw-Mensah, D.; Chen, X.; Yan, J.; Lu, X.; Zou, W. Effects of 10 Years of the Return of Corn Straw on Soil Aggregates and the Distribution of Organic Carbon in a Mollisol. Agronomy 2022, 12, 2374. https://doi.org/10.3390/agronomy12102374
Gan J, Qiu C, Han X, Kwaw-Mensah D, Chen X, Yan J, Lu X, Zou W. Effects of 10 Years of the Return of Corn Straw on Soil Aggregates and the Distribution of Organic Carbon in a Mollisol. Agronomy. 2022; 12(10):2374. https://doi.org/10.3390/agronomy12102374
Chicago/Turabian StyleGan, Jiawei, Chen Qiu, Xiaozeng Han, David Kwaw-Mensah, Xu Chen, Jun Yan, Xinchun Lu, and Wenxiu Zou. 2022. "Effects of 10 Years of the Return of Corn Straw on Soil Aggregates and the Distribution of Organic Carbon in a Mollisol" Agronomy 12, no. 10: 2374. https://doi.org/10.3390/agronomy12102374





