Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Plant Material
2.2. Climate, Soil, and Vine Water Regime
2.3. Phenology
2.4. Vine Water Status and Must Carbon Isotope Analysis
2.5. Must Oxygen Isotope Analysis
2.6. Yield Components, Pruning Weight, and Grape Quality Components
2.7. Statistical Analysis
3. Results
3.1. Must Carbon Isotope Ratio
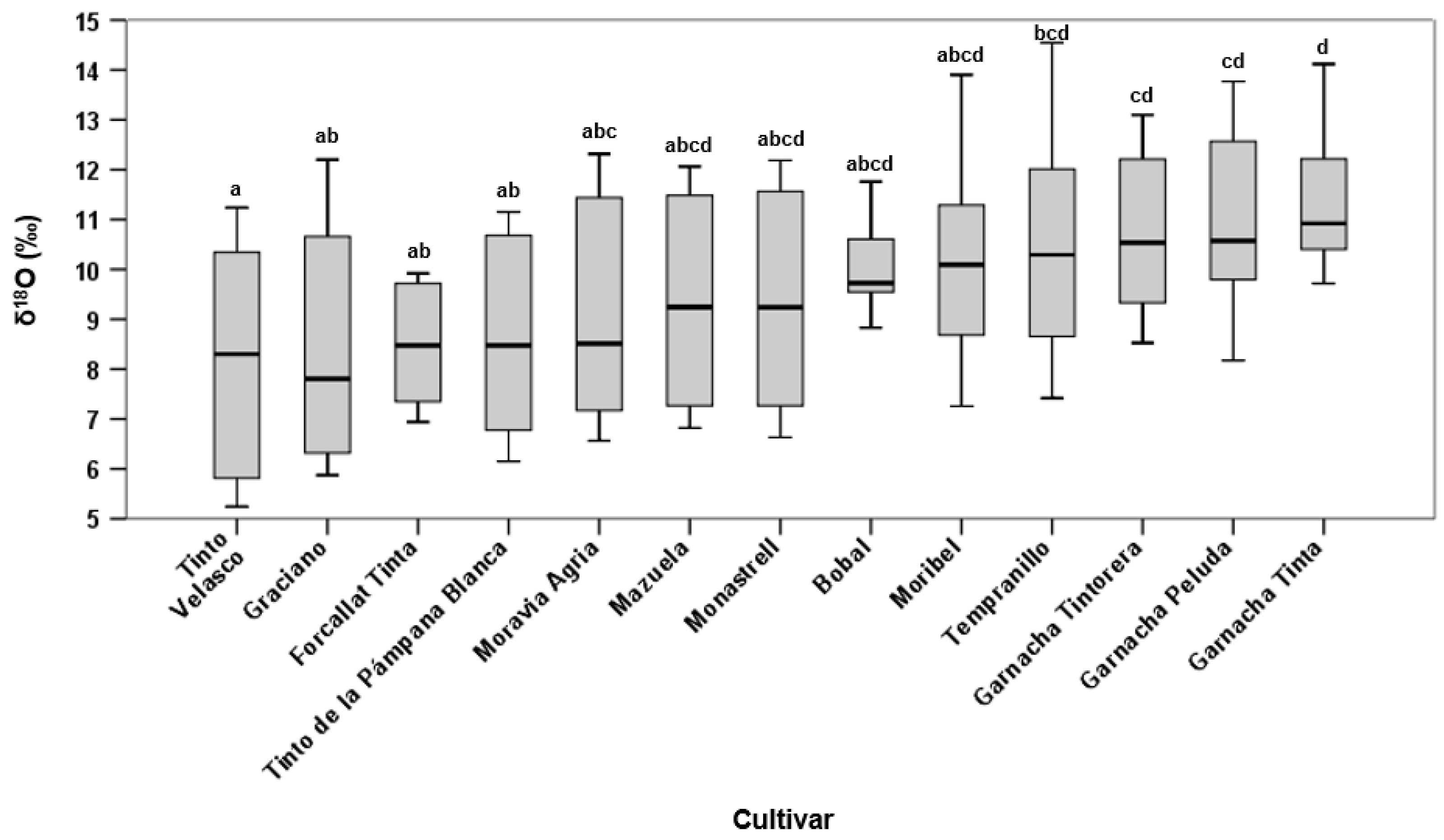
3.2. Must Oxygen Isotope Ratio
3.3. Yield Components and Pruning Weight
3.4. Must Quality Components
4. Discussion
4.1. Cultivar Water Status and Isotope Ratios
4.2. Agronomic Response
4.3. Categorizing Cultivars Based on Their Agronomic Response to Drought
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrano, H.; Escalona, J.M.; Cifre, J.; Bota, J.; Flexas, J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: Effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant Biol. 2003, 30, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.A.; Lebon, É.; Ojeda, H. Stomatal behavior of different grapevine cultivars in response to soil water status and air water vapor pressure deficit. J. Int. Sci. Vigne Vin 2010, 44, 9–20. [Google Scholar] [CrossRef]
- Costa, J.M.; Vaz, M.; Escalona, J.; Egipto, R.; Lopes, C.; Medrano, H.; Chaves, M.M. Modern viticulture in southern Europe: Vulnerabilities and strategies for adaptation to water scarcity. Agric. Water Manag. 2016, 164, 5–18. [Google Scholar] [CrossRef]
- IPCC. IPCC AR6 WGI Climate Change 2021 The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Intrigliolo, D.S.; Castel, J.R. Trunk diameter variations as water stress indicator in plum and grapevine. Acta Hortic. 2008, 792, 363–369. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef] [Green Version]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; Ramos, M.C. Projected effects of climate change on Tempranillo and Chardonnay varieties in La Mancha Designation of Origin. Agron. Sustain. Dev. 2021, 41, 24. [Google Scholar] [CrossRef]
- Morales-Castilla, I.; De Cortázar-Atauri, I.G.; Cook, B.I.; Lacombe, T.; Parker, A.; Van Leeuwen, C.; Nicholas, K.A.; Wolkovich, E.M. Diversity buffers winegrowing regions from climate change losses. Proc. Natl. Acad. Sci. USA 2020, 117, 2864–2869. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; García De Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.A.; Lacombe, T. From Pinot to Xinomavro in the world’s future wine-growing regions. Nat. Clim. Chang. 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef] [Green Version]
- Barnuud, N.N.; Zerihun, A.; Mpelasoka, F.; Gibberd, M.; Bates, B. Responses of grape berry anthocyanin and titratable acidity to the projected climate change across the Western Australian wine regions. Int. J. Biometeorol. 2014, 58, 1279–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.C.; Martínez de Toda, F. Variability in the potential effects of climate change on phenology and on grape composition of Tempranillo in three zones of the Rioja DOCa (Spain). Eur. J. Agron. 2020, 115, 126014. [Google Scholar] [CrossRef]
- Martinez De Toda, F.; Balda, P. Quantifying the effect of temperature on decoupling anthocyanins and sugars of the grape (Vitis vinifera L. ’Maturana Tinta de Navarrete’). Vitis J. Grapevine Res. 2015, 54, 117–120. [Google Scholar]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Alatzas, A.; Theocharis, S.; Miliordos, D.E.; Leontaridou, K.; Kanellis, A.K.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The Effect of Water Deficit on Two Greek Vitis vinifera L. Cultivars: Physiology, Grape Composition and Gene Expression during Berry Development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef]
- Bassoi, L.H.; De Melo Chaves, A.R.; Teixeira, R.P. Responses of “Syrah” grapevine to deficit irrigation in the Brazilian semi-arid region. Agric. Water Manag. 2021, 258, 107186. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E.; Gómez-Alonso, S.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on the Phenolic Compounds of ‘Merlot’ Grapes in a Semi-Arid Mediterranean Climate. Horticulturae 2021, 7, 161. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae 2020, 6, 95. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Intrigliolo, D.S. Grape composition under abiotic constrains: Water stress and salinity. Front. Plant Sci. 2017, 8, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy 2020, 10, 680. [Google Scholar] [CrossRef]
- Zufferey, V.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Koestel, C.; Rösti, J.; Gindro, K.; Spangenberg, J.E.; Viret, O.; et al. The impact of plant water status on the gas exchange, berry composition and wine quality of Chasselas grapes in Switzerland. OENO One 2018, 52. [Google Scholar] [CrossRef]
- Faralli, M.; Bianchedi, P.L.; Bertamini, M.; Varotto, C. Rootstock Genotypes Shape the Response of cv. Pinot gris to Water Deficit. Agronomy 2020, 11, 75. [Google Scholar] [CrossRef]
- Florez-Sarasa, I.; Clemente-Moreno, M.J.; Cifre, J.; Capo, M.; Llompart, M.Q.; Fernie, A.R.; Bota, J. Differences in Metabolic and Physiological Responses between Local and Widespread Grapevine Cultivars under Water Deficit Stress. Agronomy 2020, 10, 1052. [Google Scholar] [CrossRef]
- Martorell, S.; Diaz-Espejo, A.; Tomàs, M.; Pou, A.; El Aou-ouad, H.; Escalona, J.M.; Vadell, J.; Ribas-Carbó, M.; Flexas, J.; Medrano, H. Differences in water-use-efficiency between two Vitis vinifera cultivars (Grenache and Tempranillo) explained by the combined response of stomata to hydraulic and chemical signals during water stress. Agric. Water Manag. 2015, 156, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tramontini, S.; Vitali, M.; Centioni, L.; Schubert, A.; Lovisolo, C. Rootstock control of scion response to water stress in grapevine. Environ. Exp. Bot. 2013, 93, 20–26. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant–Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef]
- Gaudillère, J.P.; Van Leeuwen, C.; Ollat, N. Carbon isotope composition of sugars in grapevine, an integrated indicator of vineyard water status. J. Exp. Bot. 2002, 53, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Bchir, A.; Escalona, J.M.; Gallé, A.; Hernández-Montes, E.; Tortosa, I.; Braham, M.; Medrano, H. Carbon isotope discrimination (δ13C) as an indicator of vine water status and water use efficiency (WUE): Looking for the most representative sample and sampling time. Agric. Water Manag. 2016, 167, 11–20. [Google Scholar] [CrossRef]
- Brillante, L.; Martínez-Lüscher, J.; Yu, R.; Kurtural, S.K. Carbon Isotope Discrimination (δ13 C) of Grape Musts Is a Reliable Tool for Zoning and the Physiological Ground-Truthing of Sensor Maps in Precision Viticulture. Front. Environ. Sci. 2020, 8, 561477. [Google Scholar] [CrossRef]
- Gowdy, M.; Destrac-Irvine, A.; Haines, M.; Gambetta, G.; Pieri, P.; Marguerit, E.; Van Leeuwen, C. Varietal responses to soil water deficit: First results from a common-garden vineyard near Bordeaux France. E3S Web Conf. 2018, 50, 23–26. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Barbarin, I.; Royo, J.B. Application of the measurement of the natural abundance of stable isotopes in viticulture: A review. Aust. J. Grape Wine Res. 2015, 21, 157–167. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springe: Berlin, Germany, 2009. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Andersen, M.N.; Jensen, C.R. undefined Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Funct. Plant Biol. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Pascual, M.; Lordan, J.; Villar, J.M.; Fonseca, F.; Rufat, J. Stable carbon and nitrogen isotope ratios as indicators of water status and nitrogen effects on peach trees. Sci. Hortic. 2013, 157, 99–107. [Google Scholar] [CrossRef]
- Allen, R.G. Evapotranspiración del Cultivo: Guias Para la Determinación de Agua de los Cultivos; FAO: Rome, Italy, 2006; Volume 297. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale†. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- OIV Compendio de Los Métodos Internacionales de Análisis de Los Vinos y de Los Mostos. Available online: https://www.oiv.int/es/normas-y-documentos-tecnicos/metodos-de-analisis/compendio-de-los-metodos-internacionales-de-analisis-de-los-vinos-y-de-los-mostos (accessed on 13 March 2022).
- Bota, J.; Tomás, M.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Tortosa, I.; Escalona, J.M.; Bota, J.; Tomás, M.; Hernández, E.; Escudero, E.G.; Medrano, H. Exploring the genetic variability in water use efficiency: Evaluation of inter and intra cultivar genetic diversity in grapevines. Plant Sci. 2016, 251, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tardaguila, J.; Bertamini, M.; Reniero, F.; Versini, G. Oxygen isotope composition of must-water in grapevine: Effects of water deficit and rootstock. Aust. J. Grape Wine Res. 1997, 3, 84–89. [Google Scholar] [CrossRef]
- Roßmann, A.; Reniero, F.; Moussa, I.; Schmidt, H.L.; Versini, G.; Merle, M.H. Stable oxygen isotope content of water of EU data-bank wines from Italy, France and Germany. Z. Für Lebensm. Und-Forsch. A 1999, 208, 400–407. [Google Scholar] [CrossRef]
- Ingraham, N.L.; Caldwell, E.A. Influence of weather on the stable isotopic ratios of wines: Tools for weather/climate reconstruction? J. Geophys. Res. Atmos. 1999, 104, 2185–2194. [Google Scholar] [CrossRef]
- Koç, M.; Kamiloğlu, Ö.; Cangi, R.; Yıldız, K. Physiological and biochemical responses to drought stress in some autochthonous grapevines of Turkey. Acta Hortic. 2019, 1248, 531–539. [Google Scholar] [CrossRef]
- Toumi, I.; M’Sehli, W.; Bourgou, S.; Jallouli, N.; Bensalem-Fnayou, A.; Ghorbel, A.; Mliki, A. Response of ungrafted and grafted grapevine cultivans and rootstocks (Vitis sp.) to water stress. OENO One 2007, 41, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Tortosa, I.; Escalona, J.M.; Toro, G.; Douthe, C.; Medrano, H. Clonal Behavior in Response to Soil Water Availability in Tempranillo Grapevine cv: From Plant Growth to Water Use Efficiency. Agronomy 2020, 10, 862. [Google Scholar] [CrossRef]
- Bianchi, D.; Caramanico, L.; Grossi, D.; Brancadoro, L.; De Lorenzis, G. How Do Novel M-Rootstock (Vitis Spp.) Genotypes Cope with Drought? Plants 2020, 9, 1385. [Google Scholar] [CrossRef]
- Carbonneau, A. The Early Selection of Grapevine Rootstocks for Resistance to Drought Conditions. Am. J. Enol. Vitic. 1985, 36, 195–198. [Google Scholar]
- Fort, K.; Fraga, J.; Grossi, D.; Walker, M.A. Early Measures of Drought Tolerance in Four Grape Rootstocks. J. Am. Soc. Hortic. Sci. 2017, 142, 36–46. [Google Scholar] [CrossRef]
- Kucukbasmaci, A.; Sabir, A. Long-term impact of deficit irrigation on the physiology and growth of grapevine cv. “prima” grafted on various rootstocks. Acta Sci. Pol. Hortorum Cultus 2019, 18, 57–70. [Google Scholar] [CrossRef]
- Mohsen, A.T.; Stino, R.G.; Abd Allatif, A.M.; Zaid, N.M. In vitro evaluation of some grapevine rootstocks grown under drought stress. Plant Arch. 2020, 20, 1029–1034. [Google Scholar]
- Pavlousek, P. Evaluation of drought tolerance of new grapevine rootstock hybrids. J. Environ. Biol. 2011, 32, 543–549. [Google Scholar] [PubMed]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainability in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manag. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Bota, J.; Flexas, J.; Medrano, H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Ann. Appl. Biol. 2001, 138, 353–361. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M.; Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef]
- De Souza, C.R.; Maroco, J.P.; Dos Santos, T.P.; Rodrigues, M.L.; Lopes, C.M.; Pereira, J.S.; Chaves, M.M. Impact of deficit irrigation on water use efficiency and carbon isotope composition (δ13C) of field-grown grapevines under Mediterranean climate. J. Exp. Bot. 2005, 56, 2163–2172. [Google Scholar] [CrossRef] [Green Version]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillére, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? OENO One 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Guix-Hébrard, N.; Voltz, M.; Trambouze, W.; Garnier, F.; Gaudillère, J.P.; Lagacherie, P. Influence of watertable depths on the variation of grapevine water status at the landscape scale. Eur. J. Agron. 2007, 27, 187–196. [Google Scholar] [CrossRef]
- Martínez-Vergara, A.; Payan, J.-C.; Salançon, E.; Tisseyre, B. Spiderδ: An empirical method to extrapolate grapevine (Vitis vinifera L.) water status at the whole denomination scale using δ13C as ancillary data. OENO One 2014, 48, 129–140. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Guillaume, S.; Royo, J.B.; Tisseyre, B. Are precision agriculture tools and methods relevant at the whole-vineyard scale? Precis. Agric. 2013, 14, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Alonso, S.; García-Romero, E. Effect of irrigation and variety on oxygen (δ18O) and carbon (δ13C) stable isotope composition of grapes cultivated in a warm climate. Aust. J. Grape Wine Res. 2010, 16, 283–289. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. “Tempranillo”. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- De Souza, C.R.; Maroco, J.P.; Dos Santos, T.P.; Rodrigues, M.L.; Lopes, C.M.; Pereira, J.S.; Chaves, M.M. Partial rootzone drying: Regulation of stomatal aperture and carbon assimilation in field-grown grapevines (Vitis vinifera cv. Moscatel). Funct. Plant Biol. 2003, 30, 653–662. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Cuevas, M.V.; Ribas-Carbo, M.; Flexas, J.; Martorell, S.; Fernández, J.E. The effect of strobilurins on leaf gas exchange, water use efficiency and ABA content in grapevine under field conditions. J. Plant Physiol. 2012, 169, 379–386. [Google Scholar] [CrossRef]
- Gibberd, M.R.; Walker, R.R.; Blackmore, D.H.; Condon, A.G. Transpiration efficiency and carbon-isotope discrimination of grapevines grown under well-watered conditions in either glasshouse or vineyard. Aust. J. Grape Wine Res. 2001, 7, 110–117. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Pou, A.; Escalona, J.M.; Martorell, S.; Ribas-Carbó, M.; Flexas, J. Water-use efficiency in grapevine cultivars grown under controlled conditions: Effects of water stress at the leaf and whole-plant level. Aust. J. Grape Wine Res. 2012, 18, 164–172. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Urretavizcaya, I.; Royo, J.B. Carbon isotope ratio of whole berries as an estimator of plant water status in grapevine (Vitis vinifera L.) cv. “Tempranillo”. Sci. Hortic. 2012, 146, 7–13. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Voltas, J. Carbon and oxygen isotope ratios in wood constituents of Pinus halepensis as indicators of precipitation, temperature and vapour pressure deficit. Tellus B Chem. Phys. Meteorol. 2017, 57, 164–173. [Google Scholar] [CrossRef]
- Bong, Y.S.; Lee, K.S.; Shin, W.J.; Ryu, J.S. Comparison of the oxygen and hydrogen isotopes in the juices of fast-growing vegetables and slow-growing fruits. Rapid Commun. Mass Spectrom. 2008, 22, 2809–2812. [Google Scholar] [CrossRef] [PubMed]
- Kolesnov, A.; Zenina, M.; Tsimbalaev, S.; Davlyatshin, D.; Ganin, M.; Anikina, N.; Agafonova, N.; Egorov, E.; Guguchkina, T.; Prakh, A.; et al. Scientific study of 13C/12C carbon and 18O/16O oxygen stable isotopes biological fractionation in grapes in the Black Sea, Don Basin and the Western Caspian regions. BIO Web Conf. 2017, 9, 02020. [Google Scholar] [CrossRef] [Green Version]
- IAEA. Isotopic Composition of Precipitation in the Mediterranean Basin in Relation to Air Circulation Patterns and Climate; International Atomic Energy Agency: Vienna, Austria, 2005; p. 223. [Google Scholar]
- Cocco, M.; Mercenaro, L.; Cascio, M.L.; Nieddu, G. Effects of Vine Water Status and Exogenous Abscisic Acid on Berry Composition of Three Red Wine Grapes Grown under Mediterranean Climate. Horticulturae 2020, 6, 12. [Google Scholar] [CrossRef]





| Cultivar | Yield (kg vine−1) | Bunch Weight (g) | Berry Weight (g) | Pruning Weight (kg vine−1) | Ravaz Index |
|---|---|---|---|---|---|
| Monastrell | 2.06 ± 1.00 a | 120.01 ± 40.36 a | 1.05 ± 0.20 a | 0.28 ± 0.09 ab | 7.27 ± 2.62 abcd |
| Forcallat Tinta | 2.20 ± 1.13 ab | 172.05 ± 47.84 ab | 1.45 ± 0.13 b | 0.33 ± 0.13 ab | 6.80 ± 2.83 abc |
| Graciano | 2.47 ± 1.81 abc | 138.94 ± 55.47 ab | 0.97 ± 0.18 a | 0.27 ± 0.14 a | 9.39 ± 4.83 bcd |
| Tempranillo | 2.74 ± 1.85 abc | 156.83 ± 79.98 ab | 1.34 ± 0.20 b | 0.47 ± 0.25 bc | 5.64 ± 3.00 a |
| Garnacha Tintorera | 2.89 ± 2.21 abc | 114.20 ± 51.42 a | 1.49 ± 0.27 b | 0.36 ± 0.21 abc | 7.30 ± 3.05 abcd |
| Garnacha Peluda | 3.00 ± 1.74 abc | 126.95 ± 44.46 ab | 1.36 ± 0.17 b | 0.42 ± 0.20 abc | 7.26 ± 2.76 abcd |
| Garnacha Tinta | 3.11 ± 2.17 abcd | 128.10 ± 60.41 ab | 1.34 ± 0.20 b | 0.40 ± 0.20 abc | 7.15 ± 2.49 abcd |
| Bobal | 3.18 ± 0.91 abcd | 329.63 ± 98.70 d | 2.01 ± 0.21 c | 0.56 ± 0.18 c | 6.13 ± 2.08 ab |
| Mazuela | 3.56 ± 1.20 abcd | 150.90 ± 38.53 ab | 1.39 ± 0.29 b | 0.47 ± 0.14 bc | 7.56 ± 1.63 abcd |
| Moribel | 4.04 ± 2.08 bcd | 185.77 ± 47.03 bc | 1.56 ± 0.18 b | 0.41 ± 0.16 abc | 9.82 ± 2.58 cd |
| Tinto Velasco | 4.22 ± 1.28 cd | 222.29 ± 53.00 c | 1.98 ± 0.35 c | 0.52 ± 0.14 c | 8.52 ± 3.31 abcd |
| Tinto de la Pámpana Blanca | 4.38 ± 1.48 cd | 236.97 ± 54.73 c | 2.00 ± 0.19 c | 0.45 ± 0.15 abc | 10.22 ± 3.31 d |
| Moravia Agria | 4.88 ± 2.62 d | 230.01 ± 76.64 c | 1.34 ± 0.18 b | 0.39 ± 0.18 abc | 12.56 ± 3.41 e |
| Cultivar | Total Soluble Solids (°Brix) | Total Acidity (g L−1) | pH |
|---|---|---|---|
| Moravia Agria | 20.27 ± 1.59 a | 6.58 ± 1.05 f | 3.12 ± 0.12 ab |
| Garnacha Tintorera | 20.83 ± 2.39 ab | 4.29 ± 0.65 b | 3.34 ± 0.21 cd |
| Tinto de la Pámpana Blanca | 21.09 ± 1.97 abc | 4.88 ± 0.44 bc | 3.26 ± 0.13 bcd |
| Tinto Velasco | 21.65 ± 1.43 bcd | 4.85 ± 0.45 bc | 3.37 ± 0.22 cd |
| Forcallat Tinta | 22.11 ± 0.52 cde | 3.57 ± 0.35 a | 3.43 ± 0.18 cd |
| Tempranillo | 22.39 ± 1.37 cdef | 4.21 ± 0.78 b | 3.45 ± 0.20 d |
| Mazuela | 22.71 ± 1.11 defg | 6.09 ± 0.77 ef | 3.26 ± 0.19 bcd |
| Monastrell | 22.92 ± 1.00 defg | 4.81 ± 0.54 bc | 3.37 ± 0.14 cd |
| Garnacha Tinta | 23.53 ± 0.90 efg | 4.83 ± 0.59 bc | 3.26 ± 0.19 bcd |
| Moribel | 23.55 ± 1.51 efg | 5.54 ± 0.84 cde | 3.31 ± 0.18 cd |
| Bobal | 23.74 ± 1.20 fg | 5.80 ± 1.34 de | 3.36 ± 0.16 cd |
| Garnacha Peluda | 24.12 ± 1.08 g | 5.30 ± 0.71 cd | 3.23 ± 0.20 bc |
| Graciano | 26.32 ± 1.87 h | 6.52 ± 1.06 f | 3.06 ± 0.15 a |
| Trait | Category 1 (High) | Category 2 (Moderate) | Category 3 (Low) |
|---|---|---|---|
| Yield | (>4 kg vine−1) Moribel, Tinto Velasco, Tinto de la Pámpana Blanca, Moravia Agria | (3–4 kg vine−1) Garnacha Peluda, Garnacha Tinta, Bobal, Mazuela | (<3 kg vine−1) Monastrell, Forcallat Tinta, Graciano, Tempranillo, Garnacha Tintorera |
| Pruning weight | (>0.50 kg vine−1) Tinto Velasco, Bobal | (0.35–0.50 kg vine−1) Garnacha Tintorera, Moravia Agria, Garnacha Tinta, Moribel, Garnacha Peluda, Tinto de la Pámpana Blanca, Tempranillo, Mazuela | (<0.35 kg vine−1) Graciano, Monastrell, Forcallat Tinta |
| Total soluble solids | (>24.5 °Brix) Graciano | (22.5–24.5 °Brix) Mazuela, Monastrell, Garnacha Tinta, Moribel, Bobal, Garnacha Peluda | (<22.5 °Brix) Moravia Agria, Garnacha Tintorera, Tinto de la Pámpana Blanca, Tinto Velasco, Forcallat Tinta, Tempranillo |
| Total acidity | (>6.0 g L−1) Mazuela, Graciano, Moravia Agria | (4.5–6.0 g L−1) Bobal, Monastrell, Garnacha Tinta, Tinto Velasco, Tinto de la Pámpana Blanca, Garnacha Peluda, Moribel | (<4.5 g L−1) Forcallat Tinta, Tempranillo, Garnacha Tintorera |
| δ13C | (>−22.5‰) Garnacha Tintorera | (−22.5 to −23.5‰) Forcallat Tinta, Garnacha Peluda, Tinto de la Pámpana Blanca, Garnacha Tinta, Tinto Velasco, Monastrell, Moravia Agria, Tempranillo, Bobal | (<−23.5‰) Moribel, Mazuela, Graciano |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.S.; Martínez-Gascueña, J.; Alonso, G.L.; Cebrián-Tarancón, C.; Carmona, M.D.; Mena, A.; Chacón-Vozmediano, J.L. Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate. Agronomy 2022, 12, 2399. https://doi.org/10.3390/agronomy12102399
Serrano AS, Martínez-Gascueña J, Alonso GL, Cebrián-Tarancón C, Carmona MD, Mena A, Chacón-Vozmediano JL. Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate. Agronomy. 2022; 12(10):2399. https://doi.org/10.3390/agronomy12102399
Chicago/Turabian StyleSerrano, A. Sergio, Jesús Martínez-Gascueña, Gonzalo L. Alonso, Cristina Cebrián-Tarancón, M. Dolores Carmona, Adela Mena, and Juan L. Chacón-Vozmediano. 2022. "Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate" Agronomy 12, no. 10: 2399. https://doi.org/10.3390/agronomy12102399
APA StyleSerrano, A. S., Martínez-Gascueña, J., Alonso, G. L., Cebrián-Tarancón, C., Carmona, M. D., Mena, A., & Chacón-Vozmediano, J. L. (2022). Agronomic Response of 13 Spanish Red Grapevine (Vitis vinifera L.) Cultivars under Drought Conditions in a Semi-Arid Mediterranean Climate. Agronomy, 12(10), 2399. https://doi.org/10.3390/agronomy12102399





