Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection
2.2. Isolation of Endophytic Fungi
2.3. Macroscopic and Microscopic Identification of Recovered Endophytic Fungi
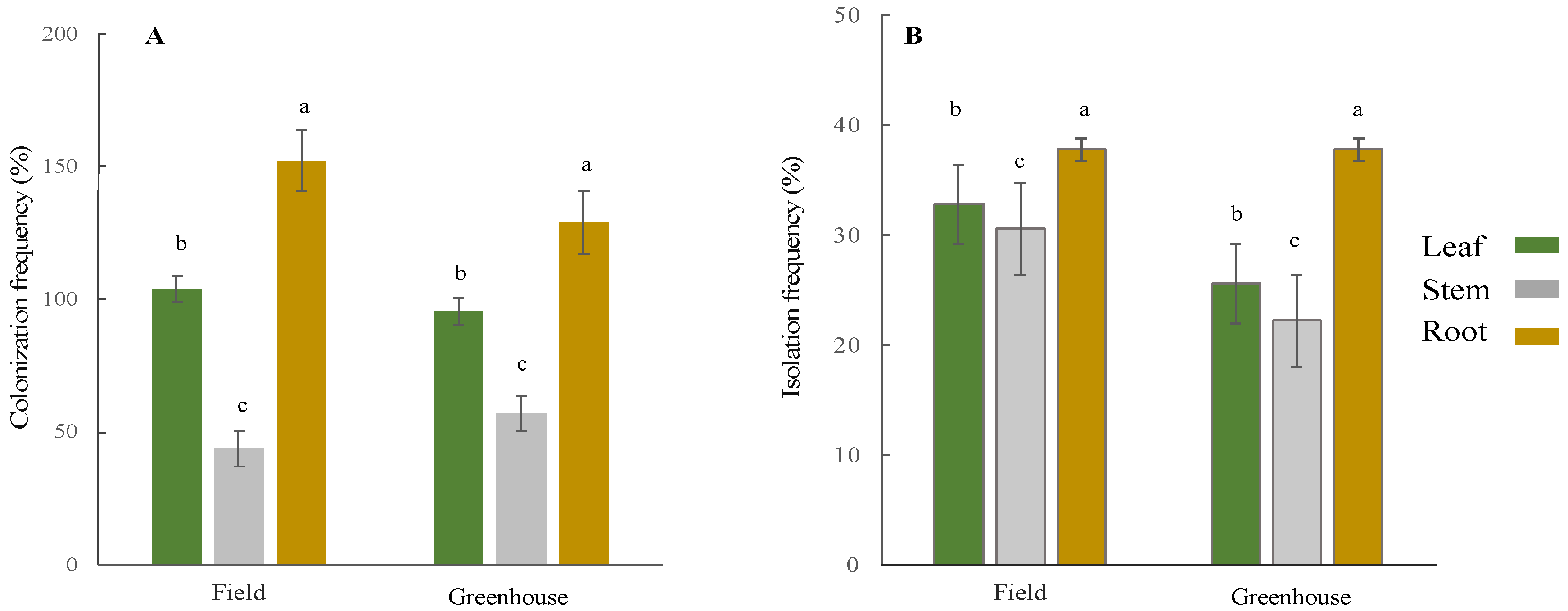
2.4. Colonization and Isolation Frequency
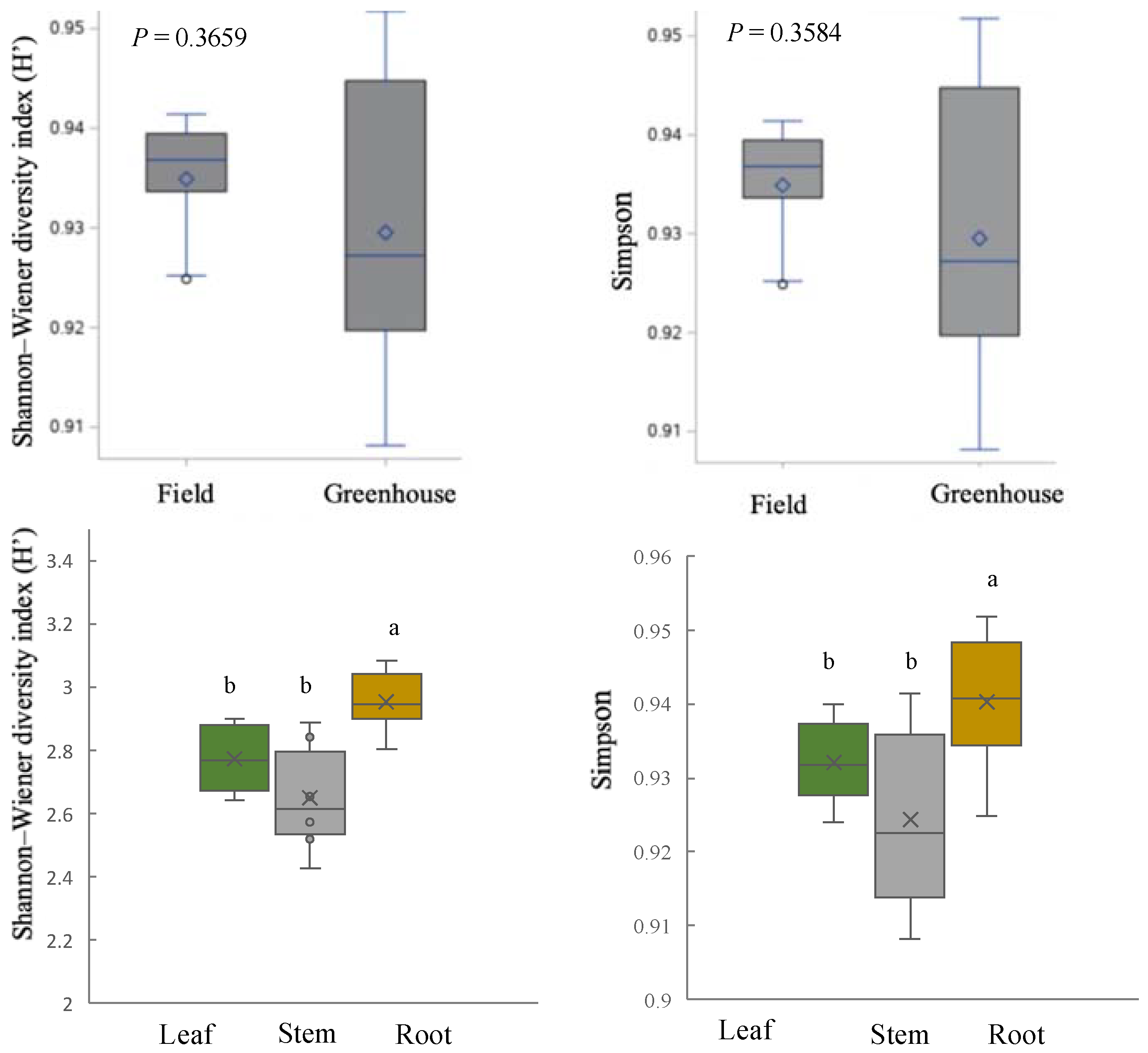
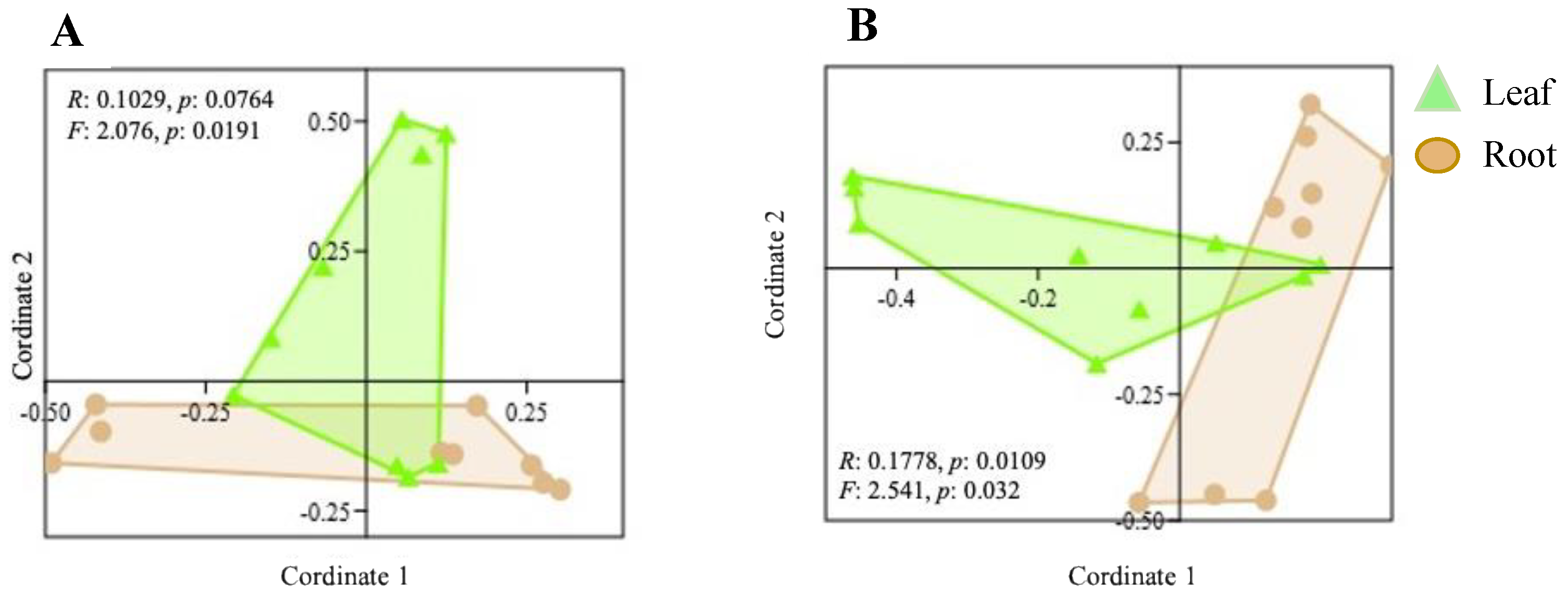
2.5. Community Structure of Culturable Endophytic Fungi
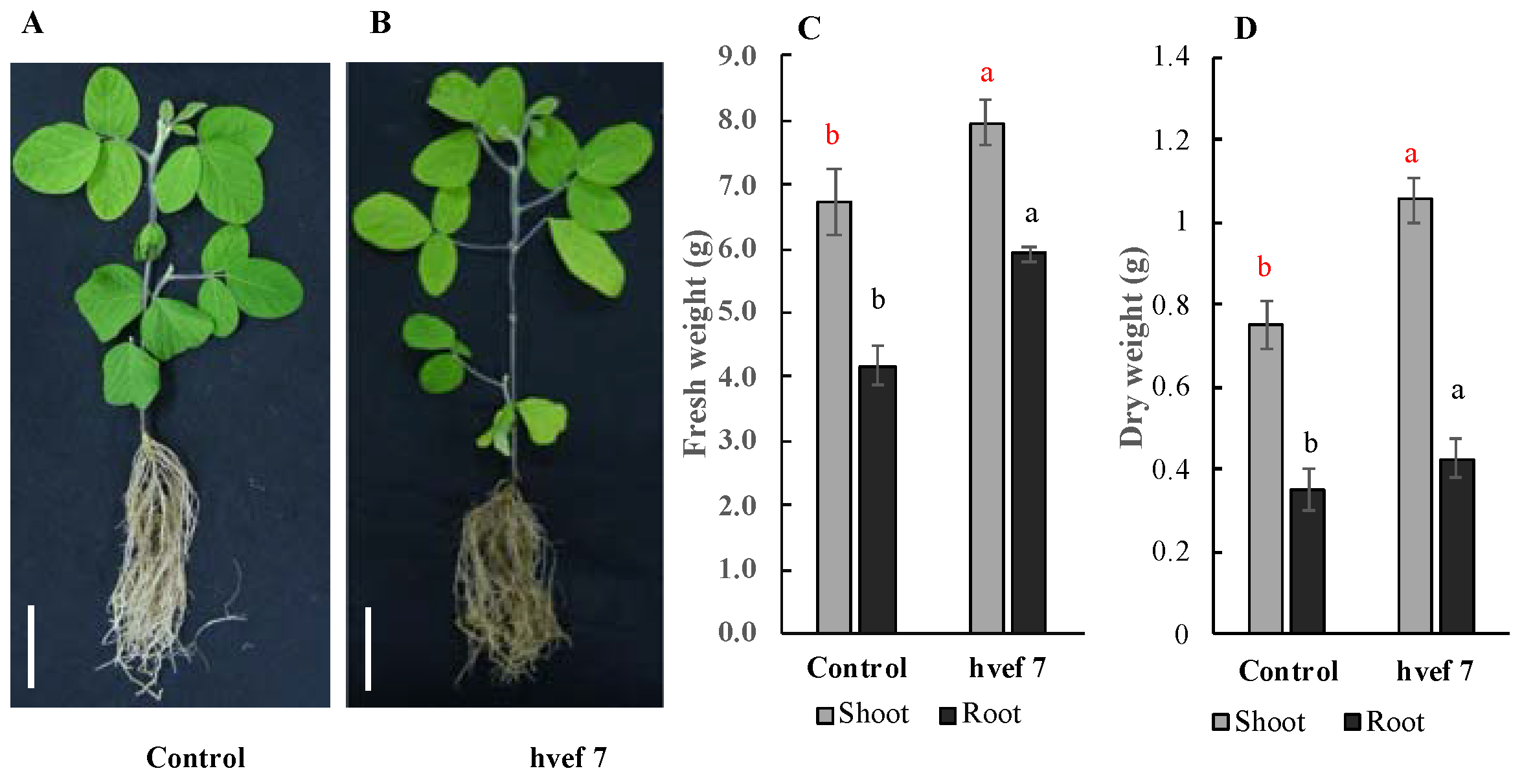
2.6. Plant Growth Promotion Potential of Endophytic Fungi
2.6.1. Phosphate and Potassium Solubilization Activity
2.6.2. Estimation of Siderophore Production
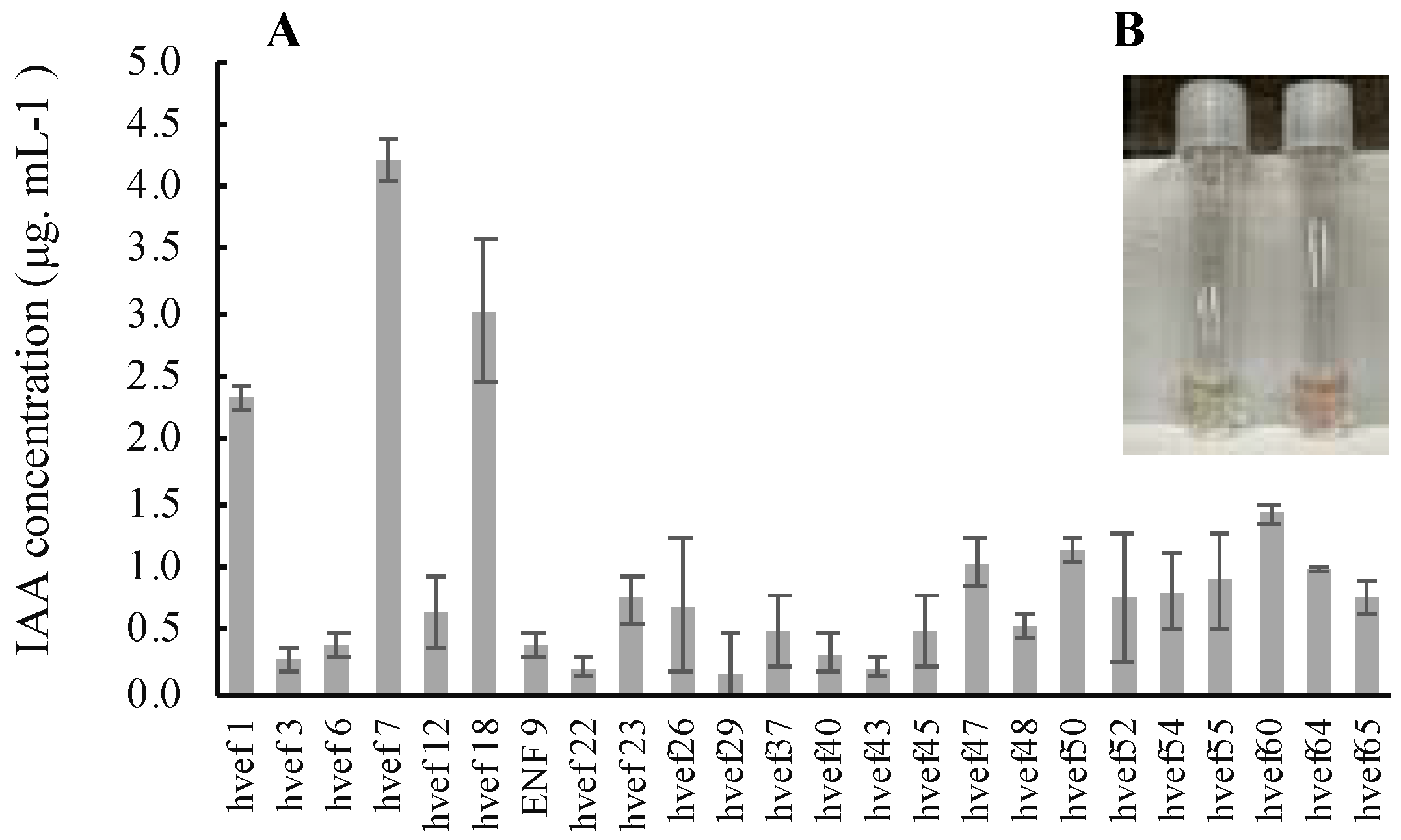
2.6.3. Qualitative and Quantitative Evaluation of Indole-3-Acetic Acid (IAA) Production
2.7. In Vitro Antagonistic Potential against Phytopathogenic Fungi
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Endophytic Fungi
3.2. Variation in Endophyte Community Composition and Assembly
3.3. Plant-Growth-Promoting Traits of Isolated Endophytes
3.3.1. Phosphate and Potassium Solubilization Activity
3.3.2. Siderophore Production Potential of Isolated Fungi
3.3.3. Qualitative and Quantitative Evaluation of Indole-3-Acetic Acid (IAA) Production
3.4. Antagonistic Potential of Fungal Isolates against Red Crown Rot (Calonectria ilicicola)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Baweja, P.; Kumar, S.; Kumar, G. Fertilizers and pesticides: Their impact on soil health and environment. In Soil Health; Giri, B., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 265–285. ISBN 978-3-030-44364-1. [Google Scholar]
- Raza, H.; Idress, M.; Yar, G.; Farah, N.; Asim, M.; Naveed, M.; Yasin, M.; Younus, M. Residual impact of pesticides on environment and health of sugarcane farmers in punjab with special reference to integrated pest management. J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 79–84. [Google Scholar] [CrossRef]
- Javanmard, A.; Amani Machiani, M.; Lithourgidis, A.; Morshedloo, M.R.; Ostadi, A. Intercropping of maize with legumes: A cleaner strategy for improving the quantity and quality of forage. Clean. Eng. Technol. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; Duan, Y.; Zhang, J.; Evers, J.B.; Zhang, Y.; Su, Z.; van der Werf, W. Intercropping Potato (Solanum tuberosum L.) with Hairy Vetch (Vicia villosa) increases water use efficiency in dry conditions. Field Crops Res. 2019, 240, 168–176. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? a meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Habibi, D.; Kashani, A.; Paknejad, F.; Jafary, H.; Al-Ahmadi, M.J.; Tookalloo, M.R.; Lamei, J. Evaluation of hairy vetch (Vicia villosa Roth) in pure and mixed cropping with barley (Hordeum vulgare l.) to determine the best combination of legume and cereal for forage production. Am. J. Agric. Biol. Sci. 2010, 5, 169–176. [Google Scholar]
- Sainju, U.M.; Singh, H.P.; Singh, B.P. Cover crop effects on soil carbon and nitrogen under bioenergy sorghum crops. J. Soil Water Conserv. 2015, 70, 410–417. [Google Scholar] [CrossRef]
- Omae, H.; Nagumo, F. Effects of Oat (Avena sativa) and Hairy Vetch (Vicia villosa) cover crops on nitrate leaching, soil water, and maize yield in subtropical islands in Japan. J. Agric. Sci. (Tor.) 2016, 8, 44–54. [Google Scholar] [CrossRef]
- Sato, T.; Sato, E.; Takakai, F.; Yokoyama, T.; Kaneta, Y. Effects of hairy vetch foliage application on nodulation and nitrogen fixation in soybean cultivated in three soil types. Soil Sci. Plant Nutr. 2011, 57, 313–319. [Google Scholar] [CrossRef]
- Bansal, S.; Yin, X.; Schneider, L.; Sykes, V.; Jagadamma, S.; Lee, J. Carbon footprint and net carbon gain of major long-term cropping systems under no-tillage. J. Environ. Manag. 2022, 307, 114505. [Google Scholar] [CrossRef] [PubMed]
- Roig, C.J. Performance of Upland Cotton Under a Hairy Vetch Regiment from a Crop Insurance Perspective. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2021. [Google Scholar]
- Hartwig, N.L.; Ammon, H.U. Cover crops and living mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Anugroho, F.; Kitou, M.; Nagumo, F.; Kinjo, K.; Tokashiki, Y. Growth, nitrogen fixation, and nutrient uptake of hairy vetch as a cover crop in a subtropical region. Weed Biol. Manag. 2009, 9, 63–71. [Google Scholar] [CrossRef]
- Seo, J.H.; Lee, H.J. Mineral nitrogen effects of hairy vetch (Vicia villosa Roth) on maize (Zea mays l.) by green manure amounts. J. Agron. 2008, 7, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.G.; Everts, K.L. Suppression of Fusarium wilt of watermelon by soil amendment with hairy vetch. Plant Dis. 2004, 88, 1357–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candole, B.L.; Rothrock, C.S. Characterization of the suppressiveness of hairy vetch-amended soils to Thielaviopsis basicola. Phytopathology 1997, 87, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamo, T.; Sakurai, S.; Yamanashi, T.; Todoroki, Y. Cyanamide is biosynthesized from l-canavanine in plants. Sci. Rep. 2015, 5, 10527. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, G.R. Weed control in irrigated corn by hairy vetch interseeded at different rates and times. Weed Biol. Manag. 2010, 10, 25–32. [Google Scholar] [CrossRef]
- Abdin, O.A.; Zhou, X.M.; Cloutier, D.; Coulman, D.C.; Faris, M.A.; Smith, D.L. Cover crops and interrow tillage for weed control in short season maize (Zea Mays). Eur. J. Agron. 2000, 12, 93–102. [Google Scholar] [CrossRef]
- Mardani-Korrani, H.; Nakayasu, M.; Yamazaki, S.; Aoki, Y.; Kaida, R.; Motobayashi, T.; Kobayashi, M.; Ohkama-Ohtsu, N.; Oikawa, Y.; Sugiyama, A.; et al. L-canavanine, a root exudate from hairy vetch (Vicia villosa) drastically affecting the soil microbial community and metabolite pathways. Front. Microbiol. 2021, 12, 2754. [Google Scholar] [CrossRef]
- Sakurai, N.; Mardani-Korrani, H.; Nakayasu, M.; Matsuda, K.; Ochiai, K.; Kobayashi, M.; Tahara, Y.; Onodera, T.; Aoki, Y.; Motobayashi, T.; et al. Metabolome analysis identified okaramines in the soybean rhizosphere as a legacy of hairy vetch. Front. Genet. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Takiuchi, K.; Murao, S.; Arai, M. Structure and insecticidal activity of new indole alkaloids, okaramines a and b, from Penicillium simplicissimum ak-40. Agric. Biol. Chem. 1989, 53, 461–469. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [Green Version]
- López, S.; Pastorino, G.; Franco, M.; Medina, R.; Lucentini, C.; Saparrat, M.; Balatti, P. Microbial endophytes that live within the seeds of two tomato hybrids cultivated in Argentina. Agronomy 2018, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-Y.; Yang, J.H.; Woo, J.-J.; Oh, S.-O.; Hur, J.-S. Diversity and distribution patterns of endolichenic fungi in jeju island, South Korea. Sustainability 2020, 12, 3769. [Google Scholar] [CrossRef]
- Mishra, S.; Bhattacharjee, A.; Sharma, S. An Ecological insight into the multifaceted world of plant-endophyte association. Crit. Rev. Plant Sci. 2021, 40, 127–146. [Google Scholar] [CrossRef]
- Nelson, J.; Shaw, A.J. Exploring the natural microbiome of the model liverwort: Fungal endophyte diversity in Marchantia polymorpha L. Symbiosis 2019, 78, 45–59. [Google Scholar] [CrossRef]
- Gibert, A.; Tozer, W.; Westoby, M. Plant performance response to eight different types of symbiosis. New Phytol. 2019, 222, 526–542. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in Arabidopsis. Curr. Opin. Microbiol. 2016, 32, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, T.R.; Doohan, F.M.; Saunders, M.J.; Murphy, B.R. Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019; ISBN 978-1-108-47176-3. [Google Scholar]
- Wolfe, E.R.; Ballhorn, D.J. Do foliar endophytes matter in litter decomposition. Microorganisms 2020, 8, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.M.A.; Hassan, S.E.-D.; Alsharif, S.M.; Eid, A.M.; Ewais, E.E.-D.; Azab, E.; Gobouri, A.A.; Elkelish, A.; Fouda, A. Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules 2021, 11, 140. [Google Scholar] [CrossRef]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; et al. Endophyte-promoted phosphorus solubilization in populus. Front. Plant Sci. 2020, 11, 567918. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; del-Val, E.; Macías-Rodríguez, L.; Alarcón, A.; González-Esquivel, C.E.; Larsen, J. Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis Son. Soil Biol. Biochem. 2018, 122, 196–202. [Google Scholar] [CrossRef]
- Timmusk, S.; Behers, L.; Muthoni, J.; Muraya, A.; Aronsson, A.-C. Perspectives and challenges of microbial application for crop improvement. Front. Plant Sci. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getachew, G.; Rewald, B.; Godbold, D.L.; Sandén, H. Endophytic fungal root colonization of Eragrostis tef in eroded croplands of the ethiopian highlands is limited by low spore density and fertilisation. Agronomy 2019, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.M.; Hallaksela, A.-M. Diversity of norway spruce needle endophytes in various mixed and pure norway spruce stands. Mycol. Res. 1998, 102, 1183–1189. [Google Scholar] [CrossRef]
- Paul, N.B.; Sundara Rao, W.V.B. Phosphate-dissolving bacteria in the rhizosphere of some cultivated legumes. Plant Soil 1971, 35, 127–132. [Google Scholar] [CrossRef]
- Aleksandrov, V.G.; Blagodyr’, R.N.; Il’ev, I.P. Phosphorus acid isolation from apatite produced by silicate bacteria. Mikrobiolohichnyi Zhurnal 1967, 29, 111–114. [Google Scholar] [PubMed]
- Milagres, A.M.F.; Machuca, A.; Napoleão, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol s (CAS) agar plate Assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Osman, Y.; Gebreil, A.; Mowafy, A.M.; Anan, T.I.; Hamed, S.M. Characterization of Aspergillus niger siderophore that mediates bioleaching of rare earth elements from phosphorites. World J. Microbiol. Biotechnol. 2019, 35, 93. [Google Scholar] [CrossRef]
- Kejela, T.; Thakkar, V.R.; Patel, R.R. Novel strain of Pseudomonas inhibits Colletotrichum gloeosporioides and Fusarium oxysporum infections and promotes germination of coffee. Rhizosphere 2017, 4, 9–15. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanile, G.; Ruscelli, A.; Luisi, N. Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta Tests. Eur. J. Plant Pathol. 2007, 117, 237–246. [Google Scholar] [CrossRef]
- Landum, M.; Felix, M.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F.; Varanda, C.M. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, B.S.; Shashikala, J.; Krishnamurthy, Y.L. Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiol. Res. 2009, 164, 290–296. [Google Scholar] [CrossRef]
- Li, J.-L.; Sun, X.; Zheng, Y.; Lü, P.-P.; Wang, Y.-L.; Guo, L.-D. Diversity and community of culturable endophytic fungi from stems and roots of desert halophytes in Northwest China. MycoKeys 2020, 62, 75–95. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Wylie, S.J. Host specificity of endophytic mycobiota of wild Nicotiana plants from arid regions of northern Australia. Microb. Ecol. 2018, 75, 74–87. [Google Scholar] [CrossRef]
- Shreelalitha, S.J.; Sridhar, K.R. Endophytic fungi of wild legume Sesbania bispinosa in coastal sand dunes and mangroves of the southwest coast of India. J. For. Res. 2015, 26, 1003–1011. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.-Q.; Liu, H.-Y.; Wei, Y.-Z.; Li, H.-L.; Su, J.; Zhao, L.-X.; Yu, L.-Y. Diversity and cold adaptation of culturable endophytic fungi from Bryophytes in the fildes region, King george island, maritime antarctica. FEMS Microbiol. Lett. 2013, 341, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Qiao, M.; Xu, J.; Yu, Z. Culture-based and culture-independent assessments of endophytic fungal diversity in aquatic plants in southwest China. Front. Fungal. Biol. 2013, 341, 52–61. [Google Scholar] [CrossRef]
- Tibpromma, S.; Karunarathna, S.; Bhat, D.J.; Suwannarach, N.; Stephenson, S.; Elgorban, A.; Al-Rejaie, S.; Xu, J.; Mortimer, P. Using culture-dependent and molecular techniques to identify endophytic fungi associated with tea leaves (Camellia spp.) in yunnan province, China. Diversity 2022, 14, 287. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Toghueo, R.M.K.; Boyom, F.F. Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications. 3 Biotech 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Patle, P.; Navnage, N.; Ramteke, P. Endophytes in plant system: Roles in growth promotion, mechanism, and their potentiality in achieving agriculture sustainability. Int. J. Chem. Stud. 2018, 6, 270–274. [Google Scholar]
- Muthuraja, R.; Muthukumar, T. Isolation and characterization of potassium solubilizing Aspergillus species isolated from saxum habitats and their effect on maize growth in different soil types. Geomicrobiol. J. 2021, 38, 672–685. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, X.-R.; Wu, X.-Q.; Li, G.-E.; Wang, Z.; Li, D.-W. The phosphate-solubilizing ability of Penicillium guanacastense and its effects on the growth of Pinus massoniana in phosphate-limiting conditions. Biol. Open 2019, 8, bio046797. [Google Scholar] [CrossRef] [Green Version]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore – a boon to agricultural sciences. Biol. Control. 2020, 144, 104214. [Google Scholar] [CrossRef]
- Joo, K.A.H. and J.H. Zinc ions affect siderophore production by fungi isolated from the Panax ginseng rhizosphere. J. Microbiol. Biotechnol. 2019, 29, 105–113. [Google Scholar] [CrossRef]
- Tamariz-Angeles, C.; Huamán, G.D.; Palacios-Robles, E.; Olivera-Gonzales, P.; Castañeda-Barreto, A. Characterization of Siderophore-producing microorganisms associated to plants from high-andean heavy metal polluted soil from Callejón de Huaylas (Ancash, Perú). Microbiol. Res. 2021, 250, 126811. [Google Scholar] [CrossRef]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Oide, S.; Moeder, W.; Krasnoff, S.; Gibson, D.; Haas, H.; Yoshioka, K.; Turgeon, B.G. NPS6, Encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic Ascomycetes. Plant Cell 2006, 18, 2836–2853. [Google Scholar] [CrossRef] [PubMed]
- Franken, A.C.W.; Lechner, B.E.; Werner, E.R.; Haas, H.; Lokman, B.C.; Ram, A.F.J.; van den Hondel, C.a.M.J.J.; de Weert, S.; Punt, P.J. Genome mining and functional genomics for siderophore production in Aspergillus niger. Brief. Funct. Genom. 2014, 13, 482. [Google Scholar] [CrossRef] [Green Version]
- Baakza, A.; Vala, A.K.; Dave, B.; Dube, H. A comparative study of siderophore production by fungi from marine and terrestrial habitats. J. Exp. Mar. Biol. Ecol. 2004, 311, 1–9. [Google Scholar] [CrossRef]
- Wright, W.; Little, J.; Liu, F.; Chakraborty, R. Isolation, and structural identification of the trihydroxamate siderophore vicibactin and its degradative products from rhizobium leguminosarum ATCC 14479 Bv. Trifolii. Biometals 2013, 26, 271–283. [Google Scholar] [CrossRef]
- Woodward, A.W. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.-M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. isolate as fungal plant growth promoting agent. Agronomy 2021, 11, 392. [Google Scholar] [CrossRef]
- Hirota, A.; Sakai, H.; Isogai, A. New Plant Growth Regulators, Cladospolide A and B, Macrolides Produced by Cladosporium cladosporioides. Agric Biol Chem. 1985, 49, 731–735. [Google Scholar] [CrossRef]
- Liang, L.-J.; Jeewon, R.; Dhandevi, P.; Durairajan, S.S.K.; Li, H.; Lin, F.-C.; Wang, H.-K. A novel species of Penicillium with inhibitory effects against Pyricularia oryzae and fungal pathogens inducing citrus diseases. Front. Cell Infect. Microbiol. 2021, 10, 604504. [Google Scholar] [CrossRef]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]

| Isolate No. | Isolates Acc. No. | Class | Closest Match (Genbank) | Query Coverage (%) | Tissue |
|---|---|---|---|---|---|
| hvef1 | MK036245 | Dothideomycetes | Cladosporium pseudocladosporioides (MK111597.1) | 99.8 | R, S |
| hvef3 | MK036247 | Eurotiomycetes | Penicillium sp. (LC133788.1) | 100 | L, S, R |
| hvef6 | MK036250 | Eurotiomycetes | Penicillium brefeldianum (MH858155.1) | 100 | L, S, R |
| hvef7 | MK036251 | Dothideomycetes | Cladosporium cladosporioides (MG669180.1) | 96.0 | R |
| hvef9 | MK036253 | Eurotiomycetes | Penicillium ochrochloron (MH137639.1) | 99.0 | L, S, R |
| hvef10 | MK036254 | Eurotiomycetes | Penicillium glaucoroseum (MH865551.1) | 100 | L, S, R |
| hvef12 | MK036256 | Agaricomycetes | Trametes versicolor (MK322281.1) | 100 | L, S |
| hvef18 | MK036262 | Eurotiomycetes | Penicillium simplicissimum (MH856014.1) | 100 | L, S, R |
| hvef22 | MK036266 | Eurotiomycetes | Penicillium cremeogriseum (MH374608.1) | 100 | L, S, R |
| hvef23 | MK036267 | Eurotiomycetes | Penicillium sp. (MK625191.1) | 100 | L, S, R |
| hvef26 | MK036270 | Dothideomycetes | Cladosporium halotolerans (MK265717.1) | 100 | L, S, R |
| hvef29 | MK036273 | Eurotiomycetes | Aspergillus flavus (MK791661.1) | 100 | R, L |
| hvef30 | MK036274 | Eurotiomycetes | Aspergillus sp. (MK817589.1) | 100 | L, S, R |
| hvef31 | MK036275 | Dothideomycetes | Cladosporium sphaerospermum (MH482916.1) | 100 | R, S |
| hvef32 | MK036276 | Sordariomycetes | Eutypella sp. (KX828160.1) | 99.0 | L, S, R |
| hvef37 | MK036281 | Sordariomycetes | Chordomyces antarcticum (KX385856.1) | 100 | L, S, R |
| hvef40 | MK036284 | Eurotiomycetes | Penicillium steckii (MH484016.1) | 99.0 | S, R |
| hvef41 | MK036285 | Eurotiomycetes | Penicillium expansum (KC456325.1) | 100 | L, S, R |
| hvef43 | MK036287 | Eurotiomycetes | Aspergillus westerdijkiae (MG733714.1) | 100 | L, S, R |
| hvef44 | MK036288 | Eurotiomycetes | Aspergillus sydowii (KX898426.1) | 99.0 | L, S, R |
| hvef45 | MK036289 | Eurotiomycetes | Penicillium italicum (DQ991463.1) | 100 | L, S, R |
| hvef46 | MK036290 | Eurotiomycetes | Penicillium svalbardense (KC346348.1) | 93.0 | L, S, R |
| hvef47 | MK036291 | Eurotiomycetes | Penicillium sp. (KY350140.1) | 100 | L, S, R |
| hvef48 | MK036292 | Agaricomycetes | Tricholoma matsutake (MF037418.1) | 100 | R |
| hvef50 | MK036294 | Eurotiomycetes | Penicillium sp. (MG551581.1) | 96.0 | L, S, R |
| hvef52 | MK036296 | Eurotiomycetes | Penicillium sp. (MH325925.1) | 98.0 | L, S, R |
| hvef54 | MK036298 | Eurotiomycetes | Penicillium sp. (MH550491.1) | 94.0 | L, S, R |
| hvef55 | MK036299 | Eurotiomycetes | Penicillium griseopurpureum (KY678777.1) | 100 | L, S, R |
| hvef58 | MK036302 | Eurotiomycetes | Penicillium sp. (KJ921867.1) | 99.0 | L, R |
| hvef60 | MK036304 | Eurotiomycetes | Penicillium oxalicum (KF152942.1) | 94.0 | L, S, R |
| hvef63 | MK036307 | Eurotiomycetes | Penicillium sp. (KY425713.1) | 97.0 | L, S, R |
| hvef64 | MK036308 | Eurotiomycetes | Penicillium crustosum (KT735107.1) | 96.0 | R |
| hvef65 | MK036309 | Eurotiomycetes | Penicillium sp. (KT369826.1) | 98.0 | L, S, R |
| No | Fugal Strain | Phosphate Solubilization Index | Potassium Solubilization Index | % Siderophore Unit |
|---|---|---|---|---|
| 1 | hvef1 | 0 | 0 | 0 |
| 2 | hvef3 | 0 | 0 | 65.4 ± 0.13 |
| 3 | hvef6 | 0 | 0 | 0 |
| 4 | hvef7 | 1.36 ± 0.01 | 0 | 0 |
| 5 | hvef9 | 0 | 0 | 0 |
| 6 | hvef10 | 0 | 0 | 0 |
| 7 | hvef12 | 0 | 0 | 62.0 ± 0.03 |
| 8 | hvef18 | 1.13 ± 0.06 | 0 | 55.3 ± 0.46 |
| 9 | hvef22 | 0 | 0 | 0 |
| 10 | hvef23 | 0 | 0 | 60.7 ± 0.07 |
| 11 | hvef26 | 0 | 0 | 60.1 ± 0.02 |
| 12 | hvef29 | 1.13 ± 0.03 | 0 | 74.7 ± 0.16 |
| 13 | hvef30 | 0 | 0 | 63.0 ± 0.25 |
| 14 | hvef31 | 0 | 0 | 0 |
| 15 | hvef32 | 0 | 0 | 64.7 ± 0.23 |
| 16 | hvef37 | 0 | 3.53 ± 0.11 | 69.4 ± 0.24 |
| 17 | hvef40 | 0 | 0 | 61.1 ± 0.04 |
| 18 | hvef41 | 0 | 15.9 ± 0.11 | 61.0 ± 0.05 |
| 19 | hvef43 | 0 | 12.6 ± 0.20 | 63.1 ± 0.25 |
| 20 | hvef44 | 0 | 0 | 57.5 ± 0.03 |
| 21 | hvef45 | 1.14 ± 0.04 | 15.1 ± 0.23 | 0 |
| 22 | hvef46 | 0 | 11.1 ± 0.11 | 0 |
| 23 | hvef47 | 0 | 4.73 ± 0.25 | 0 |
| 24 | hvef48 | 0 | 0 | 56.7 ± 0.05 |
| 25 | hvef50 | 0 | 4.10 ± 0.10 | 54.7 ± 0.05 |
| 26 | hvef52 | 0 | 0 | 0 |
| 27 | hvef54 | 0 | 0 | 54.3 ± 0.08 |
| 28 | hvef55 | 0 | 0 | 56.3 ± 0.01 |
| 29 | hvef58 | 0 | 3.33 ± 0.30 | 58.9 ± 0.17 |
| 30 | hvef60 | 1.30 ± 0.02 | 10.8 ± 0.20 | 61.7 ± 0.24 |
| 31 | hvef63 | 0 | 14.6 ± 0.20 | 0 |
| 32 | hvef64 | 0 | 0 | 0 |
| 33 | hvef65 | 0 | 0 | 0 |
| No | Fungal Isolates Code | Growth Inhibition Rates (%) | No | Fungal Isolates CODE | Growth Inhibition Rates (%) |
|---|---|---|---|---|---|
| 1 | hvef1 | 16.0 ± 0.25 | 18 | hvef41 | 49.7 ± 0.29 |
| 2 | hvef3 | 95.2 ± 0.06 | 19 | hvef43 | 93.5 ± 0.13 |
| 3 | hvef6 | 89.3 ± 0.17 | 20 | hvef44 | 56.2 ± 0.15 |
| 4 | hvef7 | 62.7 ± 0.26 | 21 | hvef45 | 1.14 ± 0.04 |
| 5 | hvef9 | 92.9 ± 0.01 | 22 | hvef46 | 43.2 ± 0.20 |
| 6 | hvef10 | 67.5 ± 0.29 | 23 | hvef47 | 92.9 ± 0.10 |
| 7 | hvef12 | 59.2± 0.20 | 24 | hvef48 | 83.4 ± 0.12 |
| 8 | hvef18 | 63.3 ± 0.21 | 25 | hvef50 | 81.1 ± 0.10 |
| 9 | hvef22 | 80.5 ± 0.17 | 26 | hvef52 | 67.5 ± 0.22 |
| 10 | hvef23 | 60.4 ± 0.25 | 27 | hvef54 | 48.5 ± 0.17 |
| 11 | hvef26 | 60.3 ± 0.12 | 28 | hvef55 | 69.8 ± 0.21 |
| 12 | hvef29 | 95.9 ± 0.06 | 29 | hvef58 | 73.4 ± 0.06 |
| 13 | hvef30 | 87.0 ± 0.25 | 30 | hvef60 | 55.6 ± 0.50 |
| 14 | hvef31 | 76.3 ± 0.15 | 31 | hvef63 | 88.7 ± 0.23 |
| 15 | hvef32 | 54.4 ± 0.21 | 32 | hvef64 | 67.5 ± 0.29 |
| 16 | hvef37 | 67.5 ± 0.76 | 33 | hvef65 | 82.2 ± 0.20 |
| 17 | hvef40 | 75.7 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taheri, P.; Kaida, R.; Dastogeer, K.M.G.; Appiah, K.S.; Yasuda, M.; Tanaka, K.; Mardani Korrani, H.; Azizi, M.; Okazaki, S.; Fujii, Y. Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth. Agronomy 2022, 12, 2417. https://doi.org/10.3390/agronomy12102417
Taheri P, Kaida R, Dastogeer KMG, Appiah KS, Yasuda M, Tanaka K, Mardani Korrani H, Azizi M, Okazaki S, Fujii Y. Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth. Agronomy. 2022; 12(10):2417. https://doi.org/10.3390/agronomy12102417
Chicago/Turabian StyleTaheri, Parisa, Rumi Kaida, Khondoker M. G. Dastogeer, Kwame Sarpong Appiah, Michiko Yasuda, Keisuke Tanaka, Hossein Mardani Korrani, Majid Azizi, Shin Okazaki, and Yoshiharu Fujii. 2022. "Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth" Agronomy 12, no. 10: 2417. https://doi.org/10.3390/agronomy12102417
APA StyleTaheri, P., Kaida, R., Dastogeer, K. M. G., Appiah, K. S., Yasuda, M., Tanaka, K., Mardani Korrani, H., Azizi, M., Okazaki, S., & Fujii, Y. (2022). Isolation and Functional Characterization of Culture-Dependent Endophytes Associated with Vicia villosa Roth. Agronomy, 12(10), 2417. https://doi.org/10.3390/agronomy12102417








