Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soils and Amendment
2.2. Treatment Plan and Experimental Set-Up
2.3. Data Collection
2.3.1. Plant Analysis
2.3.2. Enzymes Activity
2.3.3. Plant PTE Contents
2.3.4. Soil Analysis
2.3.5. Human Health Risk Assessment
2.3.6. Translocation Factor
2.3.7. Remediation Factor
2.4. Statistical Analysis
3. Results
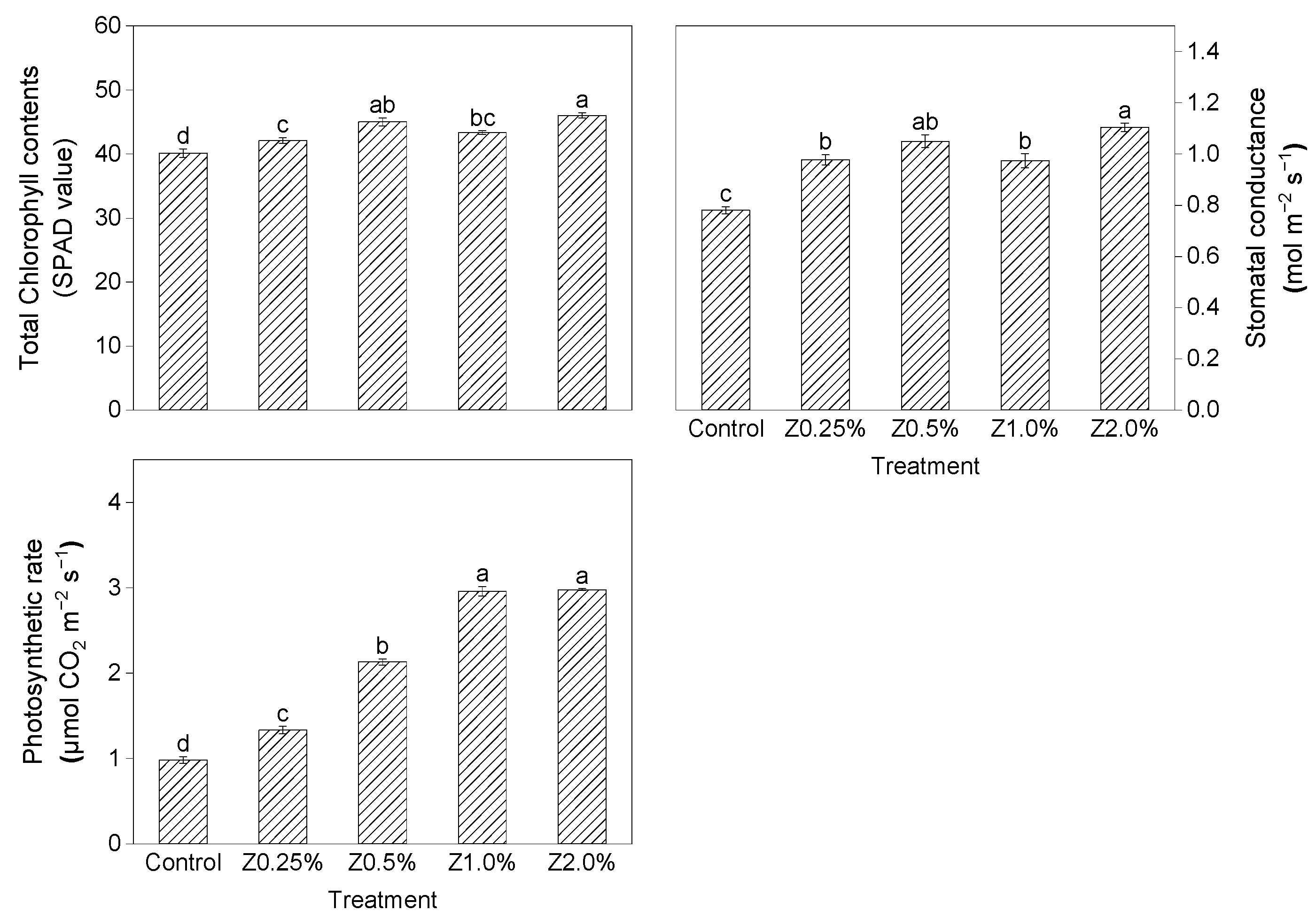
3.1. Physiological Parameters
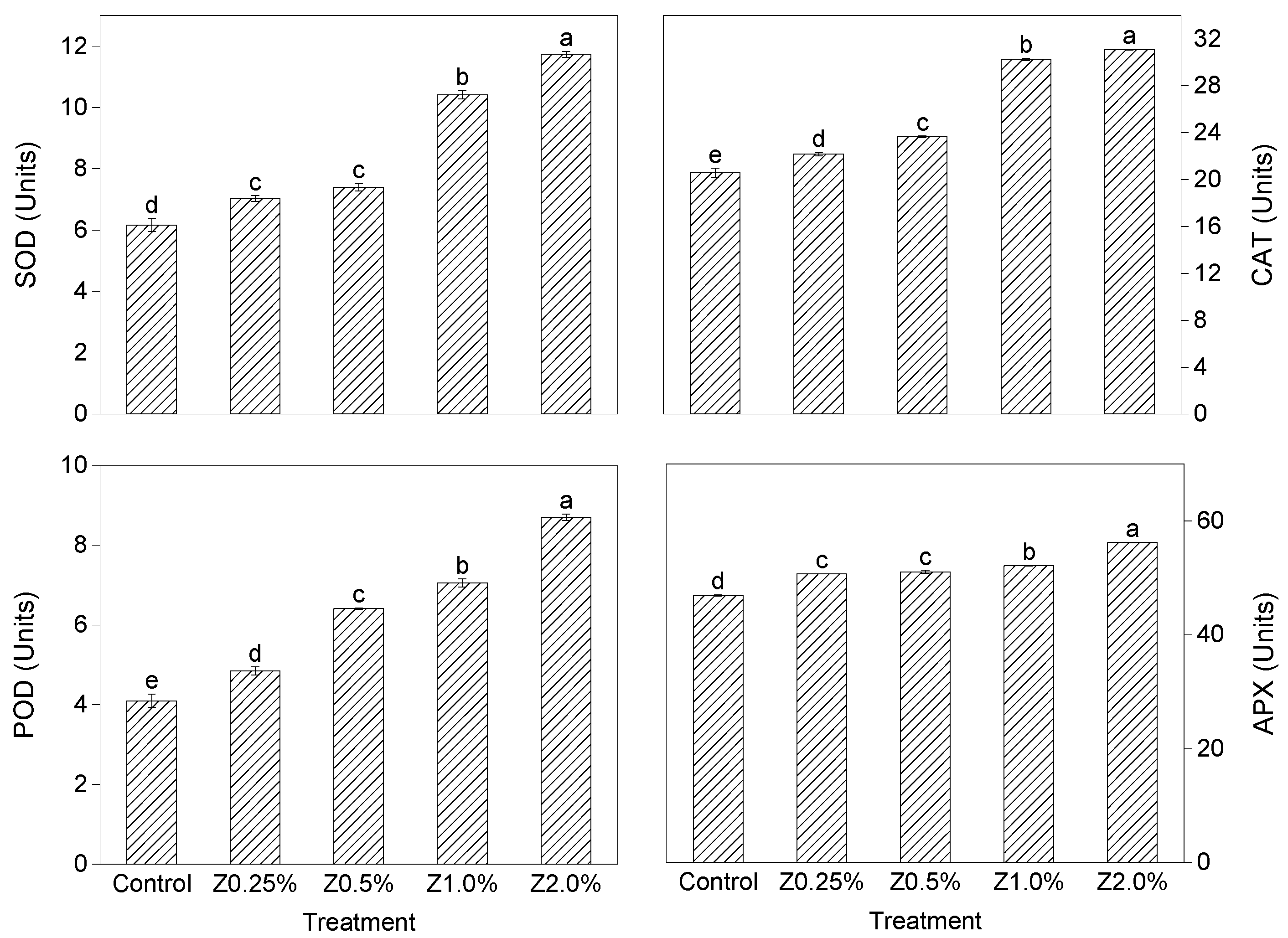
3.2. Fruit Enzymatic Activity
3.3. Growth Parameters of Plants
3.4. Post-Harvest Soil Characteristics
3.5. Plant Tissue PTE Concentrations
3.6. Human Health Risk Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonel, L.P.; Tonetti, A.L. Wastewater reuse for crop irrigation: Crop yield, soil and human health implications based on giardiasis epidemiology. Sci. Total Environ. 2021, 775, 145833. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T. Metal Pollution in the Aquatic Environment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Adimalla, N.; Chen, J.; Qian, H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: A case study from an urban region of South India. Ecotoxicol. Environ. Saf. 2020, 194, 110406. [Google Scholar] [CrossRef]
- Sarim, M.; Jan, T.; Khattak, S.A.; Mihoub, A.; Jamal, A.; Saeed, M.F.; Radicetti, E. Assessment of the Ecological and Health Risks of Potentially Toxic Metals in Agricultural Soils from the Drosh-Shishi Valley, Pakistan. Land 2022, 11, 1663. [Google Scholar] [CrossRef]
- Gill, S.S.; Mehmood, M.A.; Rashid, U.; Ibrahim, M.; Saqib ATabassum, M.R. Waste-water treatment coupled with biodiesel production using microalgae: A bio-refinery approach. Pak. J. Life Soc. Sci. 2013, 11, 179–189. [Google Scholar]
- Ul-Allah, S.; Khan, A.A.; Burkert, A.; Wachendorf, M. Socio-economic aspects of fodder production in urban and peri-urban areas of Faisalabad. Pak. J. Agric. Sci. 2014, 51, 483–490. [Google Scholar]
- Bhagat, S.K.; Tung, T.M.; Yaseen, Z.M. Development of artificial intelligence for modeling wastewater heavy metal removal: State of the art, application assessment and possible future research. J. Clean. Prod. 2020, 250, 119473. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. In Natural Resources Forum; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef] [Green Version]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Hsien, K.J.; Pascua, C.; Wan, M.W. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 528–536. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Boateng, A.A.; Qi, P.X.; Lima, I.M.; Chang, J. Heavy metal and phenol adsorptive properties of biochars from pyrolyzed switchgrass and woody biomass in correlation with surface properties. J. Environ. Manag. 2013, 118, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef] [PubMed]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Apori, O.; Hanyabui, E.; Asiamah, Y. Remediation technology for copper contaminated soil: A review. Asian Soil Res. J. 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gao, X.; Li, J.; Zuo, R.; Wang, J.; Song, L.; Wang, G. The stabilization process in the remediation of vanadium-contaminated soil by attapulgite, zeolite and hydroxyapatite. Ecol. Eng. 2020, 156, 105975. [Google Scholar] [CrossRef]
- Sparks, D.L. Kinetics of soil chemical processes: Past progress and future needs. Future Dev. Soil Sci. Res. 1978, 1, 61–73. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Baran, T.; Baran, N.Y.; Sajjadi, M.; Tahsili, M.R.; Shokouhimehr, M. Pd nanocatalyst stabilized on amine-modified zeolite: Antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Sep. Purif. Technol. 2020, 239, 116542. [Google Scholar] [CrossRef]
- Lahori, A.H.; Mierzwa-Hersztek, M.; Demiraj, E.; Sajjad, R.U.; Ali, I.; Shehnaz, H.; Zhang, Z. Direct and residual impacts of zeolite on the remediation of harmful elements in multiple contaminated soils using cabbage in rotation with corn. Chemosphere 2020, 250, 126317. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Inglezakis, V.J.; Moustakas, K.G.; Malamis, S.P.; Loizidou, M.D. Removal of Cu (II) in fixed bed and batch reactors using natural zeolite and exfoliated vermiculite as adsorbents. Desalination 2007, 215, 133–142. [Google Scholar] [CrossRef]
- Salmasi, R.; Tavassoli, A. Using different amendments to reduce heavy metals movement in soils. Int. J. Environ. Sci. Technol. 2005, 1, 295–300. [Google Scholar] [CrossRef]
- Morales, M.; Nava, V.; Velásquez, E.; Razo-Flores, E.; Revah, S. Mineralization of methyl tert-butyl ether and other gasoline oxygenates by Pseudomonads using short n-alkanes as growth source. Biodegradation 2009, 20, 271–280. [Google Scholar] [CrossRef]
- Chaturvedi, P.K.; Seth, C.S.; Misra, V. Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 2006, 64, 1109–1114. [Google Scholar] [CrossRef]
- Lahori, A.H.; Zhang, Z.; Shaheen, S.M.; Rinklebe, J.; Guo, Z.; Li, R.; Jing, R. Mono-and co-applications of Ca-bentonite with zeolite, Ca-hydroxide, and tobacco biochar affect phytoavailability and uptake of copper and lead in a gold mine-polluted soil. J. Hazard. Mater. 2019, 374, 401–411. [Google Scholar] [CrossRef]
- Hussain, K.; Khan, S.H.; Afroza, B.; Zehra, S.B.; Dar, Z.A.; Mushtaq, F.; Nazir, G. Graphical analysis for yield and yield attributing traits in brinjal (Solanum melongena L.). J. Pharmacogn. Phytochem. 2018, 7, 367–370. [Google Scholar]
- Karibasava, N.; Sreenivasulu, G.B.; Prashanth, S.J.; Jayaprakashnarayan, R.P.; Madalageri, M.B.; Ravindra, M. Studies on genetic variability and its importance in brinjal (Solanum melongena L.). Asian J. Hortic. 2009, 4, 380–382. [Google Scholar]
- Ahmad, I.; Malik, S.A.; Saeed, S.; Rehman, A.U.; Munir, T.M. Phytoextraction of Heavy Metals by Various Vegetable Crops Cultivated on Different Textured Soils Irrigated with City Wastewater. Soil Syst. 2021, 5, 35. [Google Scholar] [CrossRef]
- Marijan, M.; Jablan, J.; Jakupović, L.; Jug, M.; Marguí, E.; Dalipi, R.; Zovko Končić, M. Plants from Urban Parks as Valuable Cosmetic Ingredients: Green Extraction, Chemical Composition and Activity. Agronomy 2022, 12, 204. [Google Scholar] [CrossRef]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3 Chemical Methods; Wiley: Hoboken, NJ, USA, 1996; Volume 5, pp. 417–435. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. The hydrometer as a new method for the mechanical analysis of soils. Soil Sci. 1927, 23, 343–354. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Omeje, K.O.; Ezema, B.O.; Okonkwo, F.; Onyishi, N.C.; Ozioko, J.; Rasaq, W.A.; Okpala, C.O.R. Quantification of Heavy Metals and Pesticide Residues in Widely Consumed Nigerian Food Crops Using Atomic Absorption Spectroscopy (AAS) and Gas Chromatography (GC). Toxins 2021, 13, 870. [Google Scholar] [CrossRef]
- Giannospolitis, C.; Ries, S. Superoxide dismutase. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar] [CrossRef]
- Soltanpour, P.; Schwab, A. A new soil test for simultaneous extraction of macro-and micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Aguilar, M.; Mondaca, P.; Ginocchio, R.; Vidal, K.; Sauvé, S.; Neaman, A. Comparison of exposure to trace elements through vegetable consumption between a mining area and an agricultural area in central Chile. Environ. Sci. Pollut. Res. 2018, 25, 19114–19121. [Google Scholar] [CrossRef]
- EPA, U. Chapter 6—Inhalation Rates. In Exposure Factors Handbook 2011 Edition (Final Report); US Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- Zeng, X.; Wang, Z.; Wang, J.; Guo, J.; Chen, X.; Zhuang, J. Health risk assessment of heavy metals via dietary intake of wheat grown in Tianjin sewage irrigation area. Ecotoxicology 2015, 24, 2115–2124. [Google Scholar] [CrossRef]
- Aryal, K.K. Non Communicable Diseases Risk Factors: STEPS Survey Nepal 2013; Nepal Health Research Council (NHRC): Kathmandu, Nepal, 2014. [Google Scholar]
- Lian, M.; Wang, J.; Sun, L.; Xu, Z.; Tang, J.; Yan, J.; Zeng, X. Profiles and potential health risks of heavy metals in soil and crops from the watershed of Xi River in Northeast China. Ecotoxicol. Environ. Saf. 2019, 169, 442–448. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Murtaza, G.; Bibi, S.; Sabir, M.; Owens, G.; Saifullah Zeeshan, N. Immobilization of cadmium in soil-plant system through soil and foliar applied silicon. Int. J. Phytoremediation 2022, 24, 1193–1204. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes. J. Am. Diet. Assoc. 2001, 101, 294. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Pascual, V.C.; Hurtado, M.C.; Moreno, P.C.; Daschner, Á.; Pons, R.M.G.; Fandos, E.G.; Lázaro, D.R. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (ASEAN) on the Identification of Emerging Food Risks. Rev. Com. Cient. ASEAN 2019, 29, 19–42. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. 2015, 13, 4002. [Google Scholar]
- Nursanti, I.; Supriyanto, R. Pertumbuhan Legume Cover Crops (Puararia javanica) Pada Tanah Pasca Penambangan Batubara Plus Zeolit. J. Media Pertan. 2022, 7, 7–10. [Google Scholar]
- Mahboub, K.A. Influence of substitution of peat with Iranian zeolite (clinoptilolite) in peat medium on Ficus benjamina growth. J. Ornam. Hortic. Plants 2011, 1, 1–10. [Google Scholar]
- Karami, S.; Hadi, H.; Tajbaksh, M.; Modarres-Sanavy, S.A. Effect of zeolite on nitrogen use efficiency and physiological and biomass traits of amaranth (Amaranthus hypochondriacus) under water-deficit stress conditions. J. Soil Sci. Plant Nutr. 2020, 20, 1427–1441. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Yamamoto, T.; Inoue, M.; Eneji, A.E.; Mori, Y.; Irshad, M. Effects of zeolite on soil nutrients and growth of barley following irrigation with saline water. J. Plant Nutr. 2008, 31, 1159–1173. [Google Scholar] [CrossRef]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Moeen, M.; Qi, T.; Hussain, Z.; Ge, Q.; Maqbool, Z.; Jianjie, X.; Kaiqing, F. Use of Zeolite to Reduce the Bioavailability of Heavy Metals in a Contaminated Soil. J. Ecol. Eng. 2020, 21, 186–196. [Google Scholar] [CrossRef]
- Dang, V.M.; Van, H.T.; Duong, H.T.M.; Nguyen, D.H.; Chao, H.P.; Nguyen, L.H.; Lin, C.C. Evaluation of fly ash, apatite and rice straw derived-biochar in varying combinations for in situ remediation of soils contaminated with multiple heavy metals. Soil Sci. Plant Nutr. 2020, 66, 379–388. [Google Scholar] [CrossRef]
- Teng, Y.; Li, J.; Wu, J.; Lu, S.; Wang, Y.; Chen, H. Environmental distribution and associated human health risk due to trace elements and organic compounds in soil in Jiangxi province, China. Ecotoxicol. Environ. Saf. 2015, 122, 406–416. [Google Scholar] [CrossRef]
- Cárcamo, V.; Bustamante, E.; Trangolao, E.; de la Fuente, L.M.; Mench, M.; Neaman, A.; Ginocchio, R. Simultaneous immobilization of metals and arsenic in acidic polluted soils near a copper smelter in central Chile. Environ. Sci. Pollut. Res. 2012, 19, 1131–1143. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Bajda, T.; Kope, M. Use of biochar and a zeolite as adsorbents of mineral pollutions. Chem. Ind. 2019, 98, 1969–1972. [Google Scholar] [CrossRef]
- Edwards, R.; Rebedea, I.; Lepp, N.W.; Lovell, A.J. An investigation into the mechanism by which synthetic zeolites reduce labile metal concentrations in soils. Environ. Geochem. Health 1999, 21, 157–173. [Google Scholar] [CrossRef]
- Oste, L.A.; Lexmond, T.M.; van Riemsdijk, W.H. Metal immobilization in soils using synthetic zeolites. J. Environ. Qual. 2002, 31, 813–821. [Google Scholar] [CrossRef]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Explor. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Singh, S.; Zacharias, M.; Kalpana, S.; Mishra, S. Heavy metals accumulation and distribution pattern in different vegetable crops. J. Environ. Chem. Ecotoxicol. 2012, 4, 170–177. [Google Scholar] [CrossRef]
- Azam, F.M.S.; Al Labib, B.; Jabin, D.; Sayeed, M.S.R.; Islam, S.; Akter, S.; Rahmatullah, M. Study on synergistic effect of Zeolite and supplementary fertilizer in soil on flowering of Solanum melongena L. Am. Eurasian J. Sustain. Agric. 2013, 7, 108–114. [Google Scholar]
- Abdi, G.; Khosh-Khui, M.; Eshghi, S. Effects of natural zeolite on growth and flowering of strawberry (Fragaria × ananassa Duch.). Int. J. Agric. Res. 2010, 5, 799–804. [Google Scholar] [CrossRef]
- Moreno, J.L.; Ondoño, S.; Torres, I.; Bastida, F. Compost, leonardite, and zeolite impacts on soil microbial community under barley crops. J. Soil Sci. Plant Nutr. 2017, 17, 214–230. [Google Scholar] [CrossRef] [Green Version]
- Shahkolaie, S.S.; Baranimotlagh, M.; Dordipour, E.; Khormali, F. Effects of inorganic and organic amendments on physiological parameters and antioxidant enzymes activities in Zea mays L. from a cadmium-contaminated calcareous soil. South Afr. J. Bot. 2020, 128, 132–140. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, Q. Effects of soil amendments on the extractability and speciation of cadmium, lead, and copper in a contaminated soil. Bull. Environ. Contam. Toxicol. 2009, 83, 136–140. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Khan, S.A.; Iqbal, M.; Ramzani, P.M.A.; Fatima, M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Quartacci, M. Biochar amendment reduces oxidative stress in lettuce grown under copper excess. Agrochim. Pisa 2015, 59, 188–202. [Google Scholar] [CrossRef]
- Turan, V.; Ramzani, P.M.A.; Ali, Q.; Abbas, F.; Iqbal, M.; Irum, A.; Khan, W.U.D. Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch. Agron. Soil Sci. 2018, 64, 1053–1067. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Al-Tabbaa, A. Leachability and heavy metal speciation of 17-year old stabilised/solidified contaminated site soils. J. Hazard. Mater. 2014, 278, 144–151. [Google Scholar] [CrossRef]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Hussain, A.; Maitra, J.; Khan, K.A. Development of biochar and chitosan blend for heavy metals uptake from synthetic and industrial wastewater. Appl. Water Sci. 2017, 7, 4525–4537. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Shan, L.; Anjum, S.; Ronggui, H.; Iqbal, M.; Virk, Z.A.; Kausar, S. Improved quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Physiol. Biochem. 2017, 116, 127–138. [Google Scholar] [CrossRef]
- Tripathi, N.; Choppala, G.; Singh, R.S. Evaluation of modified chitosan for remediation of zinc contaminated soils. J. Geochem. Explor. 2017, 182, 180–184. [Google Scholar] [CrossRef]
- Rahim, H.U.; Akbar, W.A.; Alatalo, J.M. A Comprehensive Literature Review on Cadmium (Cd) Status in the Soil Environment and Its Immobilization by Biochar-Based Materials. Agronomy 2022, 12, 877. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Santos, I.R.; Silva-Filho, E.V.; Schaefer, C.E.; Albuquerque-Filho, M.R.; Campos, L.S. Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar. Pollut. Bull. 2005, 50, 185–194. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. 2000. Available online: https://www.atsdr.cdc.gov/pdns/unfilled.html (accessed on 4 September 2022).

| Properties | Unit | Value | Reference |
|---|---|---|---|
| pHs | - | 7.8 | [30] |
| ECe | dS m−1 | 1.82 | [31] |
| TSS | mmolc L−1 | 18.2 | [30] |
| SAR | (mmol L−1)1/2 | 9.13 | |
| Ca2+ + Mg2+ | mmolc L−1 | 45 | |
| Na+ | mmolc L−1 | 13.7 | |
| CO32- | mmolc L−1 | Absent | |
| HCO3- | mmolc L−1 | 40 | |
| Cl- | mmolc L−1 | 75 | |
| Texture | - | Sandy loam | [32] |
| OM | % | 0.77 | [33] |
| Cu | mg kg−1 | 86 | [34] |
| Cd | mg kg−1 | 2.27 | |
| Ni | mg kg−1 | 2.32 | |
| Pb | mg kg−1 | 2.85 |
| Factor | Description | Unit | Male | Female | Child |
|---|---|---|---|---|---|
| Cplant | Heavy metal in plant | mg kg−1 | - | - | - |
| EF | Exposure frequency | day year−1 | 350 | 350 | 350 |
| ED | Exposure duration | year | 30 | 30 | 6 |
| BW | Body weight of the exposed individual | kg | 66 | 59 | 18.6 |
| AT | Averaged time | days | 365ED | 365ED | 365ED |
| IR | Vegetable intake | kg day−1 | 0.260 | 0.260 | 0.130 |
| Treatment | pHs | ECe | HCO3− | Cl− | Ca2+ + Mg2+ |
|---|---|---|---|---|---|
| Control | 7.52 ± 0.00 | 1.16 ± 0.03 | 32.43 ± 0.72 | 68.07 ± 0.67 | 41.69 ± 0.07 |
| Z0.25% | 7.72 ± 0.01 | 1.49 ± 0.09 | 35.20 ± 0.75 | 70.10 ± 0.05 | 44.03 ± 0.03 |
| Z0.5% | 7.72 ± 0.01 | 1.57 ± 0.04 | 40.06 ± 0.23 | 74.51 ± 0.53 | 45.37 ± 0.20 |
| Z1.0% | 7.73 ± 0.01 | 1.65 ± 0.03 | 38.09 ± 0.45 | 72.31 ± 0.40 | 45.25 ± 0.66 |
| Z2.0% | 7.95 ± 0.02 | 1.73 ± 0.04 | 44.17 ± 0.14 | 76.08 ± 0.15 | 46.07 ± 0.05 |
| LSD value | 0.0428 | 0.1849 | 2.0072 | 1.6506 | 1.1935 |
| Treatment | Fruit | Shoot | Root | Soil | TF | RF |
|---|---|---|---|---|---|---|
| Cu | ||||||
| Control | 9.62 a | 18.52 a | 25.67 a | 44.84 a | 0.72 | 236 |
| Z0.25% | 9.95 a | 17.31 a | 22.20 b | 38.19 b | 0.78 | 265 |
| Z0.5% | 7.66 b | 11.3 b | 17.33 c | 32.81 c | 0.65 | 266 |
| Z1.0% | 5.34 c | 10.8 b | 11.82 d | 26.74 d | 0.91 | 281 |
| Z2.0% | 5.52 c | 9.2 b | 10.22 e | 20.28 e | 0.90 | 292 |
| Cd | ||||||
| Control | 0.36 a | 0.47 a | 0.59 a | 0.78 a | 0.80 | 236.0 |
| Z0.25% | 0.27 b | 0.43 ab | 0.43 b | 0.77 a | 1.00 | 264.7 |
| Z0.5% | 0.10 c | 0.28 bc | 0.37 b | 0.66 b | 0.76 | 266.3 |
| Z1.0% | 0.02 d | 0.15 c | 0.23 c | 0.64 b | 0.65 | 281.0 |
| Z2.0% | 0.01 d | 0.15 c | 0.17 c | 0.41 c | 0.88 | 291.7 |
| Ni | ||||||
| Control | 0.37 a | 0.50 a | 0.57 a | 0.80 a | 0.88 | 236.0 |
| Z0.25% | 0.27 b | 0.42 b | 0.58 a | 0.80 a | 0.72 | 264.7 |
| Z0.5% | 0.37 a | 0.38bc | 0.4 b | 0.80 a | 0.95 | 266.3 |
| Z1.0% | 0.18 c | 0.35 c | 0.41 b | 0.54 b | 0.85 | 281.0 |
| Z2.0% | 0.11 d | 0.23 d | 0.30 c | 0.36 c | 0.77 | 291.7 |
| Pb | ||||||
| Control | 0.47 a | 0.66 a | 0.83 a | 0.95 a | 0.79 | 236.0 |
| Z0.25% | 0.33 b | 0.55 b | 0.76 b | 0.92 a | 0.72 | 264.7 |
| Z0.5% | 0.26 c | 0.48 c | 0.53 c | 0.71 b | 0.90 | 266.3 |
| Z1.0% | 0.10 d | 0.28 d | 0.42 d | 0.53 c | 0.67 | 281.0 |
| Z2.0% | 0.00 e | 0.22 e | 0.20 e | 0.40 d | 1.10 | 291.7 |
| Treatment | DI | HQ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Cd | Pb | Ni | Cu | Cd | Pb | Ni | ||
| Children | Control | 0.015 | 0.00056 | 0.00073 | 0.00057 | 0.371 | 0.561 | 0.183 | 0.029 |
| Z0.25% | 0.014 | 0.00037 | 0.00045 | 0.00036 | 0.339 | 0.368 | 0.112 | 0.018 | |
| Z0.5% | 0.011 | 0.00015 | 0.00038 | 0.00052 | 0.280 | 0.146 | 0.095 | 0.026 | |
| Z1.0% | 0.007 | 0.00003 | 0.00014 | 0.00024 | 0.181 | 0.033 | 0.035 | 0.012 | |
| Z2.0% | 0.008 | 0.00002 | 0.00000 | 0.00017 | 0.208 | 0.015 | 0.000 | 0.008 | |
| Men | Control | 0.008 | 0.00032 | 0.00041 | 0.00032 | 0.209 | 0.316 | 0.103 | 0.016 |
| Z0.25% | 0.008 | 0.00021 | 0.00025 | 0.00020 | 0.191 | 0.207 | 0.063 | 0.010 | |
| Z0.5% | 0.006 | 0.00008 | 0.00021 | 0.00029 | 0.158 | 0.083 | 0.054 | 0.015 | |
| Z1.0% | 0.004 | 0.00002 | 0.00008 | 0.00014 | 0.102 | 0.018 | 0.020 | 0.007 | |
| Z2.0% | 0.005 | 0.00001 | 0.00000 | 0.00009 | 0.117 | 0.008 | 0.000 | 0.005 | |
| Women | Control | 0.009 | 0.00035 | 0.00046 | 0.00036 | 0.234 | 0.354 | 0.115 | 0.018 |
| Z0.25% | 0.009 | 0.00023 | 0.00028 | 0.00023 | 0.214 | 0.232 | 0.071 | 0.011 | |
| Z0.5% | 0.007 | 0.00009 | 0.00024 | 0.00033 | 0.177 | 0.092 | 0.060 | 0.016 | |
| Z1.0% | 0.005 | 0.00002 | 0.00009 | 0.00015 | 0.114 | 0.020 | 0.022 | 0.008 | |
| Z2.0% | 0.005 | 0.00001 | 0.00000 | 0.00010 | 0.131 | 0.009 | 0.000 | 0.005 | |
| Parameter | UL | TWI | TDI | TDI | |||||
| Guideline values | value | 10 mg day−1 | 2.5 µg kg−1 bw−1 week−1 | 0.3 µg kg−1 bw−1 day−1 | 2.8 µg kg−1 bw−1 day−1 | ||||
| Reference | [46] | [47] | [48] | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, Z.U.R.; Ahmad, I.; Abdul Qadir, A.; Murtaza, G.; Rafiq, S.; Jamal, A.; Zeeshan, N.; Murtaza, B.; Javed, W.; Radicetti, E.; et al. Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation. Agronomy 2022, 12, 2433. https://doi.org/10.3390/agronomy12102433
Farooqi ZUR, Ahmad I, Abdul Qadir A, Murtaza G, Rafiq S, Jamal A, Zeeshan N, Murtaza B, Javed W, Radicetti E, et al. Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation. Agronomy. 2022; 12(10):2433. https://doi.org/10.3390/agronomy12102433
Chicago/Turabian StyleFarooqi, Zia Ur Rahman, Iftikhar Ahmad, Ayesha Abdul Qadir, Ghulam Murtaza, Sana Rafiq, Aftab Jamal, Nukshab Zeeshan, Behzad Murtaza, Wasim Javed, Emanuele Radicetti, and et al. 2022. "Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation" Agronomy 12, no. 10: 2433. https://doi.org/10.3390/agronomy12102433
APA StyleFarooqi, Z. U. R., Ahmad, I., Abdul Qadir, A., Murtaza, G., Rafiq, S., Jamal, A., Zeeshan, N., Murtaza, B., Javed, W., Radicetti, E., & Mancinelli, R. (2022). Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation. Agronomy, 12(10), 2433. https://doi.org/10.3390/agronomy12102433













