The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. The Effect of HexaFrass™ Fertilizer on the Plant Performance
2.3. The Effect of HexaFrass™ on the Regrowth of Chicory and Plantain after Cutting
2.4. The Effect of HexaFrass™ Combined with Biochar on the Growth of Chicory and Plantain
2.5. Data Analysis
3. Results
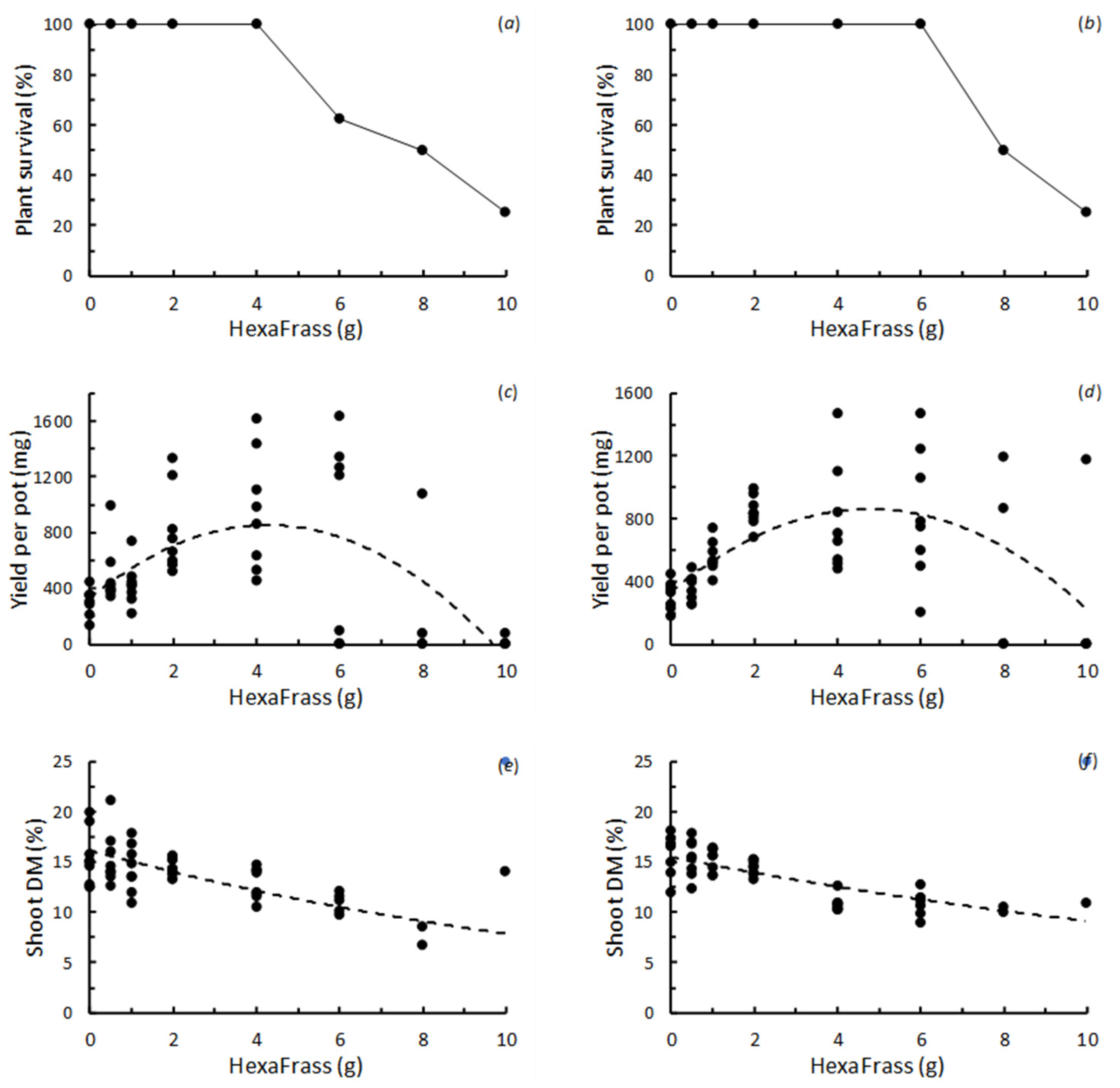
3.1. The Effect of HexaFrass™ on the Performance of Chicory and Plantain
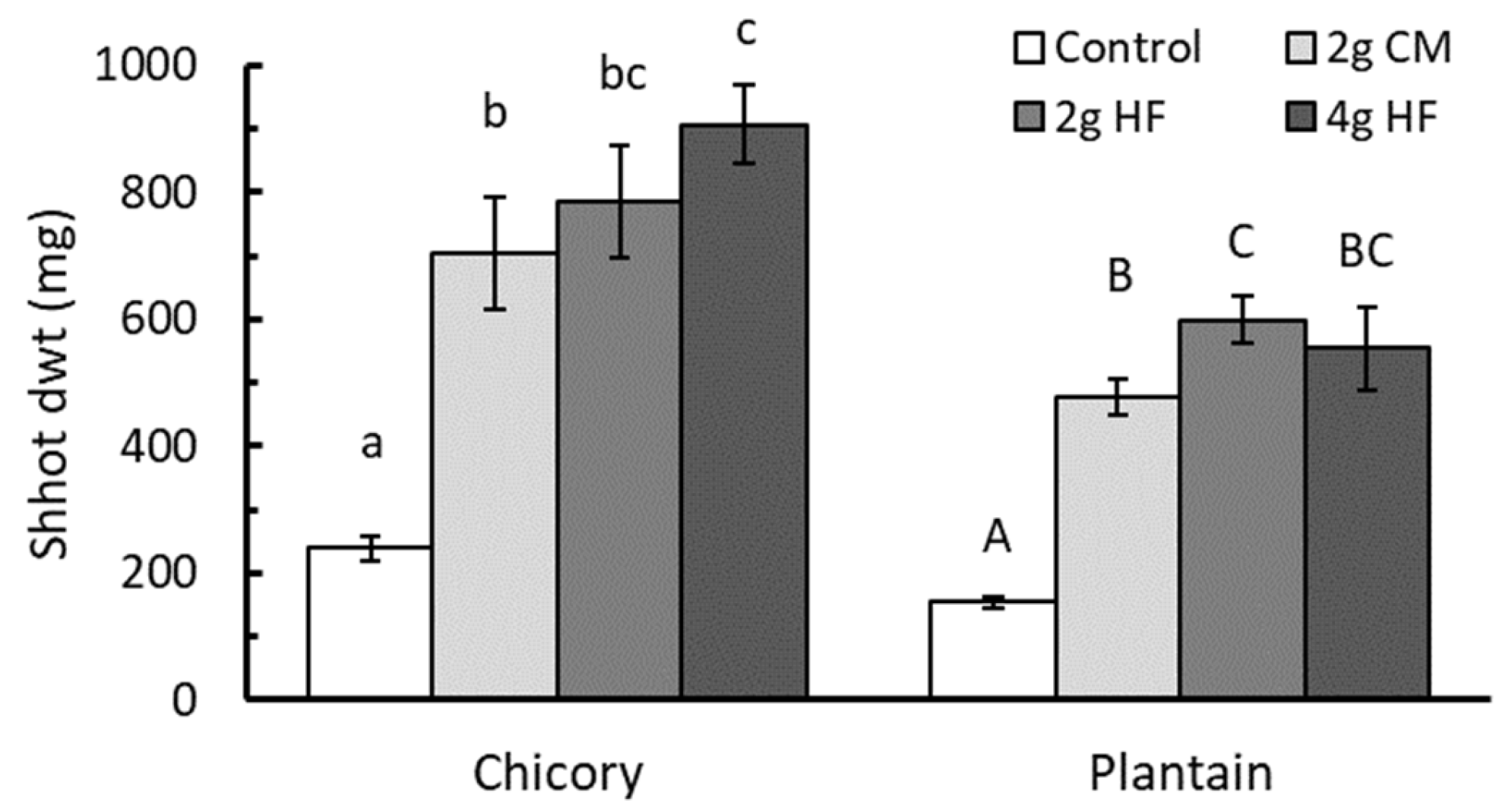
3.2. A Comparison of HexaFrass™ and a Standard Organic Fertilizer on the Shoot Growth of Chicory and Plantain
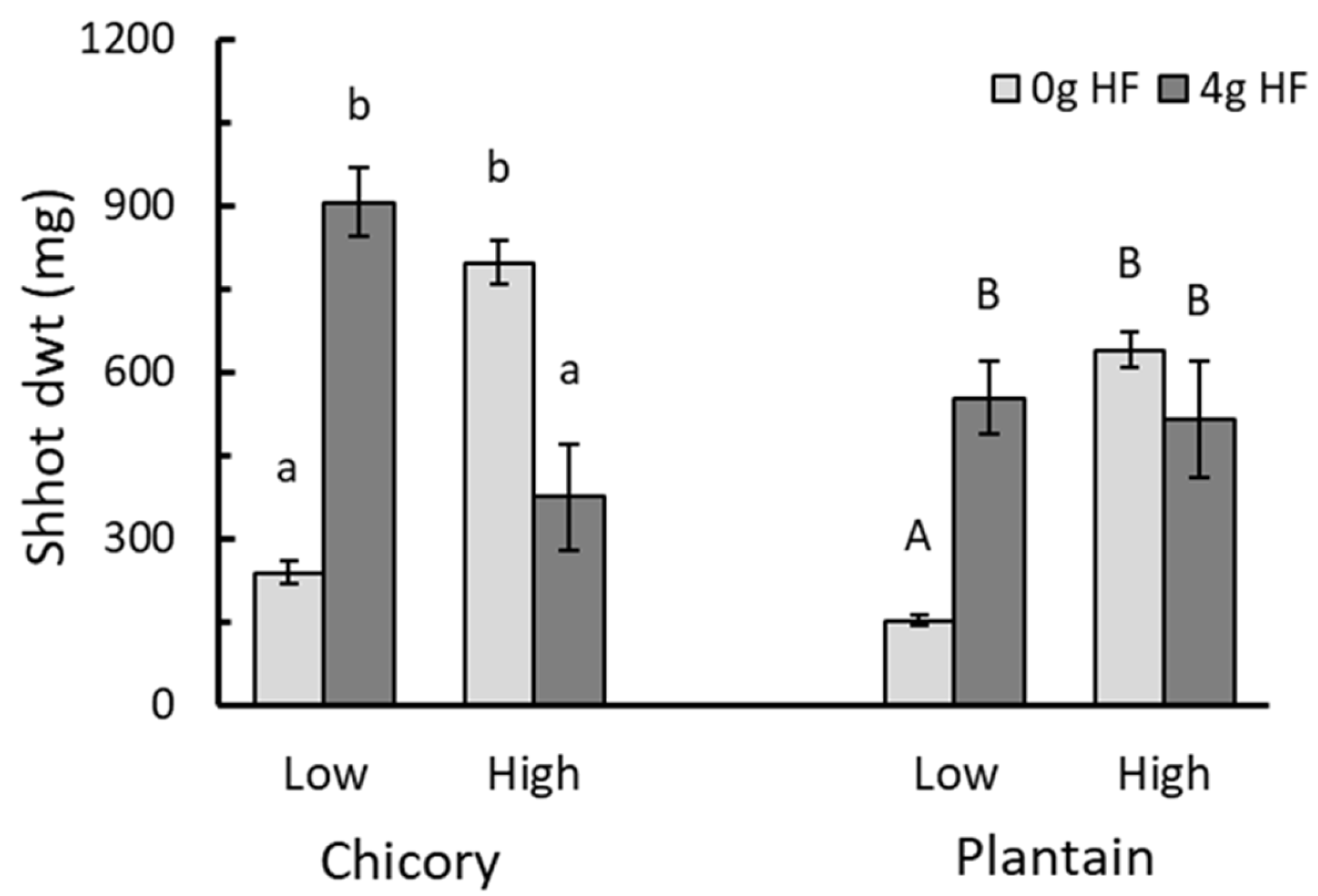
3.3. Effect of HexaFrass™ on the Shoot Growth in High- and Low-Nutrient Potting Mix
3.4. Effect of HexaFrass™ on the Shoot Growth of Chicory and Plantain after Cutting
3.5. Effect of HexaFrass™ and Biochar on the Shoot Growth of Chicory and Plantain
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef]
- Al-Marashdeh, O.; Cameron, K.; Hodge, S.; Gregorini, P.; Edwards, G. Integrating plantain (Plantago lanceolata L.) and Italian ryegrass (Lolium multiflorum Lam.) into New Zealand grazing dairy system: The effect on farm productivity, profitability, and nitrogen losses. Animals 2001, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Joy, M.K.; Rankin, D.A.; Wöhler, L.; Boyce, P.; Canning, A.; Foote, K.J.; McNie, P.M. The grey water footprint of milk due to nitrate leaching from dairy farms in Canterbury, New Zealand. Australas. J. Environ. Manag. 2022, 29, 177–199. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Internat. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Beck, M.; Garrett, K.; Barrell, G.; Al-Marashdeh, O.; Gregorini, P. Urine and fecal excretion patterns of dairy cows divergent for milk urea nitrogen breeding values consuming either a plantain or ryegrass diet. J. Dairy Sci. 2022, 105, 4218–4236. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, D.; Potts, S.; Öckinger, E. Showcasing synergies between agriculture, biodiversity and ecosystem services to help farmers capitalising on native biodiversity (SHOWCASE). Res. Ideas Outcomes 2022, 8, e90079. [Google Scholar] [CrossRef]
- McKergow, L.A.; Matheson, F.E.; Quinn, J.M. Riparian management: A restoration tool for New Zealand streams. Ecolog Manag. Restor. 2016, 17, 218–227. [Google Scholar] [CrossRef]
- Wilcock, R.J.; Monaghan, R.M.; Quinn, J.M.; Srinivasan, M.S.; Houlbrooke, D.; Duncan, M.J.; Wright-Stow, A.E.; Scarsbrook, M.R. Trends in water quality of five dairy farming streams in response to adoption of best practice and benefits of long-term monitoring at the catchment scale. Mar. Freshw. Res. 2013, 64, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Curtis, K.; Bowie, M.; Hodge, S. Can native plantings encourage native and beneficial invertebrates on Canterbury dairy farms? NZ Entomol. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Andrews, M.; Scholefield, D.; Abberton, M.; McKenzie, B.; Hodge, S.; Raven, J. Use of white clover as an alternative to nitrogen fertiliser for dairy pastures in nitrate vulnerable zones in the UK: Productivity, environmental impact and economic considerations. Ann. Appl. Biol. 2007, 151, 11–23. [Google Scholar] [CrossRef]
- Garrett, K.; Beck, M.R.; Marshall, C.J. Varied diets: Implications for lamb performance, rumen characteristics, total antioxidant status, and welfare. J. Anim. Sci. 2021, 99, skab334. [Google Scholar] [CrossRef]
- Jaramillo, D.M.; Sheridan, H.; Soder, K.; Dubeux, J.C.C.B., Jr. Enhancing the Sustainability of Temperate Pasture Systems through More Diverse Swards. Agronomy 2021, 11, 1912. [Google Scholar] [CrossRef]
- Box, L.A.; Edwards, G.R.; Bryant, R.H. Milk production and urinary nitrogen excretion of dairy cows grazing plantain in early and late lactation. N. Z. J. Agric. Res. 2017, 60, 470–482. [Google Scholar]
- Bryant, R.H.; Miller, L.A.; Greenwood, S.L.; Edwards, G.R. Milk yield and nitrogen excretion of dairy cows grazing binary and multispecies pastures. Grass Forage Sci. 2017, 72, 806–817. [Google Scholar] [CrossRef]
- Bryant, R.H.; Snow, V.O.; Shorten, P.R.; Welten, B.G. Can alternative forages substantially reduce N leaching? findings from a review and associated modelling. NZ J. Agric. Res. 2019, 63, 3–28. [Google Scholar] [CrossRef]
- Cummins, S.; Finn, J.A.; Richards, K.G.; Lanigan, G.J.; Grange, G.; Brophy, C.; Cardenas, L.M.; Misselbrook, T.H.; Reynolds, C.K.; Krol, D.J. Beneficial effects of multi-species mixtures on N2O emissions from intensively managed grassland swards. Sci Total Environ 2021, 792, 148163. [Google Scholar] [CrossRef]
- Woods, R.R.; Cameron, K.C.; Edwards, G.R.; Di, H.J.; Clough, T.J. Reducing nitrogen leaching losses in grazed dairy systems using an Italian ryegrass-plantain-white clover forage mix. Grass Forage Sci. 2018, 73, 878–887. [Google Scholar] [CrossRef]
- Beye, H.; Taube, F.; Lange, K.; Hasler, M.; Kluß, C.; Loges, R.; Diekötter, T. Species-Enriched Grass-Clover Mixtures Can Promote Bumblebee Abundance Compared with Intensively Managed Conventional Pastures. Agronomy 2022, 12, 1080. [Google Scholar] [CrossRef]
- Robertson, M.; Macdonald, B.; Farrell, M.; Norman, H.; Macdonald, L.; Vadakattu, G.; Taylor, J. What Can Science Offer the Proponents of Regenerative Agriculture Practices? Occasional Paper No. 22.01; CSIRO Agriculture & Food, Australian Farm Institute: Eveleigh, NSW, Australia, 2022. [Google Scholar]
- Cusworth, G.; Lorimer, J.; Brice, J.; Garnett, T. Green rebranding: Regenerative agriculture, future-pasts, and the naturalisation of livestock. Trans Inst Br Geogr. 2022. [Google Scholar] [CrossRef]
- Burggraaf, V.T.; Lucci, G.M.; Ledgard, S.F.; Antille, D.L.; Snow, V.O.; De Klein, C.A.M. Application of circular economy principles to New Zealand pastoral farming systems. J. NZ Grassl. 2020, 82, 53–59. [Google Scholar] [CrossRef]
- Holden, N.M.; Neill, A.M.; Stout, J.C.; O’Brien, D.; Morris, M.A. Biocircularity: A Framework to Define Sustainable, Circular Bioeconomy. Circ. Econ. Sustain. 2022. [Google Scholar] [CrossRef]
- Howard, M.; Bohm, S.; Eatherley, D. Systems resilience and SME multilevel challenges: A place-based conceptualization of the circular economy. J. Bus. Res. 2022, 145, 757–768. [Google Scholar] [CrossRef]
- Smitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture: A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Borkent, S.; Hodge, S. Glasshouse evaluation of the Black Soldier Fly waste product HexaFrass™ as an organic fertilizer. Insects 2021, 12, 977. [Google Scholar] [PubMed]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.; de Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B.A.; van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Hodge, S. Beetles for sale: Could insect farming feed us all and help save the planet? The Wētā 2022, 56, 1–12. [Google Scholar]
- Franco, A.; Sciezo, C.; Salvia, R.; Mancini, I.M.; Caniani, D.; Masi, S.; Falabella, P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull Insectology 2022, 75, 75–82. [Google Scholar]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangarajan, R.; Bolan, N.S.; Kunhikrishnan, A.; Wijesekara, H.; Xu, Y.; Tsang, D.C.; Song, H.; Ok, Y.S.; Hou, D. The potential value of biochar in the mitigation of gaseous emission of nitrogen. Sci. Total Environ. 2018, 612, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis”. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Env. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef]
- Ivins, J.D. The relative palatability of herbage plants. J. Br. Grassl. Soc. 1952, 7, 43–54. [Google Scholar] [CrossRef]
- Deaker, J.M.; Young, M.J.; Fraser, T.J.; Rowarth, J.S. Carcass, liver and kidney characteristics of lambs grazing plantain (Plantago lanceolata),chicory (Cichorium intybus), white clover or perennial ryegrass. Proc. NZ Soc. Anim. Prod. 1994, 54, 197–200. [Google Scholar]
- Barry, T.N. The feeding value of chicory (Cichorium intybus) for ruminant livestock. J. Agric. Sci. 1998, 131, 251–257. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Labreveux, M.; Hall, M.H.; Elwinger, G.F. Nutritive Value of Chicory and English Plantain Forage. Crop. Sci. 2003, 43, 1797–1804. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Navarrete, S.; Horne, D.J.; Donaghy, D.J.; Kemp, P.D. Forage plantain (Plantago lanceolata L.): Meta-analysis quantifying the decrease in nitrogen excretion, the increase in milk production, and the changes in milk composition of dairy cows grazing pastures containing plantain. Anim. Feed Sci. Tech. 2022, 285, 115244. [Google Scholar] [CrossRef]
- Milton, W.E.J. The yield of ribwort plantain (ribgrass) when sown in pure plots and with grass and clover species. Welsh J. Agric. 1943, 17, 109–116. [Google Scholar]
- Stewart, A.V. Plantain (Plantago lanceolata)—A potential pasture species. Proc. NZ Grassl. Assoc. 1996, 58, 77–86. [Google Scholar] [CrossRef]
- Grace, C.; Lynch, M.B.; Sheridan, H.; Lott, S.; Fritch, R.; Boland, T.M. Grazing multispecies swards improves ewe and lamb performance. Animal 2018, 13, 1721–1729. [Google Scholar] [CrossRef]
- Hodge, S.; Merfield, C.N.; Liu, W.Y.Y.; Tan, H.W. Seedling responses to organically-derived plant growth promoters: An effects-based approach. Plants 2021, 10, 660. [Google Scholar] [CrossRef]
- Kozak, M.; Piepho, H.-P. What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop. Sci. 2018, 204, 86–98. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring black soldier fly frass as novel fertilizer for improved growth, yield, and nitrogen use efficiency of maize under field conditions. Front. Plant Sci. 2020, 11, 574592. [Google Scholar] [CrossRef]
- Agustiyani, D.; Agandi, R.; Nugroho, A.A.; Antonius, S. The effect of application of compost and frass from Black Soldier Fly Larvae (Hermetia illucens L.) on growth of Pakchoi (Brassica rapa L.). Earth Environ. Sci. 2021, 762, 012036. [Google Scholar] [CrossRef]
- Chiam, Z.; Lee, J.T.E.; Tan, J.K.N.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T.W. Evaluating the potential of okara-derived black soldier fly larval frass as a soil amendment. J. Environ. Manag. 2021, 286, 112163. [Google Scholar] [CrossRef]
- Song, S.; Wei, A.; Koon, J.; Cheong, J.C.; Chiam, Z.; Arora, S.; Lam, W.N.; Tan, H.T.W. Upcycling food waste using black soldier fly larvae: Effects of further composting on frass quality, fertilising effect and its global warming potential. J. Clean. Prod. 2021, 288, 125664. [Google Scholar] [CrossRef]
- Terfa, G.N. Role of black soldier fly (Hermetia illucens) larvae frass bio-fertilizer on vegetable growth and sustainable farming in sub-Saharan Africa. Rev. Agric. Sci. 2021, 9, 92–102. [Google Scholar] [CrossRef]
- Belesky, D.P.; Ruckle, J.M.; Clapham, W.M. Dry-Matter Production, Allocation and Nutritive Value of Forage Chicory Cultivars as a Function of Nitrogen. J. Agron. Crop Sci. 2004, 190, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Freijsen, A.H.J.; Otten, H.A. comparison of the responses of two Plantago species to nitrate availability in culture experiments with exponential nutrient addition. Oecologia 1987, 74, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; Bryant, R.H.; Hodge, S.; Edwards, G.R. Effect of autumn regrowth interval and nitrogen fertiliser on dry matter yield and plant characteristics of six forage species. J. NZ Grassl. 2017, 79, 61–66. [Google Scholar] [CrossRef]
- Martin, K.; Edwards, G.; Bryant, R.; Hodge, M.; Moir, J.; Chapman, D.; Cameron, K. Herbage dry matter (DM) yield and nitrogen concentration of grass, legume and herb species grown at different nitrogen fertiliser rates under irrigation. Anim. Prod. Sci. 2017, 57, 1283–1288. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three-year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Laird, D.; Novak, J.; Collins, H.; Ippolito, J.; Karlen, D.; Lentz, R.; Sistani, K.; Spokas, K.; Van Pelt, R. Multi-year and multi-location soil quality and crop biomass yield responses to hardwood fast pyrolysis biochar. Geoderma 2017, 289, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ali, K.; Wang, X.; Riaz, M.; Islam, B.; Khan, Z.H.; Shah, F.; Munsif, F.; Haq, S.I.U. Biochar: An eco-friendly approach to improve wheat yield and associated soil properties on sustainable basis. Pak. J. Bot. 2019, 54, 1255–1261. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, S.; Iwabuchi, K.; Itoh, T.; Watanabe, T.; Osaki, M.; Taniguro, K. Effects of Different Feedstock Type and Carbonization Temperature of Biochar on Oat Growth and Nitrogen Uptake in Coapplication with Compost. J. Soil Sci. Plant Nutrit. 2021, 21, 276–285. [Google Scholar] [CrossRef]
- Gathorne-Hardy, A.; Knight, J.; Woods, J. Biochar as a soil amendment positively interacts with nitrogen fertiliser to improve barley yields in the UK. Earth Environ. Sci. 2009, 6, 372052. [Google Scholar] [CrossRef]
- Edmeades, D.C. The effects of liquid fertilisers derived from natural products on crop, pasture, and animal production: A review. Aust. J. Agric. Res. 2002, 53, 965–976. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.-P.; Dulaurent, A.-M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the Short-Term Fertilizer Potential of Mealworm Frass Using a Pot Experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Gärttling, D.; Schulz, H. Compilation of Black Soldier Fly Frass Analyses. J. Soil Sci. Plant Nutr. 2022, 22, 937–943. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengel, K. Alternative or Complementary Role of Foliar Supply in Mineral Nutrition. Acta Hortic. 2002, 594, 33–47. [Google Scholar] [CrossRef]
- Ravi, I.; Kamaraju, K.; Kumar, S.; Nori, S.S. Foliar application of seaweed bio formulation enhances growth and yield of banana cv. Grand Naine (AAA). Indian J. Nat. Sci. 2018, 8, 13482–13488. [Google Scholar]
- Menino, R.; Felizes, F.; Castelo-Branco, M.A.; Fareleira, P.; Moreira, O.; Nunes, R.; Murta, D. Agricultural value of Black Soldier Fly larvae frass as organic fertilizer on ryegrass. Heliyon 2021, 7, e05855. [Google Scholar] [CrossRef]
- Nicksy, J.; Amiro, B.; Entz, M. Recycled nutrients supply phosphorus for organically-managed wheat and forage crops. Nutr. Cycl Agroecosyst 2022, 123, 37–151. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodge, S.; Conway, J. The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards. Agronomy 2022, 12, 2459. https://doi.org/10.3390/agronomy12102459
Hodge S, Conway J. The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards. Agronomy. 2022; 12(10):2459. https://doi.org/10.3390/agronomy12102459
Chicago/Turabian StyleHodge, Simon, and John Conway. 2022. "The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards" Agronomy 12, no. 10: 2459. https://doi.org/10.3390/agronomy12102459
APA StyleHodge, S., & Conway, J. (2022). The Effects of Insect Frass Fertilizer and Biochar on the Shoot Growth of Chicory and Plantain, Two Forage Herbs Commonly Used in Multispecies Swards. Agronomy, 12(10), 2459. https://doi.org/10.3390/agronomy12102459






