Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Material and SE Preparation
2.2. Plant Material and Growing Conditions
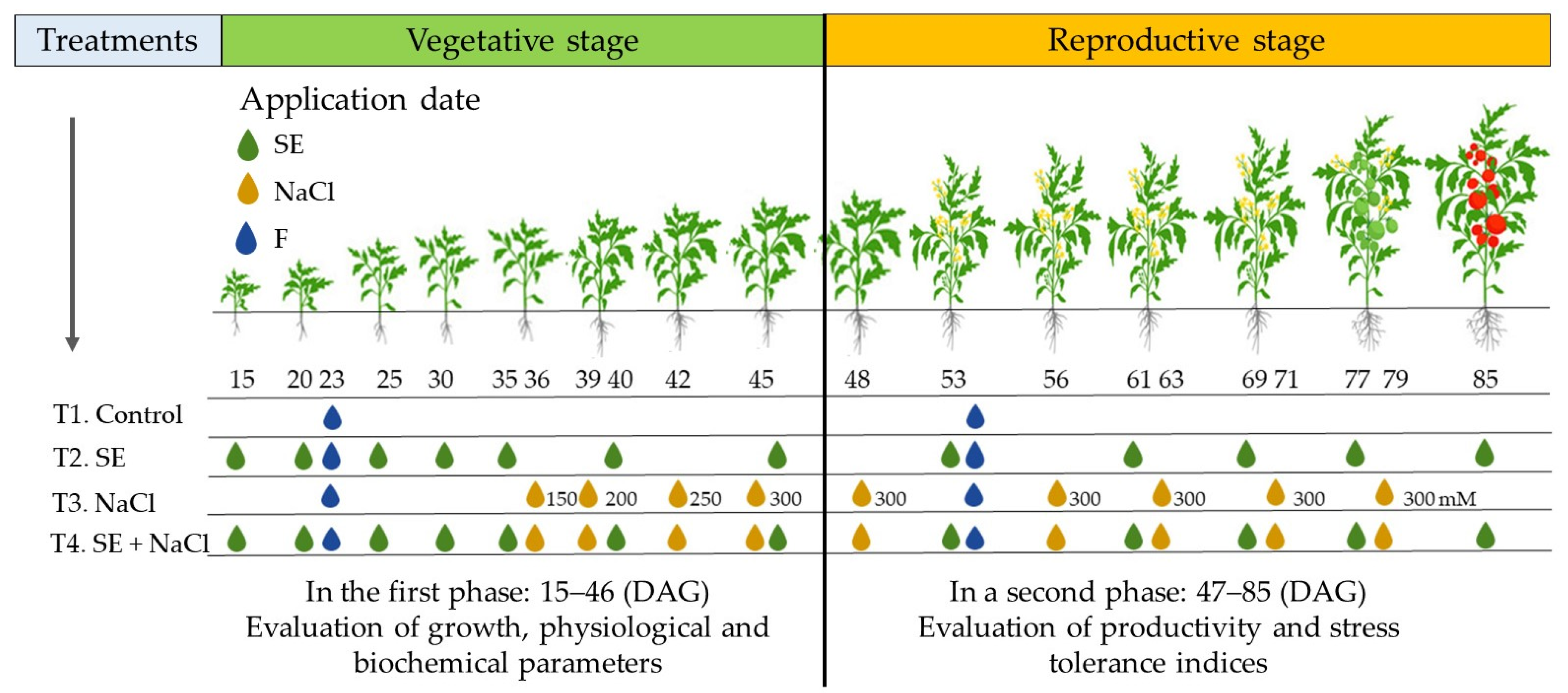
2.3. Experimental Design
2.4. SE Application and Salinity (NaCl) Stress Experiments
2.5. Physiological Measurements
2.5.1. Photosynthetic Performance
2.5.2. Chlorophyll Measurements
2.6. Plant Growth Measurements
2.7. Biochemical Measurements
2.7.1. Metabolite Analyses
2.7.2. Antioxidant Capacity
2.7.3. Antioxidant Enzyme Activity
2.7.4. Na+, K+, and Cl− Concentrations
2.8. Productivity Evaluation and Stress Tolerance Indices
2.9. Statistical Analysis
3. Results
3.1. Effect of the SE on the Physiological Responses of Tomato Plants
3.2. Effects of the SE on the Morphological Attributes of Tomato Plants
3.3. Effects of the SE on the Biochemical Characteristics of Tomato Plants
3.3.1. Metabolites
3.3.2. Antioxidant Activity
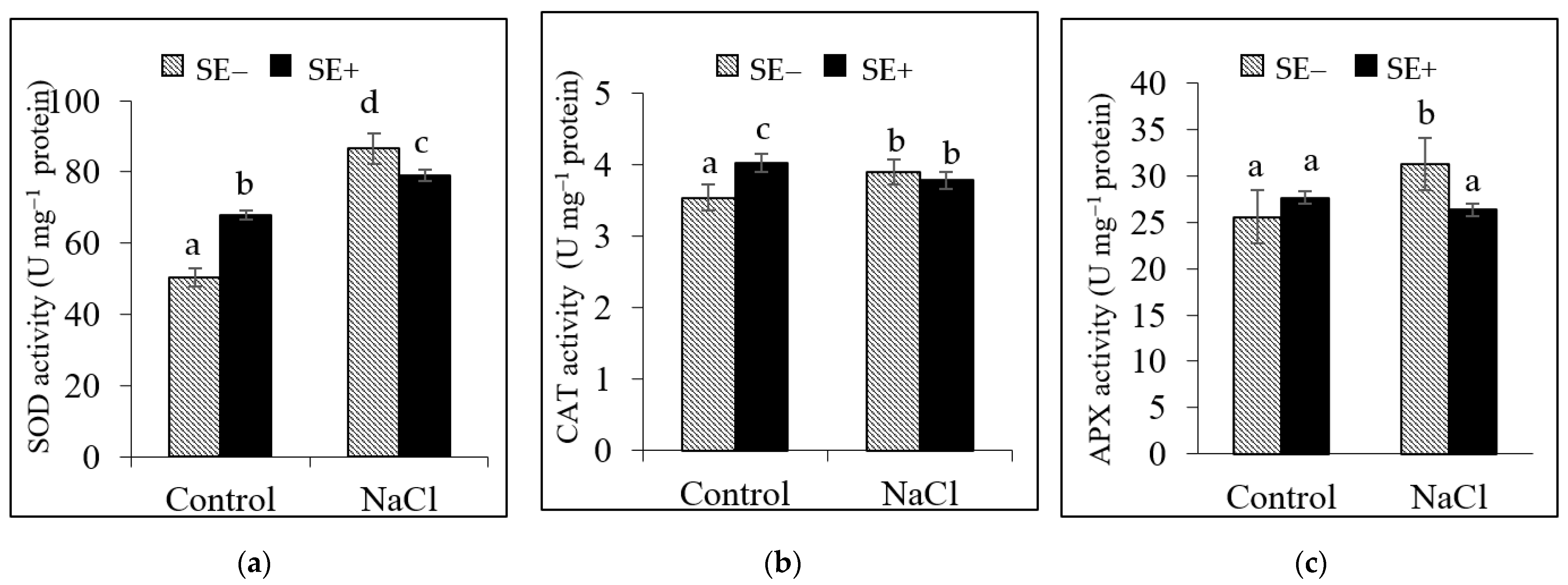
3.3.3. Antioxidant Enzyme Activity
3.3.4. Measurement of Na+, K+, and Cl− Concentrations
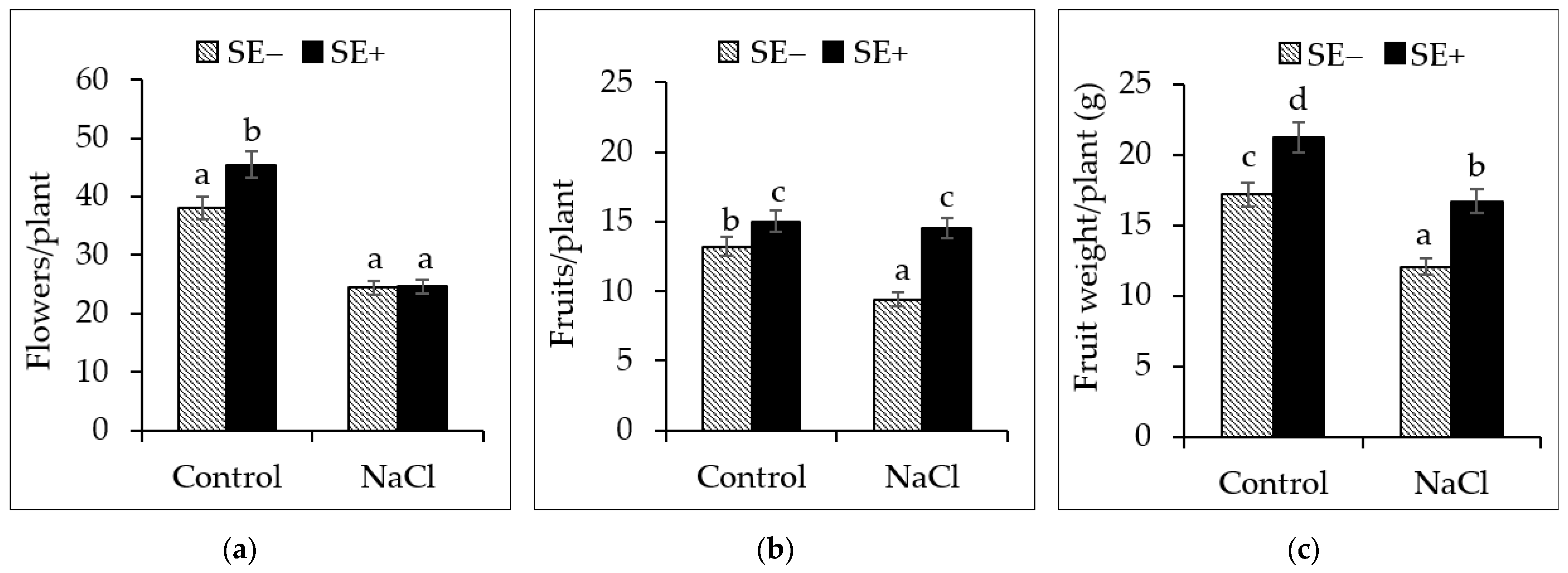
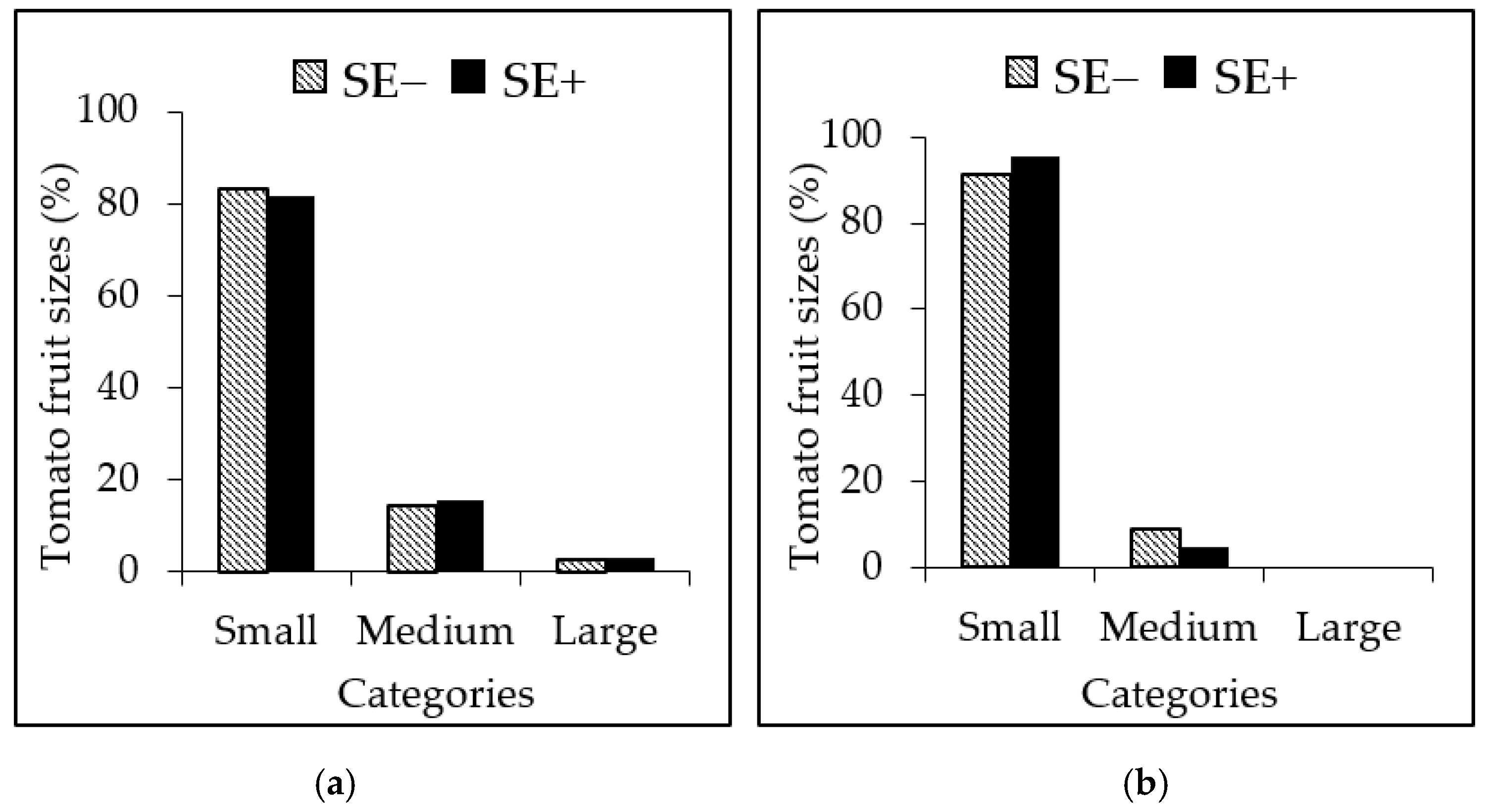
3.4. Effects of the SE on the Reproductive Attributes of the Tomato Plants
3.5. Effects of the SE on the Tolerance and Productivity of Tomato Plants as Determined by Stress Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Takhur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer Nature Singapore Private Ltd.: Singapore, 2018; pp. 27–46. [Google Scholar]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production [Internet]; Hasanuzzaman, M., Filho, M.C.M.T., Fujita, M., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2020; pp. 1–21. [Google Scholar]
- FAO & ITPS Status of the World’s Soil Resources (SWSR)—Main Report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome, Italy. 2015. Available online: http://www.fao.org/3/a-i5199e.pdf (accessed on 16 May 2022).
- Simoes, A.J.G.; Hidalgo, C.A. The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. 2022. Available online: https://oec.world/en/profile/hs/tomatoes?redirect=true (accessed on 24 July 2022).
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2011, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Devkar, V.; Thirumalaikumar, V.P.; Xue, G.P.; Vallarino, J.G.; Turěcková, V.; Strnad, M.; Fernie, A.R.; Hoefgen, R.; Mueller-Roeber, B.; Balazadeh, S. Multifaceted regulatory function of tomato SlTAF1 in the response to salinity stress. New Phytol. 2020, 225, 1681–1698. [Google Scholar] [CrossRef]
- Pailles, Y.; Awlia, M.; Julkowska, M.M.; Passone, L.; Zemmouri, K.; Negrão, S.; Schmöckel, S.M.; Tester, M. Diverse traits contribute to salinity tolerance of wild tomato seedlings from the Galapagos Islands. Plant Physiol. 2020, 182, 534–546. [Google Scholar] [CrossRef]
- Ibrahimova, U.F.; Mammadov, A.C.; Feyziyev, Y.M. The effect of NaCl on some physiological and biochemical parameters in Triticum aestivum L. genotypes. Plant Physiol. Rep. 2019, 24, 370–375. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salinity stress under the scalpel—Dissecting the genetics of salt tolerance. Plant J. 2019, 97, 148–163. [Google Scholar] [CrossRef]
- Massange-Sánchez, J.A.; Sánchez-Hernández, C.V.; Hernández-Herrera, R.M.; Palmeros-Suárez, P.A. The Biochemical Mechanisms of Salt Tolerance in Plants. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2021. [Google Scholar]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafque, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortifed composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. South. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Borusiewicz, A.; Łozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Derbali, W.; Goussi, R.; Koyro, H.W.; Abdelly, C.; Manaa, A. Physiological and biochemical markers for screening salt tolerant quinoa genotypes at early seedling stage. J. Plant Interact. 2020, 15, 27–38. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and timing of application of biostimulant substances to enhance fruit tree tolerance toward environmental stresses and fruit quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Scienc; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Drira, M.; Mohamed, J.B.; Hlima, H.B.; Hentati, F.; Michaud, P.; Abdelkafi, S.; Fendri, I. Improvement of Arabidopsis thaliana salt tolerance using a polysaccharidic extract from the brown algae Padina pavonica. Algal Res. 2021, 56, 102324. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Zhao, H.; Yuan, Y.; Meng, L.; Zhang, C.; Li, Y. Polysaccharides derived from the brown algae Lessonia nigrescens enhance salinity stress tolerance to wheat seedlings by enhancing the antioxidant system and modulating intracellular ion concentration. Front. Plant Sci. 2019, 10, 48. [Google Scholar] [CrossRef]
- Bi, F.; Iqbal, S.; Arman, M.; Ali, A.; Hassan, M.-U. Carrageenan as an elicitor of induced secondary metabolites and its effects on various growth characters of chickpea and maize plants. J. Saudi. Chem. Soc. 2011, 15, 269–273. [Google Scholar] [CrossRef]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarkar, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yavad, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Sharma, L.; Banerjee, M.; Malik, G.C.; Gopalakrishnan, V.A.K.; Zodape, S.T.; Ghosh, A. Sustainable agro-technology for enhancement of rice production in the red and lateritic soils using seaweed based biostimulants. J. Clean. Prod. 2017, 149, 968–975. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hussein, M.H.; Eltanahy, E.; Al Bakry, A.F.; Elsafty, N.; Elshamy, M.M. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sensis and Zea mays. J. Appl. Phycol. 2021, 33, 1273–1291. [Google Scholar] [CrossRef]
- Kasim, W.A.E.A.; Saad-Allah, K.M.; Hamouda, M. Seed priming with extracts of two seaweeds alleviates the physiological and molecular impacts of salinity stress on radish (Raphanus sativus). Int. J. Agric. Biol. 2016, 18, 653–660. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Ud-Din, A.; Sajid, Z.A.; Khilji, S.; Rehman, A.; Tabassum, A.; et al. Sargassum wightii aqueous extract improved salinity stress tolerance in Abelmoschus esculentus by mediating metabolic and ion rebalance. Front. Mar. Sci. 2022, 9, 853272. [Google Scholar] [CrossRef]
- Rai, N.; Rai, S.P.; Sarma, B.K. Prospects for abiotic stress tolerance in crops utilizing phyto- and bio-stimulants. Front. Sustain. Food. Syst. 2021, 5, 754853. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Martínez-Gallardo, N.A.; Montero-Vargas, J.M.; Gómez-Leyva, J.F.; Délano-Frier, J.P. The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Negrulescu, A.; Patrulea, V.; Mincea, M.M.; Cosmin, I.; Beatrice, A.V.; Vasile, O. Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 2012, 23, 2176–2182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutierrez, A.; Gomez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crops. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; Nieves-Soto, M.; Manzano-Sarabia, M.M.; Hernández-Garibay, E.; Lizardi-Mendoza, J.; Burgos-Hernández, A.; Hurtado-Oliva, M.A. Gross chemical composition, fatty acids, sterols, and pigments in tropical seaweed species off Sinaloa, Mexico. Cienc. Mar. 2019, 45, 101–120. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell. Physiol. 1981, 22, 867–880. [Google Scholar]
- Zhang, J.; Zeng, L.; Chen, S.; Sun, H.; Ma, S. Transcription profile analysis of Lycopersicum esculentum leaves, unravels volatile emissions and gene expression under salinity stress. Plant Physiol. Biochem. 2018, 126, 11–21. [Google Scholar] [CrossRef]
- Mohr, C.F. Neue massanalytische Bestimmung des chlors in Verbindungen. Ann.Chem. Pharm. 1856, 97, 335–338. [Google Scholar]
- Alam, M.S.; Tester, M.; Fiene, G.; Mousa, M.A.A. Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plant 2021, 10, 712. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars: I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetable and Other Food Crops in Temperature and Water Stress, Tainan, Taiwan, 13–18 August 1992; pp. 257–270. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non- stress environment. Crop. Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybean. Part 1: Evaluation of three screening techniques for heat and drought tolerance. Crop. Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Golestani Araghi, S.; Assad, M.T. Evaluation of four screening techniques for drought resistance and their relationship to yield reduction ratio in wheat. Euphytica 1998, 103, 293–299. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in seaweeds: An overview on biosynthesis to biomedical applications. Int. J. Mol. Sci. 2021, 22, 12691. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Briceño-Domínguez, D.R.; Filippo-Herrera, D.A.; Hernández-Carmona, G. Seaweed as potential plant growth stimulants for agriculture in Mexico. Hidrobiologica 2018, 28, 129–140. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic Physiological Phenotyping of Drought-Stressed Pepper Plants Treated with “Productivity-Enhancing” and “Survivability-Enhancing” Biostimulants. Front. Plant. Sci. 2019, 10, 905. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mansour, H.A.; El-Khawas, S.A.; Hassanein, R.A. The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salinity stress tolerance of canola (Brassica napus L.) plants. Agronomy 2019, 9, 146. [Google Scholar]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; Hussein, M.M.; El-Baroty, G.S. Algal extracts improve antioxidant defense abilities and salt tolerance of wheat plant irrigated with sea water. Afr. J. Biomed. Res. 2008, 2, 151–164. [Google Scholar]
- Erulan, V.; Thirumaran, G.; Soundarapandian, P.; Ananthan, G. Studies on the effect of Sargassum polycystum (C. Agardh, 1824) extract on the growth and biochemical composition of Cajanus Cajan (L.) Mill sp. Am. -Eurasian J. Agric. Environ Sci. 2009, 6, 392–399. [Google Scholar]
- Ramya, S.S.; Nagaraj, S.; Vijayanand, N. Biofertilizing Efficiency of brown and green algae on growth, biochemical and yield parameters of Cyamopsis Tetragonolaba (L.) Taub. Recent Res. Sci. Technol. 2010, 2, 45–52. [Google Scholar]
- Gharib, F.A.E.L.; Zeid, I.M.; Salem, O.M.A.H.; Ahmed, E.Z. Effects of Sargassum latifolium extract on growth, oil content and enzymatic activities of rosemary plants under salinity stress. Life Sci. 2014, 11, 933–945. [Google Scholar]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ramarajan, S.; Joseph, L.H.; Ganthi, A.S. Effect of seaweed liquid fertilizer on the germination and pigment concentration of soybean. J. Crop Sci. Technol. 2012, 1, 1–5. [Google Scholar]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. Trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Becker, D.F. Connecting proline metabolism and signaling pathways in plant senescence. Front. Plant Sci. 2015, 6, 552. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salinity stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Jaarsma, R.; de Vries, R.S.; de Boer, A.H. Effect of salinity stress on growth, Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLoS ONE 2013, 8, e60183. [Google Scholar]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salinity stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Chernane, H.; Latique, S.; Mansori, M.; El Kaoua, M. Salinity stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. J. Agric. Vet. 2015, 8, 36–44. [Google Scholar]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Gossett, D.R.; Millhollon, E.P.; Lueas, C.; Banks, S.W.; Marney, M.M. The effects of NaCI on antioxidant enzyme activities in callus tissue of salt-tolerant and salt-sensitive cotton genotypes (Gossypium hirsutum L.). Plant Cell. Rep. 1994, 13, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Fadzilla, N.A.M.; Finch, R.P.; Burdon, R.H. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J. Exp. Bot. 1997, 48, 325–331. [Google Scholar] [CrossRef]
- Shalata, A.; Neumann, P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salinity stress and reduces lipid peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chowdhury, N. Salt stress in plants and amelioration strategies: A critical review. In Abiotic Stress in Plants; Fahad, S., Saudy, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: London, UK, 2020; pp. 1–23. [Google Scholar]
- Shanmuganathan, B.; Pandima-Devi, K. Evaluation of the nutritional profile and antioxidant and anti-cholinesterase activities of Padina gymnospora (Phaeophyceae). Eur. J. Phycol. 2016, 51, 482–490. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant. Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Venkatesan, K.; Selvakumari, P. Seasonal influence of seaweed gel on growth and yield of tomato (Solanum lycopersicum Mill.) Hybrid COTH 2. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 55–66. [Google Scholar] [CrossRef]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory Activities of Ascophyllum nodosum Extract in Tomato and Sweet Pepper Crops in a Tropical Environment. PLoS ONE 2019, 14, e0216710. [Google Scholar]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Jayaraman, J.; Ali, N. Use of Seaweed Extracts for Disease Management of Vegetable Crops. In Sustainable Crop Disease Management Using Natural Products; CABI: Wallingford, UK, 2015; pp. 160–173. [Google Scholar]
- Mitra, J. Genetics and genetic improvement of drought resistance in crop plants. Curr. Sci. 2001, 80, 758–762. [Google Scholar]
- Porch, T.G. Application of stress indices for heat tolerances screening of common bean. J. Agron. Crop. Sci. 2006, 192, 390–394. [Google Scholar] [CrossRef]
- Singh, S.; Sengar, R.S.; Kulshreshtha, N.; Datta, D.; Tomar, R.S.; Rao, V.P.; Garg, D.; Ojha, A. Assessment of multiple tolerance indices for salinity stress in bread wheat (Triticum aestivum L.). J. Agric. Sci. 2015, 7, 49–57. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Gautam, R.K.; Sharma, P.C.; Sharma, D.K. Effect of different salinity stresses on agro-morphological traits and utilization of salinity stress indices for reproductive stage salt tolerance in rice. Field Crops Res. 2016, 190, 26–33. [Google Scholar] [CrossRef]


| Stress Tolerance Index | Formula | Reference |
|---|---|---|
| Stress susceptibility index (SSI) | [1 − (Ys/Yns)]/[1 − (Xs/Xns)] | Fischer and Maurer [57] |
| Stress tolerance index (STI) | (Yns × Ys)/(Xns)2 | Fernandez [58] |
| Mean productivity (MPI) | (Ys + Yns)/2 | Rosielle and Hamblin [59] |
| Yield stability index (YSI) | (Ys/Y ns) | Bouslama and Schapaugh [60] |
| Yield reduction (YR) | 1 − (Ys/Y ns) | Golestani and Assad [61] |
| Growth Condition | FV/FM | ETRMAX | Chlorophyll | EK | NPQ | |
|---|---|---|---|---|---|---|
| Control | SE− | 0.781 ± 0.007 c | 124.02 ± 11.50 bc | 43.54 ± 2.8 c | 569.69 ± 138.65 b | 0.674 ± 0.164 b |
| SE+ | 0.786 ± 0.007 c | 130.28 ± 18.42 c | 46.10 ± 3.7 c | 610.31 ± 127.97 b | 0.713 ± 0.137 b | |
| NaCl | SE− | 0.734 ± 0.028 a | 92.66 ± 13.86 a | 34.46 ± 4.1 a | 386.21 ± 162.74 a | 0.455 ± 0.147 a |
| SE+ | 0.755 ± 0.015 b | 118.79 ± 13.17 b | 39.95 ± 4.0 b | 557.68 ± 110.62 b | 0.442 ± 0.117 a | |
| Growth Condition | Length (cm) | Area (cm2) | Fresh Weight (g) | ||||
|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | ||
| Control | SE− | 14.6 ± 1.7 a | 14.00 ± 1.1 c | 17.9 ± 3.4 a | 102.7 ± 17.4 b | 0.56 ± 0.14 a | 3.8 ± 0.44 b |
| SE+ | 16.9 ± 1.2 b | 16.76 ± 0.8 d | 28.3 ± 6.2 b | 146.7 ± 22.9 c | 0.91 ± 0.21 b | 5.3 ± 0.95 c | |
| NaCl | SE− | 14.3 ± 1.5 a | 10.31 ± 0.9 a | 18.0 ± 5.9 a | 63.2 ± 9.3 a | 0.55 ± 0.15 a | 2.2 ± 0.41 a |
| SE+ | 18.0 ± 1.0 c | 12.79 ± 0.8 b | 33.1 ± 6.2 c | 98.6 ± 17.7 b | 1.09 ± 0.34 c | 3.8 ± 0.59 b | |
| Primary Metabolites | Secondary Metabolites | |||||
|---|---|---|---|---|---|---|
| Growth Condition | Proline (μmol/mg DW) | TRS (mg/g DW) | Phenols (mg/g DW) | Flavonoids (mg/g DW) | Carotenoids (μmol/g FW) | |
| Control | SE− | 43.8 ± 1.8 a | 22.84 ± 0.82 a | 10.30 ± 0.58 a | 4.86 ± 0.12 b | 0.045 ± 0.002 a |
| SE+ | 38.5 ± 1.3 a | 42.68 ± 2.00 c | 11.30 ± 0.12 b | 4.38 ± 0.23 a | 0.046 ± 0.001 a | |
| NaCl | SE− | 161.3 ± 5.4 b | 74.27 ± 0.85 d | 14.64 ± 0.48 d | 5.00 ± 0.14 b | 0.072 ± 0.001 c |
| SE+ | 180.0 ± 7.7 c | 30.60 ± 1.34 b | 13.91 ± 0.65 c | 5.42 ± 0.14 c | 0.065 ± 0.006 b | |
| Antioxidant Activity (mg ET/g DW) | |||
|---|---|---|---|
| Growth Condition | DPPH | ABTS | |
| Control | SE− | 11.23 ± 0.14 a | 26.52 ± 0.57 a |
| SE+ | 12.02 ± 0.10 b | 30.31 ± 0.12 b | |
| NaCl | SE− | 12.55 ± 0.13 c | 33.16 ± 0.71 c |
| SE+ | 12.56 ± 0.11 c | 34.72 ± 0.48 d | |
| Treatment | Stress Tolerance Index (STI) | Mean ProductivityIndex (MPI) | Yield Stability Index (YSI) | Stress Susceptibility Index (SSI) | Yield Reduction (YR) |
|---|---|---|---|---|---|
| SE− | 0.15 | 13.93 | 0.70 | 1.12 | 0.30 |
| SE+ | 0.22 | 16.79 | 0.76 | 0.90 | 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy 2022, 12, 2495. https://doi.org/10.3390/agronomy12102495
Hernández-Herrera RM, Sánchez-Hernández CV, Palmeros-Suárez PA, Ocampo-Alvarez H, Santacruz-Ruvalcaba F, Meza-Canales ID, Becerril-Espinosa A. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy. 2022; 12(10):2495. https://doi.org/10.3390/agronomy12102495
Chicago/Turabian StyleHernández-Herrera, Rosalba Mireya, Carla Vanessa Sánchez-Hernández, Paola Andrea Palmeros-Suárez, Héctor Ocampo-Alvarez, Fernando Santacruz-Ruvalcaba, Iván David Meza-Canales, and Amayaly Becerril-Espinosa. 2022. "Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress" Agronomy 12, no. 10: 2495. https://doi.org/10.3390/agronomy12102495
APA StyleHernández-Herrera, R. M., Sánchez-Hernández, C. V., Palmeros-Suárez, P. A., Ocampo-Alvarez, H., Santacruz-Ruvalcaba, F., Meza-Canales, I. D., & Becerril-Espinosa, A. (2022). Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy, 12(10), 2495. https://doi.org/10.3390/agronomy12102495









