Impact of Different Rootstocks on Antioxidant Properties and Volatile Profile of Honeydew Melons (Cucumis melo L.) during Postharvest Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Melon Fruits and Postharvest Storage
2.2. Melon Sample Preparation
2.3. Total Phenol Content
2.4. Ascorbic Acid Content
2.5. Total Chlorophyl Content
2.6. Antioxidant Scavenging Activities
2.7. Volatile Compounds
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Changes in Antioxidants in Grafted and Non-Grafted Honeydew Melons during Postharvest Storage
3.1.1. Total Phenols
3.1.2. Ascorbic Acid Content
3.1.3. Total Chlorophyll Content
3.2. Changes in Antioxidant Activity of Grafted and Un-Grafted Honeydew Melons during Postharvest Storage
3.3. Volatile Compounds
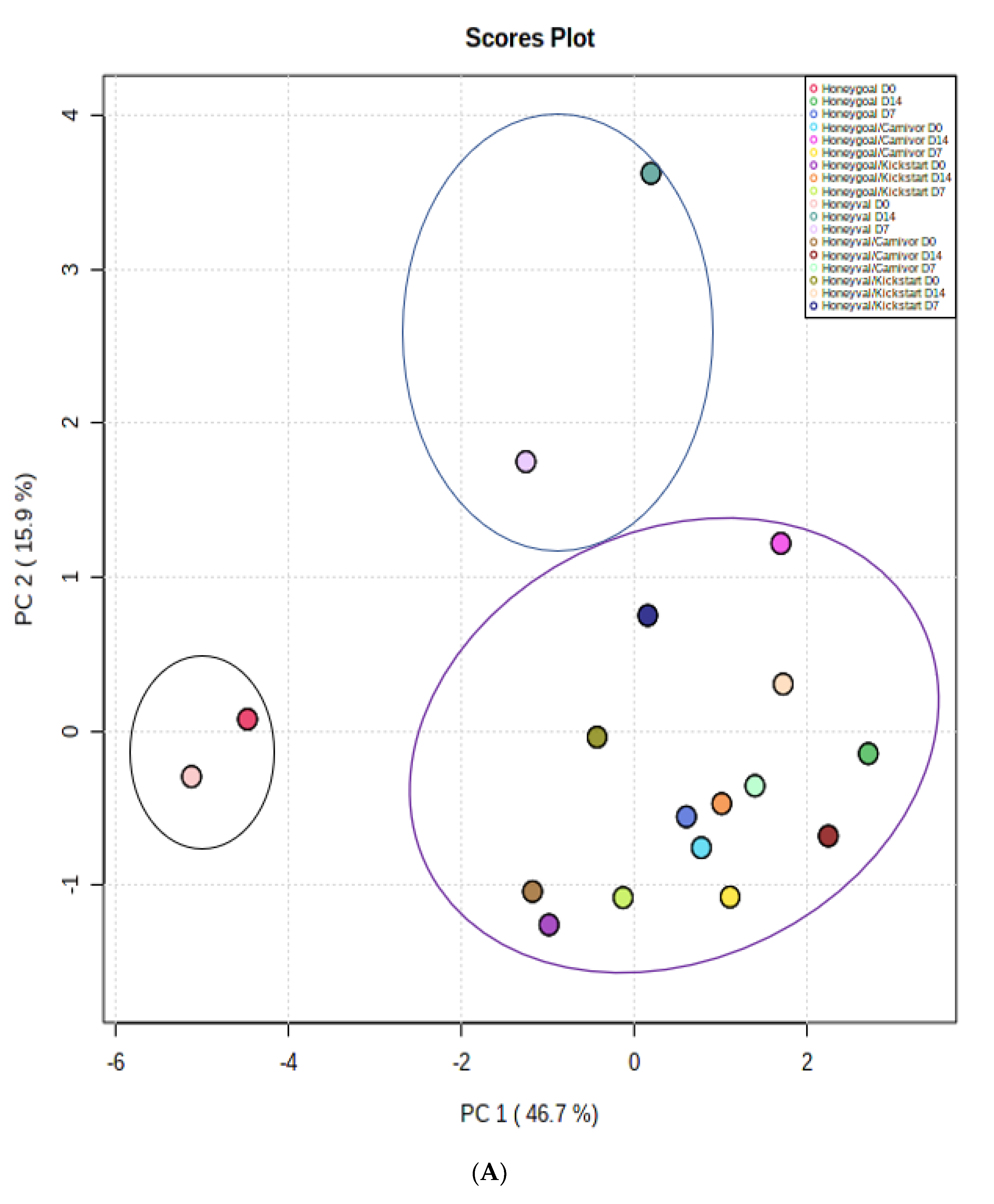
3.4. Multivariate Analysis
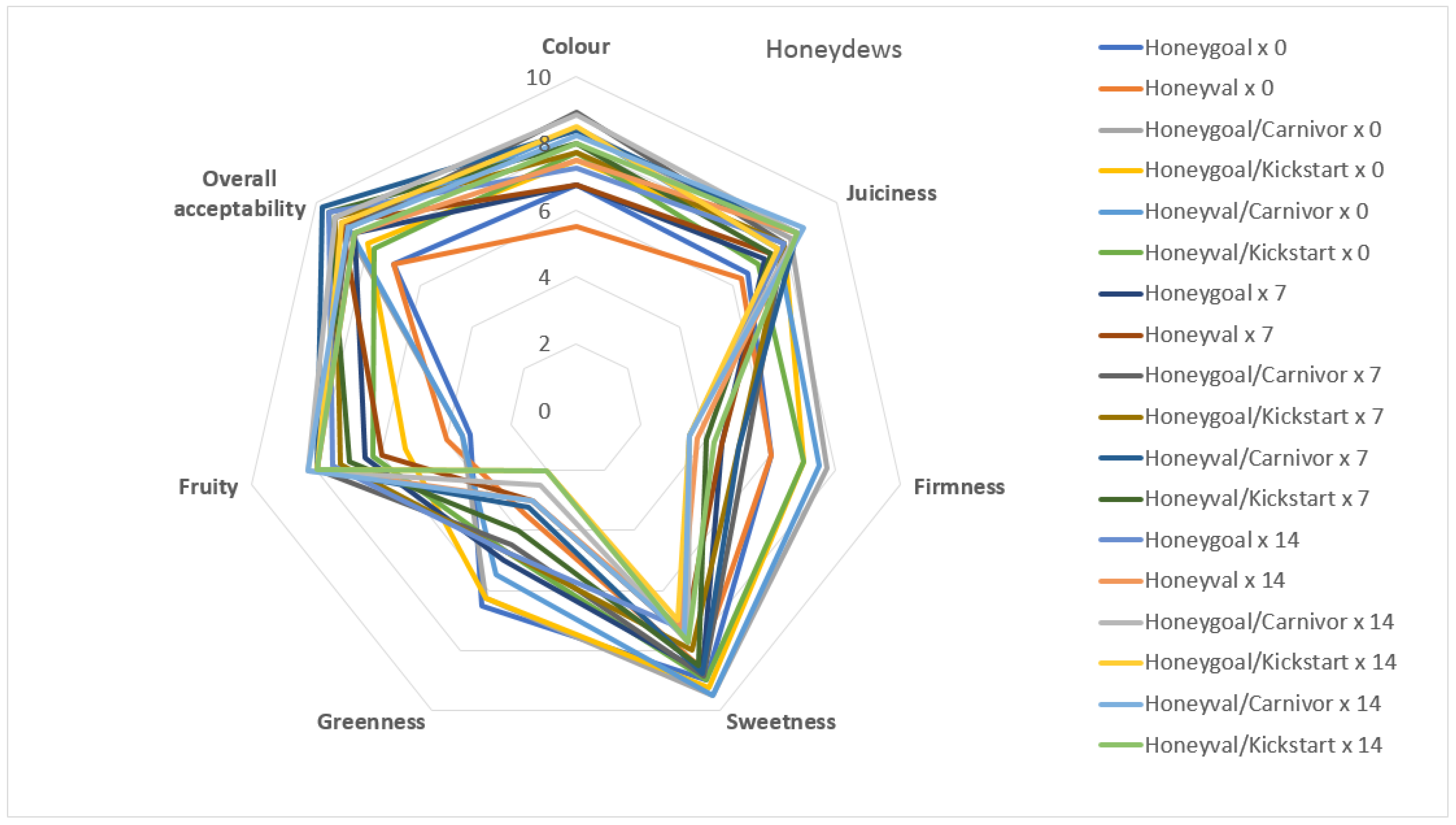
3.5. Sensory Attributes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perera, T.; Madhujith, T. The pattern of consumption of fruits and vegetables by undergraduate students: A case study. Trop. Agric. Res. 2012, 23, 261. [Google Scholar] [CrossRef] [Green Version]
- Ruel, M.T.; Minot, N.; Smith, L. Patterns and Determinants of Fruit and Vegetable Consumption in Sub-Saharan Africa: A Multicountry Comparison; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Park, M.-H.; Chang, E.-H.; Yang, H.-J.; Lee, J.-S.; Do, G.-R.; Song, H.J.; Chang, M.-S.; Ku, K.-M. Modified atmosphere and humidity film reduces browning susceptibility of oriental melon suture tissue during cold storage. Foods 2020, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Sugiyama, M.; Ohara, T.; Morishita, M. Influence of rootstocks on the resistance of grafted cucumber (Cucumis sativus L.) scions to powdery mildew (Podosphaera xanthii U. Braun & N. Shishkoff). Jpn. Soc. Hortic. Sci. 2006, 75, 135–140. [Google Scholar]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock—Scion interactions. Sci. Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of different rootstock on plant growth, yield and quality of watermelon. Aust. J. Exp. Agric. 2003, 43, 1269–1274. [Google Scholar] [CrossRef]
- USDA. Cantaloupe, Honeydew, Honey Ball and Other Similar Melons. In Shipping Point and Market Inspection Instructions; USDA: Washington, DC, USA, 2021. [Google Scholar]
- Alsmeirat, N.; El-Assi, N. Changes in esters, alcohols and acetaldehyde in two cultivars of Charentais melon as influenced by harvest date and storage. Int. J. Bot. 2010, 6, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.; Gao, H.; Jiang, Y. Insights into metabolomics in quality attributes of postharvest fruit. Curr. Opin. Food Sci. 2022, 45, 100836. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Juma, S.; Du, X. Consumer acceptance of watermelon flesh-rind blends and the effect of rind on refreshing perception. J. Food Sci. 2021, 86, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef] [PubMed]
- Lignou, S.; Parker, J.K.; Baxter, C.; Mottram, D.S. Sensory and instrumental analysis of medium and long shelf-life Charentais cantaloupe melons (Cucumis melo L.) harvested at different maturities. Food Chem. 2014, 148, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Obando-Ulloa, J.M.; Nicolai, B.; Lammertyn, J.; Bueso, M.C.; Monforte, A.J.; Fernández-Trujillo, J.P. Aroma volatiles associated with the senescence of climacteric or non-climacteric melon fruit. Postharvest Biol. Technol. 2009, 52, 146–155. [Google Scholar] [CrossRef]
- Lecholocholo, N.; Shoko, T.; Manhivi, V.E.; Maboko, M.M.; Akinola, S.A.; Sivakumar, D. Influence of different rootstocks on quality and volatile constituents of cantaloupe and honeydew melons (Cucumis melo L.) grown in high tunnels. Food Chem. 2022, 393, 133388. [Google Scholar] [CrossRef]
- Park, E.; Luo, Y.; Marine, S.C.; Everts, K.L.; Micallef, S.A.; Bolten, S.; Stommel, J. Consumer preference and physicochemical evaluation of organically grown melons. Postharvest Biol. Technol. 2018, 141, 77–85. [Google Scholar] [CrossRef]
- Managa, M.G.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote Leaf (Sechium edule) and pineapple (Ananas comosus) smoothies. Front. Nutr. 2021, 8, 649189. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Sivakumar, D.; Beukes, M.; van Rensburg, W.J. Effect of modified atmosphere packaging on the quality and bioactive compounds of Chinese cabbage (Brasicca rapa L. ssp. chinensis). J. Sci. Food Agric. 2013, 93, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Phahlane, C.J.; Laurie, S.M.; Shoko, T.; Manhivi, V.E.; Sivakumar, D. An evaluation of phenolic compounds, carotenoids, and antioxidant properties in leaves of South African cultivars, Peruvian 199062.1 and USA’s Beauregard. Front. Nutr. 2021, 8, 773550. [Google Scholar] [CrossRef]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Kolayli, S.; Kara, M.; Tezcan, F.; Erim, F.B.; Sahin, H.; Ulusoy, E.; Aliyazicioglu, R. Comparative study of chemical and biochemical properties of different melon cultivars: Standard, hybrid, and grafted melons. J. Agric. Food Chem. 2010, 58, 9764–9769. [Google Scholar] [CrossRef] [PubMed]
- Mannozzi, C.; Glicerina, V.; Tylewicz, U.; Castagnini, J.M.; Canali, G.; Dalla Rosa, M.; Romani, S. Influence of two different coating application methods on the maintenance of the nutritional quality of fresh-cut melon during storage. Appl. Sci. 2021, 11, 8510. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Dixon, W.R.; Goulette, T.R. Modeling the degradation kinetics of ascorbic acid. Crit. Rev. Food Sci. Nutr. 2018, 58, 1478–1494. [Google Scholar] [CrossRef] [PubMed]
- Touati, N.; Barba, F.J.; Louaileche, H.; Frigola, A.; Esteve, M.J. Effect of storage time and temperature on the quality of fruit nectars: Determination of nutritional loss indicators. J. Food Qual. 2016, 39, 209–217. [Google Scholar] [CrossRef] [Green Version]
- San Bautista, A.; Calatayud, A.; Nebauer, S.G.; Pascual, B.; Vicente Maroto, J. Effects of simple and double grafting melon plants on mineral absorption, photosynthesis, biomass and yield. Sci. Horticulturae 2013, 130, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Rosenbloom, R.A.; Wang, W.; Zhao, Y. Delaying ripening of ‘Bartlett’ pears (Pyrus communis) during long-term simulated industrial cold storage: Mechanisms and validation of chitosan coatings with cellulose nanocrystals Pickering emulsions. LWT Food Sci. Technol. 2020, 122, 109053. [Google Scholar] [CrossRef]
- Ahlawat, Y.; Liu, T. Varied Expression of senescence-associated and ethylene-related genes during postharvest storage of Brassica vegetables. Int. J. Mol. Sci. 2021, 22, 839. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Fan, J.; Liu, H.; Zhou, J.; Chang, X. iTRAQ proteomics reveals changes in the lettuce (Lactuca sativa L. Grand Rapid) proteome related to colour and senescence under modified atmosphere packaging. J. Sci. Food Agric. 2019, 99, 1908–1918. [Google Scholar] [CrossRef]
- Tlili, I.; Riadh, I.; Rached, Z.; Ali, A.B.; Arfaoui, K.; R’him, T. Effect of The Storage Period on the Antioxidant Properties of Different Watermelon Cultivars Grown in Tunisia. Turk. J. Agric.—Food Sci. Technol. 2022, 10, 1138–1141. [Google Scholar] [CrossRef]
- Kubota, N.; Yakuhiji, H.; Nishiyama, N.; Mimura, H.; Shimamaura, K. Phenolic contens and L-phenylalanine ammonia -lyase activity in peach fruit as affected by rootstocks. J. Jpn. Soc. Hort. Sci. 2001, 70, 151–156. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zeng, J.; Li, W.; Dong, Y. Effect of ethanol fumigation on pericarp browning associated with phenol metabolism, storage quality, and antioxidant systems of wampee fruit during cold storage. Food Sci. Nutr. 2020, 8, 3380–3388. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A. The Pherobase: Database of Pheromones and Semiochemicals; HortResearch: Lincoln, New Zealand, 2021. [Google Scholar]
- Mahattanatawee, K.; Goodner, K.L.; Baldwin, E.A. Volatile constituents and character impact compounds of selected Florida’s tropical fruit. Proc. Proc. Fla. State Hortic. Soc. 2005, 118, 414–418. [Google Scholar]
- Wang, Y.-H.; Behera, T.K.; Kole, C. Genetics, Genomics and Breeding of Cucurbits; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Obando-Ulloa, J.M.; Ruiz, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Aroma profile of a collection of near-isogenic lines of melon (Cucumis melo L.). Food Chem. 2010, 118, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Saftner, R.; Abbott, J.A.; Lester, G.; Vinyard, B. Sensory and analytical comparison of orange-fleshed honeydew to cantaloupe and green-fleshed honeydew for fresh-cut chunks. Postharvest Biol. Technol. 2006, 42, 150–160. [Google Scholar] [CrossRef]
- Derossi, A.; Mastrandrea, L.; Amodio, M.; Pati, S.; Colelli, G. Reaction mechanisms for volatiles responsible of off-odors of fresh cut melons. In Proceedings of the VI International Symposium on Applications of Modelling as an Innovative Technology in the Horticultural Supply Chain Model-IT 1311, Molfetta, Italy, 9–12 June 2019; ISHS: Leuven, Belgium, 2019; pp. 15–22. [Google Scholar]
- Beaulieu, J.C. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 2006, 41, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.C.; Grimm, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Gonda, I.; Burger, Y.; Schaffer, A.A.; Ibdah, M.; Tadmor, Y.A.; Katzir, N.; Fait, A.; Lewinsohn, E. Biosynthesis and perception of melon aroma. Biotechnol. Flavor Prod. 2016, 281–305. [Google Scholar] [CrossRef]
- Bett-Garber, K.; Beaulieu, J.; Ingram, D. Effect of storage on sensory properties of fresh-cut cantaloupe varieties. J. Food Qual. 2003, 26, 323–335. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G. Quality and postharvest performance of watermelon fruit in response to grafting on interspecific cucurbit rootstocks. J. Food Qual. 2015, 38, 21–29. [Google Scholar] [CrossRef]
- Bett-Garber, K.; Greene, J.; Lamikanra, O.; Ingram, D.; Watson, M. Effect of storage temperature variations on sensory quality of fresh-cut cantaloupe melon. J. Food Qual. 2011, 34, 19–29. [Google Scholar] [CrossRef]
- Beaulieu, J.; Baldwin, E. Flavor and aroma of fresh-cut fruits and vegetables. In Fresh Cut Fruits and Vegetables: Science, Technology and Market; CRC Press: Boca Raton, FL, USA, 2002; pp. 391–425. [Google Scholar]
- Zhou, D.; Li, T.; Cong, K.; Suo, A.; Wu, C. Influence of cold plasma on quality attributes and aroma compounds in fresh-cut cantaloupe during low temperature storage. LWT 2022, 154, 112893. [Google Scholar] [CrossRef]
- Amaro, A.L.; Beaulieu, J.C.; Grimm, C.C.; Stein, R.E.; Almeida, D.P. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012, 130, 49–57. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Kappel, N.; Mozafarian, M. Effects of Different Rootstocks and Storage Temperatures on Postharvest Quality of Eggplant (Solanum melongena L. cv. Madonna). Horticulturae 2022, 8, 862. [Google Scholar] [CrossRef]


| TPC (mg/100 g) | % Loss (−) | AA (mg/100 g) | % Loss (−) | Total Chlorophyll (mg/mL) | % Loss (−) | |
|---|---|---|---|---|---|---|
| Honeygoal × 0 d | 24.14 ± 0.36 i | 17.52 ± 1.15 e | 1.94 ± 0.56 d | |||
| Honeygoal × 7 d | 19.41 ± 0.63 j | −19.6 ± 0.42 d | 12.11 ± 0.78 g | −30.87 ± 1.08 c | 1.47 ± 1.62 f | −24.22 ± 0.09 f |
| Honeygoal × 14 d | 15.51 ± 0.85 k | −35.74 ± 0.86 b | 9.20 ± 0.83 h | −47.48 ± 0.72 a | 1.47 ± 1.62 f | −24.22 ± 0.62 f |
| Honeygoal/Carnivor × 0 d | 32.04 ± 1.47 a | 20.34 ± 1.05 b | 3.31 ± 0.59 a | |||
| Honeygoal/Carnivor × 7 d | 30.97 ± 0.66 b | −3.33 ± 0.94 i | 19.71 ± 0.61 b | −3.09 ± 1.23 j | 3.11 ± 0.48 b | −6.04 ± 0.88 i |
| Honeygoal/Carnivor × 14 d | 30.01 ± 0.32 d | −6.33 ± 1.08 f | 19.08 ± 1.49 c | −6.19 ± 1.09 i | 2.35 ± 1.26 c | −29.00 ± 0.94 e |
| Honeygoal/Kickstart × 0 d | 30.38 ± 0.47 c | 19.73 ± 0.69 b | 2.23 ± 0.74 c | |||
| Honeygoal/Kickstart × 7 d | 28.32 ± 0.72 f | −6.78 ± 0.43 f | 15.94 ± 0.52 e | −19.20 ± 0.43 f | 2.16 ± 1.22 c | −3.13 ± 0.15 j |
| Honeygoal/Kickstart × 14 d | 26.16 ± 0.73 h | −13.89 ± 0.63 e | 13.62 ± 0.64 f | −30.96 ± 0.81 c | 1.39 ± 0.55 f | −37.66 ± 0.12 c |
| Honeyval × 0 d | 27.33 ± 1.10 g | 17.02 ± 1.21 e | 1.74 ± 0.09 e | |||
| Honeyval × 7 d | 21.33 ± 0.73 i | −21.95 ± 0.94 c | 12.09 ± 0.83 g | −28.97 ± 1.32 d | 1.13 ± 1.21 g | −35.05 ± 0.94 b |
| Honeyval × 14 d | 12.70 ± 0.74 l | −53.53 ± 1.32 a | 9.43 ± 0.66 h | −44.59 ± 1.72 b | 0.64 ± 1.45 h | −63.21 ± 1.72 a |
| Honeyval/Carnivor × 0 d | 31.24 ± 0.34 b | 22.93 ± 0.89 a | 3.32 ± 1.22 a | |||
| Honeyval/Carnivor × 7 d | 31.44 ± 1.01 b | −1.29 ± 0.92 k | 22.50 ± 0.16 a | −1.87 ± 1.44 k | 3.06 ± 1.04 b | −7.83 ± 0.83 h |
| Honeyval/Carnivor × 14 d | 29.94 ± 0.81 d | −3.54 ± 1.04 h | 18.93 ± 0.73 c | −17.44 ± 1.09 h | 2.33 ± 1.06 c | −29.81 ± 0.12 e |
| Honeyval/Kickstart × 0 d | 31.16 ± 0.68 b | 22.52 ± 1.04 a | 2.03 ± 0.48 d | |||
| Honeyval/Kickstart × 7 d | 30.37 ± 0.96 c | −2.53 ± 0.43 j | 18.25 ± 1.02 d | −18.96 ± 1.73 g | 1.78 ± 0.92 e | −12.31 ± 0.91 g |
| Honeyval/Kickstart × 14 d | 29.25 ± 0.36 e | −6.12 ± 0.68 g | 16.48 ± 0.61 e | −26.82 ± 0.14 e | 1.42 ± 0.74 f | −30.04 ± 1.21 d |
| Cultivar × Rootstock × storage time | 0.36 * | 0.63 * | 0.16 * |
| Cantaloupes | DPPH (IC50 mg/mL) | ABTS (IC50 mg/mL) | FRAP (µM TEAC/g) |
|---|---|---|---|
| Honeygoal × 0 d | 17.37 ± 1.15 g | 2.23 ± 0.39 c | 2.59 ± 0.04 f |
| Honeygoal × 7 d | 21.63 ± 0.06 d | 2.94 ± 0.23 b | 2.04 ± 0.04 i |
| Honeygoal × 14 d | 28.87 ± 0.02 a | 3.90 ± 0.62 a | 1.22 ± 0.01 k |
| Honeygoal/Carnivor × 0 d | 13.55 ± 0.57 i | 0.94 ± 0.39 h | 3.47 ± 0.15 a |
| Honeygoal/Carnivor × 7 d | 15.47 ± 0.02 h | 1.06 ± 0.22 g | 3.22 ± 0.03 c |
| Honeygoal/Carnivor × 14 d | 18.05 ± 0.01 f | 1.15 ± 0.96 f | 2.91 ± 0.03 d |
| Honeygoal/Kickstart × 0 d | 13.84 ± 0.67 i | 0.83 ± 0.50 h | 2.72 ± 0.02 e |
| Honeygoal/Kickstart × 7 d | 16.01 ± 0.08 h | 1.01 ± 0.45 g | 2.33 ± 0.06 g |
| Honeygoal/Kickstart × 14 d | 18.41 ± 0.08 f | 1.22 ± 0.33 f | 2.02 ± 0.01 i |
| Honeyval × 0 d | 18.70 ± 0.45 e | 2.13 ± 0.51 c | 2.76 ± 0.03 e |
| Honeyval × 7 d | 22.87 ± 0.01 c | 3.02 ± 0.40 b | 1.74 ± 0.04 j |
| Honeyval × 14 d | 28.42 ± 1.92 a | 3.82 ± 0.61 a | 1.13 ± 0.05 l |
| Honeyval/Carnivor × 0 d | 16.54 ± 0.56 h | 1.13 ± 0.40 f | 3.30 ± 0.04 b |
| Honeyval/Carnivor × 7 d | 18.42 ± 2.11 f | 1.25 ± 0.62 f | 3.01 ± 0.16 d |
| Honeyval/Carnivor × 14 d | 22.22 ± 0.34 c | 1.49 ± 0.50 e | 2.35 ± 0.04 g |
| Honeyval/Kickstart × 0 d | 16.62 ± 1.02 h | 1.21 ± 0.46 f | 3.20 ± 0.04 c |
| Honeyval/Kickstart × 7 d | 19.12 ± 0.02 e | 1.43 ± 0.74 e | 2.75 ± 0.03 e |
| Honeyval/Kickstart × 14 d | 23.46 ± 0.10 b | 1.84 ± 0.50 d | 2.22 ± 0.07 h |
| Cultivar × Rootstock × storage time | 0.59 * | 0.18 * | 0.08 * |
| Volatile compounds (b IUPAC Name) | Ethyl Butanoate | Ethyl Hexanoate | Hexyl Acetate | Methyl Acetate | Benzyl Butanoate | 2-Ethyl hexyl Acetate | Propyl Acetate | Methyl-3- nonenoate | 2-Methyl Propyl Acetate | Total Esters |
|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated KI | 799 | 1001 | 1007 | 523 | 1344 | 1160 | 712 | 1228 | 768 | |
| Literature KI (DB-5) | 800 | 1002 | 1008 | 522 | 1354 | 1159 | 713 | 1227 | 767 | |
| Honeygoal × 0 d | 2.42 ± 0.15 i | 0.58 ± 0.35 k | 8.96 ± 0.05 n | 0.67 ± 0.29 n | 3.49 ± 0.25 h | 0.65 ± 0.44 l | 3.66 ± 0.59 k | 11.36 ± 1.08 j | 4.96 ± 0.73 m | 36.75 |
| Honeygoal × 7 d | 7.46 ± 0.30 g | 33.04 ± 0.54 b | 10.61 ± 0.10 m | 7.59 ± 0.28 h | 6.78 ± 0.73 g | 4.98 ± 0.16 i | 12.60 ± 1.09 e | 18.83 ± 1.13 i | 16.60 ± 0.99 k | 118.49 |
| Honeygoal × 14 d | 35.39 ± 0.40 a | 38.51 ± 0.79 a | 21.58 ± 0.11 g | 34.97 ± 1.69 a | 8.56 ± 0.38 f | 14.17 ± 0.07 e | 21.91 ± 0.68 a | 30.58 ± 0.57 a | 29.62 ± 0.91 b | 235.29 |
| Honeygoal/Carnivor × 0 d | 2.48 ± 0.33 i | 13.78 ± 1.63 e | 30.54 ± 0.58 e | 21.97 ± 0.39 d | 17.37 ± 0.46 c | 24.21 ± 0.44 c | 8.58 ± 0.52 i | 24.66 ± 0.11 e | 24.07 ± 1.31 f | 167.66 |
| Honeygoal/Carnivor × 7 d | 4.38 ± 0.24 h | 16.95 ± 0.52 d | 44.19 ± 0.60 c | 24.34 ± 0.83 c | 27.37 ± 0.46 b | 34.65 ± 1.00 b | 10.05 ± 0.70 g | 26.49 ± 0.75 d | 26.92 ± 0.45 d | 215.34 |
| Honeygoal/Carnivor × 14 d | 7.44 ± 0.39 g | 29.15 ± 0.52 c | 62.97 ± 1.68 b | 25.02 ± 0.42 c | 30.52 ± 0.18 a | 39.85 ± 0.56 a | 11.76 ± 0.46 f | 27.55 ± 0.11 c | 28.07 ± 0.54 c | 262.33 |
| Honeygoal/Kickstart × 0 d | 0.37 ± 0.09 k | 2.45 ± 0.26 j | 14.54 ± 0.48 j | 2.56 ± 0.29 l | 11.04 ± 0.68 e | 4.38 ± 0.36 i | 6.47 ± 0.70 j | 20.56 ± 0.76 h | 21.26 ± 1.43 j | 83.63 |
| Honeygoal/Kickstart × 7 d | 2.37 ± 0.09 i | 7.80 ± 0.05 g | 21.20 ± 0.12 g | 4.38 ± 0.25 j | 17.11 ± 0.45 c | 9.15 ± 0.01 h | 8.06 ± 0.49 i | 22.39 ± 1.37 g | 23.73 ± 0.54 g | 116.19 |
| Honeygoal/Kickstart × 14 d | 7.37 ± 0.09 g | 10.37 ± 0.29 f | 28.85 ± 0.62 f | 6.50 ± 0.31 i | 15.44 ± 0.46 d | 10.31 ± 0.44 g | 10.42 ± 1.34 g | 24.59 ± 0.53 e | 26.44 ± 0.47 d | 140.29 |
| Honeyval × 0 d | 1.09 ± 0.01 j | 0.45 ± 0.33 k | 8.65 ± 0.05 n | 1.36 ± 0.05 m | ND | 0.70 ± 0.14 l | 2.58 ± 0.44 l | 9.51 ± 0.45 k | 8.37 ± 0.42 l | 33.01 |
| Honeyval × 7 d | 4.48 ± 0.29 h | 2.45 ± 0.33 j | 42.28 ± 1.33 d | 2.32 ± 0.20 l | ND | 2.16 ± 0.32 k | 10.87 ± 0.47 g | 23.56 ± 2.27 f | 30.20 ± 1.05 b | 118.32 |
| Honeyval × 14 d | 26.46 ± 0.34 b | 7.66 ± 0.28 g | 68.32 ± 0.58 a | 3.32 ± 0.20 k | ND | 3.70 ± 0.33 j | 21.87 ± 0.47 a | 28.03 ± 0.76 b | 42.52 ± 0.95 a | 201.88 |
| Honeyval/Carnivor × 0 d | 8.40 ± 0.35 f | 4.23 ± 0.20 i | 10.38 ± 0.32 m | 17.00 ± 0.54 f | ND | 9.14 ± 0.25 h | 7.99 ± 1.01 i | 22.52 ± 1.05 g | 22.44 ± 1.05 i | 102.1 |
| Honeyval/Carnivor × 7 d | 11.28 ± 0.56 d | 4.33 ± 0.19 i | 13.52 ± 0.57 j | 20.91 ± 0.03 e | ND | 11.69 ± 1.02 f | 9.62 ± 0.55 h | 24.05 ± 1.02 e | 25.19 ± 1.11 e | 120.51 |
| Honeyval/Carnivor × 14 d | 14.38 ± 0.26 c | 5.11 ± 0.31 h | 16.60 ± 0.99 i | 26.24 ± 0.61 b | ND | 24.08 ± 1.23 c | 11.36 ± 0.59 f | 26.07 ± 0.36 d | 27.99 ± 0.39 c | 151.83 |
| Honeyval/Kickstart × 0 d | 4.22 ± 0.25 h | 2.48 ± 0.19 j | 11.65 ± 0.93 l | 1.28 ± 0.18 m | ND | 3.17 ± 0.55 j | 15.36 ± 0.34 d | 20.19 ± 0.34 h | 20.96 ± 1.47 j | 79.31 |
| Honeyval/Kickstart × 7 d | 8.80 ± 0.01 f | 4.58 ± 0.30 i | 12.93 ± 0.62 k | 3.36 ± 0.29 l | ND | 17.20 ± 1.22 d | 16.96 ± 0.56 c | 22.80 ± 0.82 g | 23.28 ± 0.57 h | 109.91 |
| Honeyval/Kickstart × 14 d | 9.44 ± 0.46 e | 4.82 ± 0.07 i | 19.36 ± 0.22 h | 8.78 ± 0.23 g | ND | 17.35 ± 0.99 d | 19.38 ± 0.15 b | 24.88 ± 0.56 e | 25.32 ± 0.53 e | 129.33 |
| Cultivar × Rootstock × storage time | 0.64 * | 2.69 * | 1.28 * | 1.06 * | 1.78 * | 0.68 * | 1.04 * | 0.49 * | 0.34 * |
| Volatile Compounds (IUPAC name) | Hexadecane | Decane | Nonane | Pentadecane | 2-methyl Decane | 2-methyl Nonane | Total Alkanes |
|---|---|---|---|---|---|---|---|
| Calculated (KI) | 1601 | 1001 | 901 | 1499 | 1060 | 961 | |
| Literature KI (DB-5) | 1600 | 999 | 899 | 1500 | 1061 | 962 | |
| Aroma description | Non-specific odor | Gasoline-like | Gasoline-like odor | Green citrus honey | Gasoline-like to odorles | Sharp | |
| Honeygoal × 0 d | 1.25 ± 0.83 i | 0.67 ± 0.29 l | 0.7 ± 0.13 e | 0.24 ± 0.31 l | 0.24 ± 0.22 k | 0.12 ± 0.21 m | 3.22 ± 0.33 e |
| Honeygoal × 7 d | 4.80 ± 0.68 g | 2.32 ± 0.21 k | 1.79 ± 0.12 c | 4.24 ± 0.31 j | 4.55 ± 0.19 e | 2.18 ± 0.05 i | 19.88 ± 0.26 d |
| Honeygoal × 14 d | 5.38 ± 0.57 f | 3.32 ± 0.20 j | 5.06 ± 0.57 a | 26.44 ± 0.46 a | 11.27 ± 0.52 b | 17.61 ± 0.54 c | 69.08 ± 0.47 bc |
| Honeygoal/Carnivor × 0 d | 1.23 ± 1.81 i | 25.02 ± 0.42 b | 0.42 ± 0.21 f | 22.71 ± 0.66 c | 0.74 ± 0.08 i | 2.35 ± 0.25 i | 52.47 ± 0.57 c |
| Honeygoal/Carnivor × 7 d | 8.56 ± 0.65 e | 54.97 ± 1.41 a | 0.48 ± 0.67 f | 24.28 ± 0.32 b | 2.30 ± 0.12 g | 4.20 ± 0.19 g | 94.79 ± 0.56 a |
| Honeygoal/Carnivor × 14 d | 19.92 ± 0.54 c | 54.34 ± 0.83 a | 0.57 ± 0.55 e | 24.28 ± 0.81 b | 3.77 ± 0.12 f | 7.42 ± 0.36 e | 110.3 ± 0.53 a |
| Honeygoal/Kickstart × 0 d | 1.26 ± 0.12 i | 2.56 ± 0.29 k | 0.52 ± 0.13 e | 1.29 ± 0.38 k | 1.60 ± 0.12 h | 4.34 ± 0.12 g | 11.57 ± 0.19 e |
| Honeygoal/Kickstart × 7 d | 5.03 ± 0.32 f | 7.59 ± 0.28 g | 0.44 ± 0.04 f | 4.29 ± 0.38 j | 1.24 ± 0.21 h | 4.85 ± 0.69 g | 23.44 ± 0.32 d |
| Honeygoal/Kickstart × 14 d | 14.03 ± 1.12 d | 15.91 ± 0.03 e | 0.53 ± 0.10 e | 9.69 ± 0.24 g | 1.70 ± 0.34 h | 5.69 ± 0.38 f | 47.55 ± 0.38 c |
| Honeyval × 0 d | 1.63 ± 0.33 i | 1.28 ± 0.18 l | ND | 1.54 ± 0.02 k | 0.42 ± 0.11 j | 0.97 ± 0.12 k | 5.84 ± 0.16 e |
| Honeyval × 7 d | 3.08 ± 0.01 h | 4.38 ± 0.25 i | ND | 6.04 ± 0.01 i | 0.96 ± 0.29 i | 1.69 ± 0.38 j | 16.15 ± 0.19 d |
| Honeyval × 14 d | 3.61 ± 0.37 h | 6.50 ± 0.31 h | ND | 8.56 ± 0.27 h | 14.08 ± 0.58 a | 65.83 ± 0.99 a | 98.58 ± 0.50 a |
| Honeyval/Carnivor × 0 d | 23.96 ± 1.21 b | 3.97 ± 0.29 i | 0.26 ± 0.21 g | 13.30 ± 0.42 f | 1.66 ± 1.31 h | 0.30 ± 0.00 l | 43.45 ± 0.57 c |
| Honeyval/Carnivor × 7 d | 42.48 ± 0.34 a | 8.78 ± 0.23 f | 1.26 ± 0.21 d | 14.53 ± 0.61 e | 4.11 ± 0.49 e | 3.41 ± 0.33 h | 74.57 ± 0.33 b |
| Honeyval/Carnivor × 14 d | 42.37 ± 2.12 a | 26.24 ± 0.61 b | 3.31 ± 0.21 b | 19.55 ± 0.40 d | 5.23 ± 0.01 d | 10.97 ± 0.79 d | 83.67 ± 0.65 b |
| Honeyval/Kickstart × 0 d | 5.88 ± 0.57 f | 1.36 ± 0.05 l | 0.22 ± 0.01 g | 9.11 ± 0.41 g | 0.84 ± 0.21 i | 0.69 ± 0.38 k | 18.12 ± 0.27 d |
| Honeyval/Kickstart × 7 d | 5.97 ± 0.39 f | 17.00 ± 0.54 d | 0.37 ± 0.22 f | 9.24 ± 0.31 g | 3.27 ± 0.04 f | 5.79 ± 0.26 f | 41.64 ± 0.29 c |
| Honeyval/Kickstart × 14 d | 15.04 ± 0.79 d | 21.97 ± 0.39 c | 0.58 ± 0.42 e | 15.08 ± 0.48 e | 10.25 ± 0.51 c | 34.67 ± 1.41 b | 97.59 ± 1.32 a |
| Cultivar × Rootstock × storage time | 0.23 * | 0.64 * | 0.26 * | 1.05 * | 0.28 * | 0.49 * | 4.58 * |
| Ketone | Alkene | Total Alkene | Aldehyde | Total Aldehyde | ||||
|---|---|---|---|---|---|---|---|---|
| Volatile compounds (IUPAC name) | 3- Hydroxybutan-2-one | 4,5 Dimethyl -1-hexene | 4-methylundec-1-ene | 2-Heptenal (Z) | Benzaldehyde | Pentanal | ||
| Calculated KI | 719 | 747 | 951 | 963 | 701 | |||
| Literature KI (DB-5) | 718 | 952 | 961 | 699 | ||||
| Aroma description | Pleasant, buttery | Green fruity sweet fatty fresh apple pear | Fatty and fruity | Powerful sweet | Musty, apricot like | |||
| Honeygoal × 0 d | 0.80 ± 0.52 l | 0.93 ± 0.34 k | 0.94 ± 0.13 h | 2.67 | 2.64 ± 0.25 g | 2.71 ± 0.19 j | 0.94 ± 0.16 j | 6.29 ± 0.19 i |
| Honeygoal × 7 d | 28.72 ± 0.76 c | 1.67 ± 1.03 k | 2.55 ± 0.25 g | 32.94 | 4.28 ± 0.14 e | 5.98 ± 0.77 h | 2.55 ± 0.05 h | 12.81 ± 1.16 g |
| Honeygoal × 14 d | 60.52 ± 0.29 a | 38.60 ± 1.24 a | 5.49 ± 0.77 e | 104.61 | 12.76 ± 0.07 a | 25.03 ± 0.13 a | 5.49 ± 0.39 f | 43.28 ± 2.08 c |
| Honeygoal/Carnivor × 0 d | 3.02 ± 0.35 k | 6.38 ± 0.33 h | 7.59 ± 0.40 d | 16.99 | 5.23 ± 0.02 d | 9.77 ± 0.24 f | 7.59 ± 1.75 e | 22.59 ± 1.30 e |
| Honeygoal/Carnivor × 7 d | 4.46 ± 0.26 j | 11.09 ± 0.01 f | 31.04 ± 0.12 b | 46.59 | 6.40 ± 0.58 c | 15.61 ± 0.51 d | 31.04 ± 0.70 b | 53.05 ± 3.62 b |
| Honeygoal/Carnivor × 14 d | 17.07 ± 0.07 e | 13.69 ± 0.64 d | 73.17 ± 0.73 a | 103.93 | 8.39 ± 0.38 b | 15.61 ± 0.08 d | 73.17 ± 0.46 a | 97.17 ± 2.64 a |
| Honeygoal/Kickstart × 0 d | 1.29 ± 0.02 l | 0.47 ± 0.38 l | 0.67 ± 0.13 h | 2.43 | 3.61 ± 0.36 f | 9.26 ± 0.06 f | 0.67 ± 0.38 jk | 13.54 ± 0.68 g |
| Honeygoal/Kickstart × 7 d | 3.60 ± 0.31 k | 2.35 ± 0.35 j | 3.34 ± 1.03 f | 9.29 | 5.38 ± 0.43 d | 15.66 ± 0.43 d | 3.34 ± 0.55 h | 24.38 ± 0.34 e |
| Honeygoal/Kickstart × 14 d | 22.54 ± 0.57 d | 5.67 ± 0.55 i | 9.34 ± 1.10 d | 37.55 | 8.38 ± 0.43 b | 17.79 ± 0.77 c | 9.34 ± 0.55 d | 35.51 ± 0.18 d |
| Honeyval × 0 d | 2.04 ± 0.23 k | 0.87 ± 0.50 k | ND | 2.91 | 2.12 ± 0.03 g | 4.48 ± 0.05 i | 0,87 ± 0.23 j | 7.47 ± 0.10 i |
| Honeyval × 7 d | 4.03 ± 0.34 j | 12.29 ± 0.21 e | 4.36 ± 0.40 f | 20.68 | 6.50 ± 0.57 c | 8.27 ± 0.13 g | 4.36 ± 0.21 g | 19.13 ± 0.22 f |
| Honeyval × 14 d | 32.28 ± 1.07 b | 28.33 ± 0.20 b | 6.46 ± 1.15 d | 67.07 | 8.13 ± 0.76 b | 15.60 ± 0.50 d | 6.46 ± 0.36 f | 30.19 ± 0.54 d |
| Honeyval/Carnivor × 0 d | 2.64 ± 0.51 k | 3.45 ± 0.32 j | 1.16 ± 0.50 h | 7.25 | 4.77 ± 0.57 e | 12.13 ± 0.01 e | 1.16 ± 0.02 j | 18.06 ± 1.42 f |
| Honeyval/Carnivor × 7 d | 5.75 ± 0.54 i | 4.73 ± 0.11 i | 6.16 ± 0.42 d | 16.64 | 6.12 ± 0.03 c | 18.27 ± 0.45 c | 6.16 ± 0.59 f | 30.55 ± 2.04 d |
| Honeyval/Carnivor × 14 d | 6.62 ± 0.03 h | 5.08 ± 0.44 i | 25.09 ± 2.92 c | 36.79 | 8.82 ± 0.15 b | 25.09 ± 0.48 a | 25.09 ± 0.56 c | 59.0 ± 1.55 b |
| Honeyval/Kickstart × 0 d | 9.84 ± 0.36 g | 2.76 ± 0.11 j | 2.21 ± 0.70 g | 14.81 | 2.45 ± 0.24 g | 5.27 ± 0.13 h | 2.21 ± 0.11 hi | 9.93 ± 0.29 h |
| Honeyval/Kickstart × 7 d | 12.79 ± 0.55 f | 8.87 ± 0.50 g | 6.25 ± 0.52 d | 27.91 | 6.34 ± 0.27 c | 12.50 ± 0.28 e | 6.25 ± 0.52 f | 25.09 ± 0.26 e |
| Honeyval/Kickstart × 14 d | 17.20 ± 0.58 e | 25.06 ± 0.39 c | 7.64 ± 0.63 d | 49.9 | 8.16 ± 0.16 b | 21.65 ± 0.89 b | 7.64 ± 0.27 e | 37.45 ± 0.19 d |
| Cultivar × Rootstock × storage time | 0.87 * | 1.20 * | 1.05 * | 0.61 * | 1.50 * | 1.02* | 2.90* | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecholocholo, N.; Shoko, T.; Manhivi, V.E.; Akinola, S.A.; Maboko, M.M.; Sivakumar, D. Impact of Different Rootstocks on Antioxidant Properties and Volatile Profile of Honeydew Melons (Cucumis melo L.) during Postharvest Storage. Agronomy 2022, 12, 2498. https://doi.org/10.3390/agronomy12102498
Lecholocholo N, Shoko T, Manhivi VE, Akinola SA, Maboko MM, Sivakumar D. Impact of Different Rootstocks on Antioxidant Properties and Volatile Profile of Honeydew Melons (Cucumis melo L.) during Postharvest Storage. Agronomy. 2022; 12(10):2498. https://doi.org/10.3390/agronomy12102498
Chicago/Turabian StyleLecholocholo, Nkamo, Tinotenda Shoko, Vimbainashe E. Manhivi, Stephen A. Akinola, Martin M. Maboko, and Dharini Sivakumar. 2022. "Impact of Different Rootstocks on Antioxidant Properties and Volatile Profile of Honeydew Melons (Cucumis melo L.) during Postharvest Storage" Agronomy 12, no. 10: 2498. https://doi.org/10.3390/agronomy12102498
APA StyleLecholocholo, N., Shoko, T., Manhivi, V. E., Akinola, S. A., Maboko, M. M., & Sivakumar, D. (2022). Impact of Different Rootstocks on Antioxidant Properties and Volatile Profile of Honeydew Melons (Cucumis melo L.) during Postharvest Storage. Agronomy, 12(10), 2498. https://doi.org/10.3390/agronomy12102498







