Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Salinity and CS Treatments
2.3. Growth Attributes Measurements
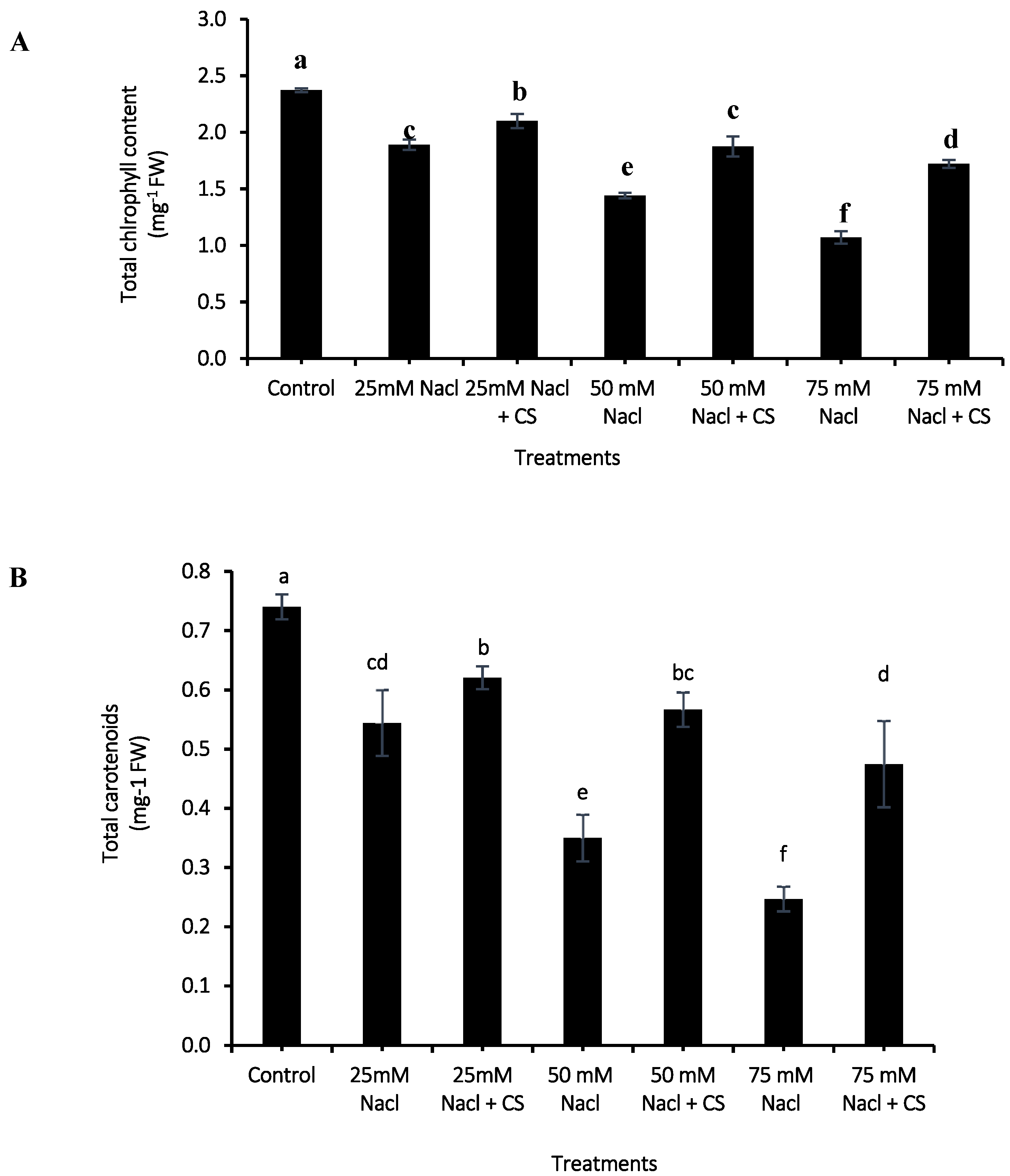
2.4. Photosynthetic Pigments Assessment
2.5. H2O2 Production
2.6. Lipid Peroxidation Assessment
2.7. Membrane Stability Index (MSI)
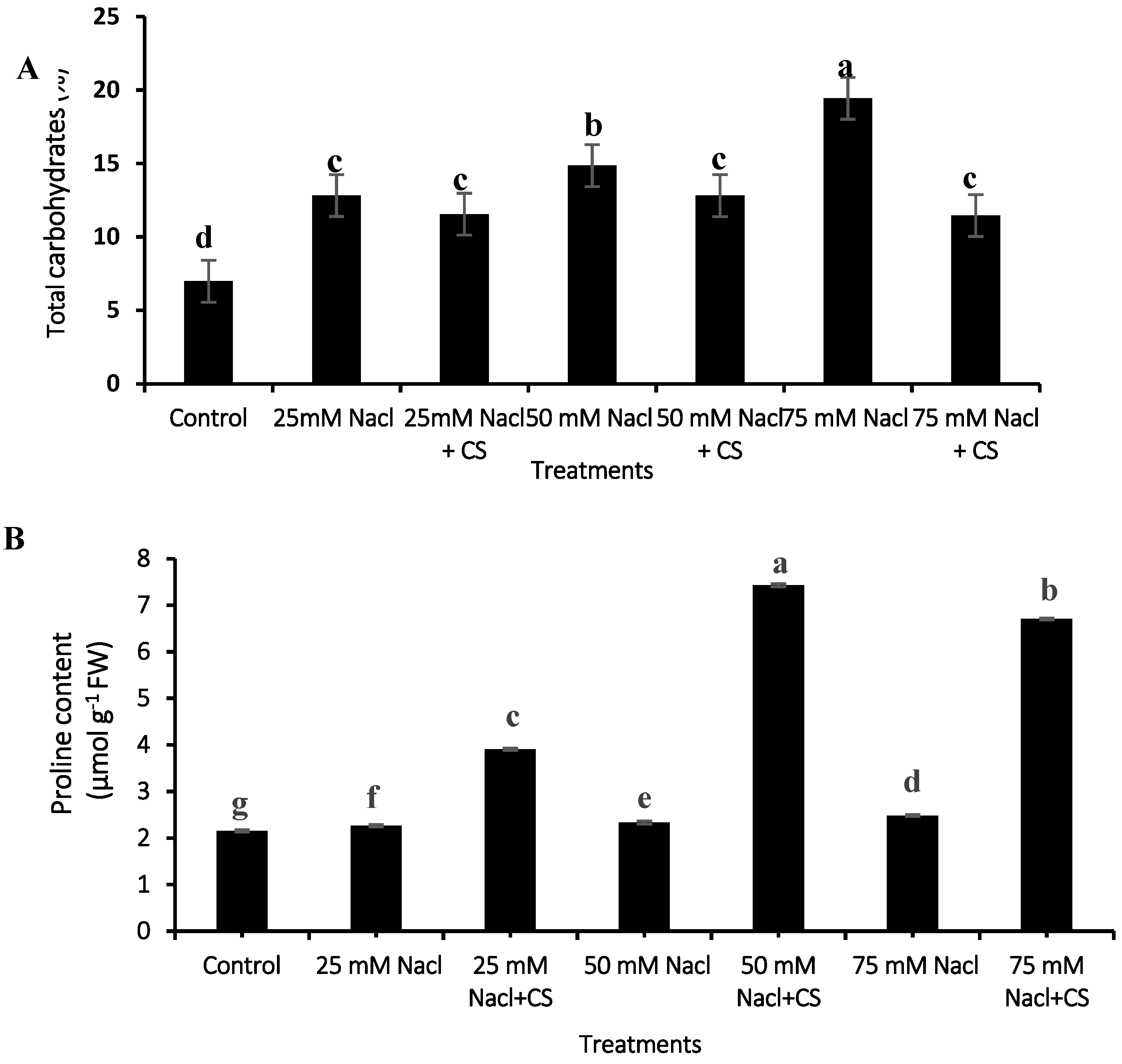
2.8. Total Carbohydrates
2.9. Proline Determination
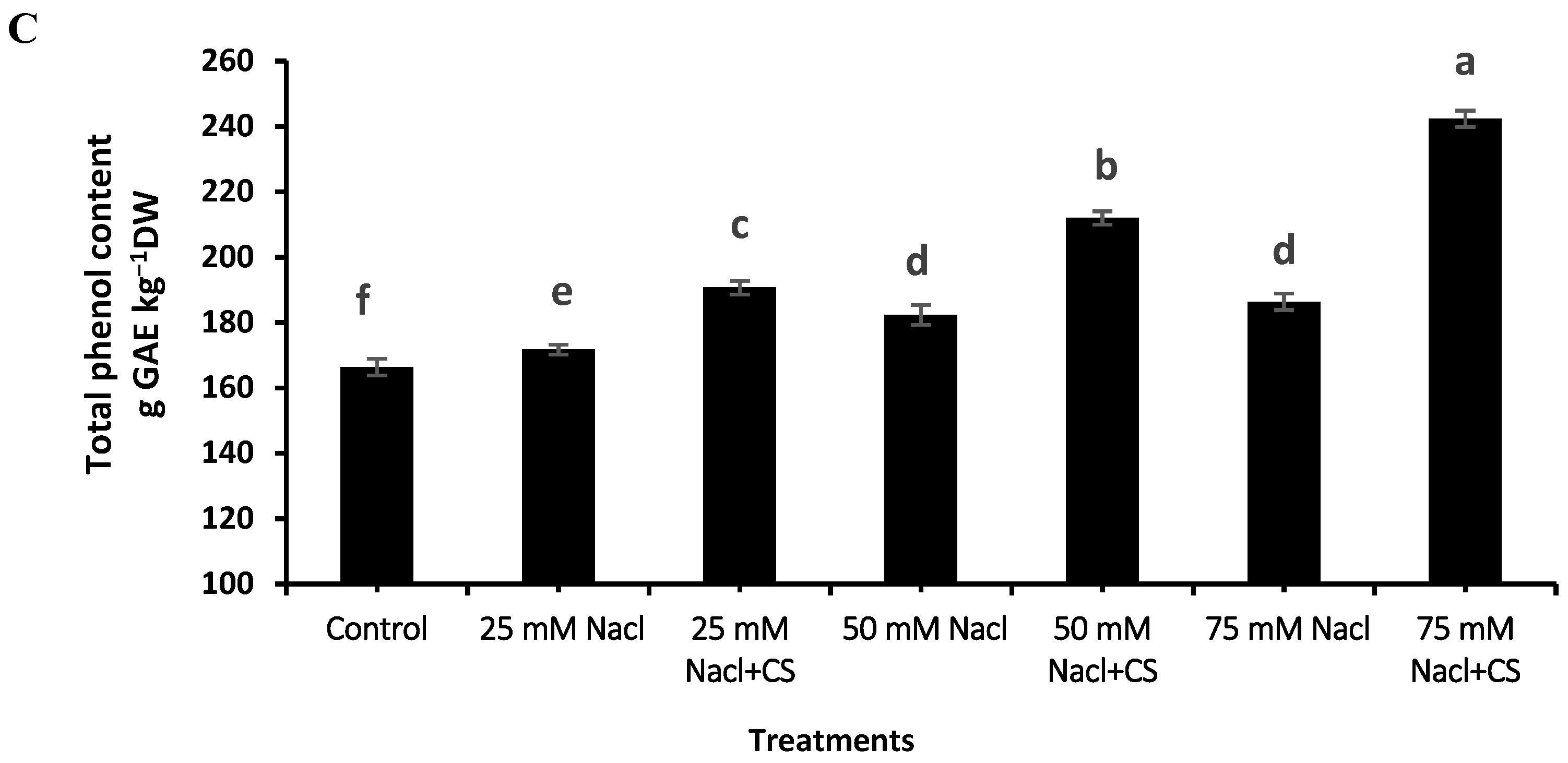
2.10. Total Phenol Content Assay
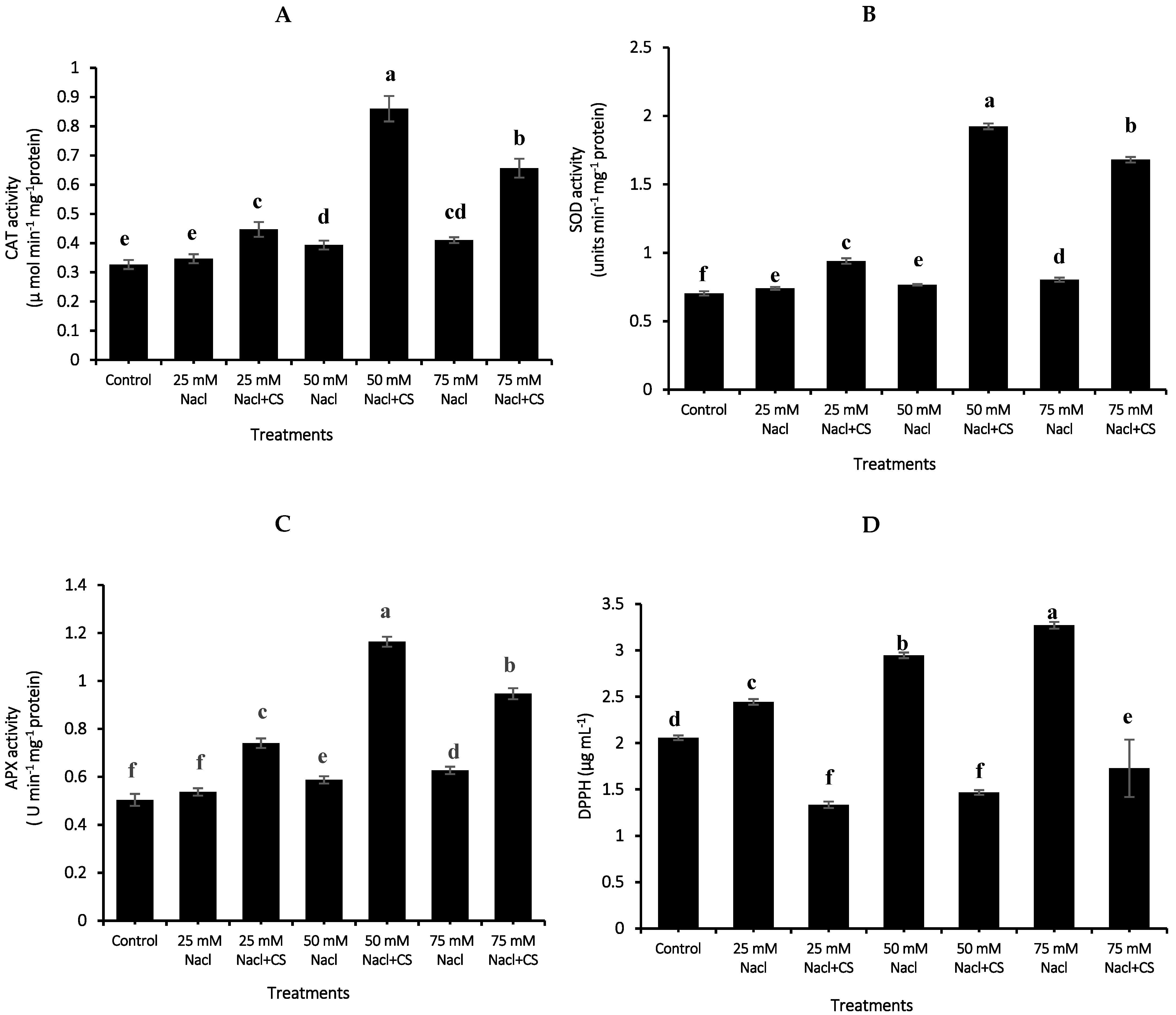
2.11. Antioxidant Enzyme Assays
2.12. Radical Scavenging Capacity (DPPH Assay)
2.13. Mineral Content
2.14. Statistical Analysis
3. Results
3.1. Plant Height and Leaf Number
3.2. Fresh and Dry Weights of Shoots and Roots
3.3. Photosynthetic Pigments
3.4. H2O2 Production
3.5. Malondialdehyde (MDA) Content
3.6. Membrane Stability Index (MSI)
3.7. Total Carbohydrates
3.8. Proline Content
3.9. Total Phenol Content
3.10. Activity of Antioxidant Enzymes
3.11. Radical Scavenging Activity (DPPH)
3.12. Mineral Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yisehak, K.; Solomon, M.; Tadelle, M. Contribution of Moringa (Moringa stenopetala, Bac.), a highly nutritious vegetable tree, for food security in South Ethiopia: A review. Asian J. Appl. Sci. 2011, 4, 477–488. [Google Scholar] [CrossRef]
- Tahany, M.A.; Hegazy, A.; Sayed, A.; Kabiel, H.; El-Alfy, T.; El-Komy, S. Study on combined antimicrobial activity of some biologically active constituents from wild Moringa peregrina Forssk. J. Yeast Fungal Res. 2010, 1, 15–24. [Google Scholar]
- El-Awady, M.A.; Hassan, M.M.; Abdel-Hameed, E.S.; Ahmed, G. Comparison of the antioxidant activities, phenolic and flavonoids contents of the leaves-crud extracts of Moringa peregrine and Moringa oleifera. Int. J. Biosci. 2016, 8, 55–62. [Google Scholar]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Fahey, J.W. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005, 1, 1–15. [Google Scholar]
- Hassan, F.A.S.; Mazrou, R.; Gaber, A.; Hassan, M.M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Hassan, F.; Al-Yasi, H.; Ali, E.F.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2021, 101, 157–165. [Google Scholar] [CrossRef]
- Metwally, S.A.; Ezzo, M.I.; Leila, B.H.A.; Abdalla, A.M. Effect of Diluted Red Sea Water on Growth Behavior and Chemical Component of Moringa Plants. Annu. Res. Rev. Biol. 2021, 36, 72–82. [Google Scholar] [CrossRef]
- De, F.; Carballo-Méndez, J.; Olivares-Sáenz, E.; Eustacio Vázquez-Alvarado, R.; Zavala-García, F.; Benavides-Mendoza, A.; Bolivar-Duarte, M. Silicon improves seedling production of Moringa oleifera lam. under saline stress. Pak. J. Bot. 2022, 54, 751–757. [Google Scholar] [CrossRef]
- Osman, A.; El-Naggar, H. Enhancing salinity stress tolerance and phenylalanine ammonia lyase gene activity using osmolytes in Moringa seedling production. Ann. Agric. Sci. 2022, 67, 127–135. [Google Scholar] [CrossRef]
- Nouman, W.; Siddiqui, M.T.; Basra, S.M.A.; Khan, R.A.; Gull, T.; Olson, M.E.; Munir, H. Response of Moringa oleifera to saline conditions. Int. J. Agric. Biol. 2012, 14, 757–762. [Google Scholar]
- Hassan, F.; Ali, E. Effects of salt stress on growth, antioxidant enzyme activity and some other physiological parameters in jojoba [Simmondsia chinensis (Link) Schneider] plant. Aust. J. Crop Sci. 2014, 8, 1615–1624. [Google Scholar]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Cirillo, C.; De Micco, V.; Arena, C.; De Pascale, S.; Rouphael, Y. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. trained to different canopy shapes. Agric. Water Manag. 2019, 212, 12–22. [Google Scholar] [CrossRef]
- Attia, H.; Al-Yasi, H.; Alamer, K.; Esmat, F.; Hassan, F.; Elshazly, S.; Hessini, K. Induced anti-oxidation efficiency and others by salt stress in Rosa damascena Miller. Sci. Hortic. 2020, 274, 109681. [Google Scholar] [CrossRef]
- Bernstein, N.; Shoresh, M.; Xu, Y.; Huang, B. Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radic. Biol. Med. 2010, 49, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Misra, R.; Mariam, A.; Yusuf, K.; Yusuf, L. Salicylic acid alters antioxidant and phenolics metabolism in Catharanthus roseus grown under salinity stress. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 118–125. [Google Scholar]
- Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The efficacies of 1-methylcyclopropene and chitosan nanoparticles in preserving the postharvest quality of Damask rose and their underlying biochemical and physiological mechanisms. Biology 2022, 11, 242. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Esmat, F.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Rehman, H.U.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Song, L.; Li, K.; Zhang, X.; Liu, S.; Qin, Y.; Li, P. Polysaccharides from Grateloupia filicina enhance tolerance of rice seeds (Oryza sativa L.) under salt stress. Int. J. Biol. Macromol. 2019, 124, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Ghasemi, N.; Omidi, H.; Bostani, A. Morphological Properties of Catharanthus roseus L. Seedlings Affected by Priming Techniques under Natural Salinity Stress. J. Plant Growth Regul. 2021, 40, 550–557. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.F.; Mahfouz, S. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of coriander plant. Aust. J. Basic Appl. Sci. 2012, 6, 600–615. [Google Scholar]
- Kahil, A.A.; Hassan, F.A.S.; Ali, E.F. Influence of bio-fertilizers on growth, yield and anthocyanin content of Hibiscus sabdariffa L. plant under Taif region conditions. Annu. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Mazrou, R.; Ali, E.F.; Hassan, S.; Hassan, F.A.S. A pivotal role of chitosan nanoparticles in enhancing the essential oil productivity and antioxidant capacity in Matricaria chamomilla L. Horticulturae 2021, 7, 574. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ullah, F.; Majeed, I.; Ayaz, A.; Munis, M. Organometallic assembling of chitosan-Iron oxidenanoparticles with their antifungal evaluation against Rhizopus oryzae. Appl. Organomet. Chem. 2019, 33, e5190. [Google Scholar] [CrossRef]
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729. [Google Scholar] [CrossRef]
- Heidari, J.; Amooaghaie, R.; Kiani, S. Impact of chitosan on nickel bioavailability in soil, the accumulation and tolerance of nickel in Calendula tripterocarpa. Int. J. Phytoremediat. 2020, 22, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.A.; de Paula, A.C.C.F.F.; Silva, V.N.B.; de Alvarenga, A.A.; Bertolucci, S.K.V. Biostimulating effect of chitosan and acetic acid on the growth and profile of the essential oil of Mentha arvensis L. Ind. Crop. Prod. 2021, 171, 113987. [Google Scholar] [CrossRef]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-induced physiological and biochemical regulations confer drought tolerance in pot marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Unter suchungen zur synchronisier barteit einzelner pigmentan angel mutanten von chlorela. Planta 1965, 65, 186. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–178. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrate in the plant extract by anthrone reagent. J. Biochem. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mcdonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chandlee, J.M.; Scandalios, J.G. Analysis of variants affecting the catalase developmental program in maize scutellum. Theoret. Appl. Genet. 1984, 69, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists, 6th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1967. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Cai, X.; Niu, G.; Starman, T.; Hall, C. Response of six garden roses (Rosa× hybrida L.) to salt stress. Sci. Hortic. 2014, 168, 27–32. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. Growth and mineral status of moringa plants as affected by silicate and salicylic acid under salt stress. Int. J. Plant Soil Sci. 2014, 3, 163–177. [Google Scholar] [CrossRef]
- Ashour, H.A.; Sanaa, E.; Mohamed, S. Alleviative effects of chitosan or humic acid on Vitex trifolia ‘Purpurea’ grown under salinity stress. Ornam. Hortic. 2020, 27, 88–102. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Li, P. Metabolite Profiling of Wheat Seedlings Induced by Chitosan: Revelation of the Enhanced Carbon and Nitrogen Metabolism. Front. Plant Sci. 2017, 8, 02017. [Google Scholar] [CrossRef] [PubMed]
- Safikhan, S.; Khoshbakht, K.; Chaichi, M.R.; Amini, A.; Motesharezadeh, B. Role of chitosan on the growth, physiological parameters and enzymatic activity of milk thistle (Silybum marianum (L.) Gaertn.) in a pot experiment. J. Appl. Res. Med. Arom. Plants 2018, 10, 49–58. [Google Scholar] [CrossRef]
- Arshad, M.A.; Akhtar, G.; Rajwana, I.A.; Ullah, S.; Hussain, M.B.; Amin, M.; Faried, N.; Razzaq, K.; Shehzad, M.A.; Ahsan, M.; et al. Foliar application of chitosan improves biomass, physiological and biochemical attributes of Rose (Gruss-an-Teplitz). Kuwait J. Sci. 2022, 49, 11655. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Morsi, M.M.; Aljoudi, N.G.S. Alleviating the Adverse Effects of Salt Stress in Rosemary by Salicylic Acid Treatment. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1980–1995. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, UK, 2015; p. 761. [Google Scholar]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, C.F.d.; Oliveira, E.V.d.; Neves, A.L.R.; Gheyi, H.R.; Bezerra, M.A.; Costa, C.A.G. Morphophysiological responses and mechanisms of salt tolerance in four ornamental perennial species under tropical climate. Rev. Bras. Eng. Agrícola Ambient. 2020, 24, 656–663. [Google Scholar] [CrossRef]
- Van, S.N.; Minh, H.D.; Anh, D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S.; Lotrakul, P.; Bunjongrat, R.; Chaidee, A.; Bangyeekhun, T. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hortic. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Rashidi, N.; Khavari-Nejad, R.A.; Ramak, P.; Saadatmand, S. The effect of chitosan on gene expression, some morphological and physiological traits of sweet basil (Ocimum basilicum l.) under salinity stress. Acta Sci. Pol. Hortorum Cultus 2020, 19, 21–30. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.A.; Prajapati, D.; Bhagat, D.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Chitosan nanofertilizer to foster source activity in maize. Int. J. Biol. Macromol. 2020, 145, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Behdad, A.; Mohsenzadeh, S.; Azizi, M.; Moshtaghi, N. Salinity effects on physiological and phytochemical characteristics and gene expression of two Glycyrrhiza glabra L. populations. Phytochemistry 2020, 171, 112236. [Google Scholar] [CrossRef] [PubMed]
- Bonacina, C.; da Cruz, R.M.S.; Nascimento, A.B.; Barbosa, L.N.; Gonçalves, J.E.; Gazim, Z.C.; de Souza, S.G.H. Salinity modulates growth, oxidative metabolism, and essential oil profile in Curcuma longa L. (Zingiberaceae) rhizomes. S. Afr. J. Bot. 2022, 146, 1–11. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Sen, S.K.; Chouhan, D.; Das, D.; Ghosh, R.; Mandal, P. Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. Int. J. Biol. Macromol. 2020, 145, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Gerami, M.; Majidian, P.; Ghorbanpour, A.; Alipour, Z. Stevia rebaudiana Bertoni responses to salt stress and chitosan elicitor. Physiol. Mol. Biol. Plants 2020, 26, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Pereira, S. High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot. 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Khalid, K.A.; Cai, W. The effects of mannitol and salinity stresses on growth and biochemical accumulations in lemon balm. Acta Ecol. Sin. 2011, 31, 112–120. [Google Scholar] [CrossRef]
- Feng, X.; Hussain, T.; Guo, K.; An, P.; Liu, X. Physiological, morphological and anatomical responses of Hibiscus moscheutos to non-uniform salinity stress. Environ. Exp. Bot. 2021, 182, 104301. [Google Scholar] [CrossRef]
- Shen, Z.; Pu, X.; Wang, S.; Dong, X.; Cheng, X.; Cheng, M. Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci. Rep. 2022, 12, 5089. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Kaya, C.; Dikilitas, M.; Higgs, D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ. Exp. Bot. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Abou El-Leel, O.F.; El-Shayeb, N.S.; El-Azzony, E.A. Effect of proline on growth and the active ingredients of moringa oleifera lam. plant under salt stress. N. Egypt. J. Microbiol. 2018, 51, 56–85. [Google Scholar]
- Sheikhalipour, M.; Esmaielpour, B.; Gohari, G.; Haghighi, M.; Jafari, H.; Farhadi, H.; Kalisz, A. Salt stress mitigation via the foliar application of chitosan-functionalized selenium and anatase titanium dioxide nanoparticles in stevia (Stevia rebaudiana Bertoni). Molecules 2021, 26, 4090. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Eldin, R.M.B. Chitosan improves morphological and physiological attributes of grapevines under deficit irrigation conditions. J. Hortic. Res. 2021, 29, 9–22. [Google Scholar] [CrossRef]
- Jahan, I.; Parvin, S.; Miah, M.G.; Ahmed, J.U. Effect of salinity on the physiological and biochemical responses of neem. Int. J. Environ. Agric. Res. 2018, 4, 47–54. [Google Scholar]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds, and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; Hamid, R. Fertilizer source and chitosan effect on productivity, nutrient accumulation, and phenolic compounds of Thymus daenensis Celak. Agron. J. 2021, 113, 5499–5515. [Google Scholar] [CrossRef]
- Idrees, M.; Naeem, M.; Aftab, T.; Khan, M.M.A. Moinuddin Salicylic acid mitigates salinity stress by improving antioxidant defence system and enhances vincristine and vinblastine alkaloids production in periwinkle [Catharanthus roseus (L.) G. Don]. Acta Physiol. Plant. 2011, 33, 987–999. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, T.; Cheng, Y.; Wang, F.; Zhao, X. Morphological and metabolic responses of four Iris germanica cultivars under salinity stress. Sci. Hortic. 2021, 281, 109960. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A. Marjoram physiological and molecular performance under water stress and chitosan treatment. Acta Physiol. Plant. 2019, 41, 1–8. [Google Scholar] [CrossRef]
- Khalvandi, M.; Amerian, M.; Pirdashti, H.; Keramati, S.; Hosseini, J. Essential oil of peppermint in symbiotic relationship with Piriformospora indica and methyl jasmonate application under saline condition. Ind. Crop. Prod. 2019, 127, 195–202. [Google Scholar] [CrossRef]
- Tarighaleslami, M.; Zarghami, R.; Boojar, M.M.A.; Oveysi, M. Effects of drought stress and different nitrogen levels on morphological traits of proline in leaf and protein of corn seed (Zea mays L.). Am.-Eurasian J. Agric. Environ. Sci. 2012, 12, 49–56. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: New York, NY, USA, 1995; pp. 15–22. [Google Scholar]
- Botella, M.A.; Martinez, V.; Pardines, J.; Cerda, A. Salinity induced potassium deficiency in maize plants. J. Plant Physiol. 1997, 150, 200–205. [Google Scholar] [CrossRef]
- Roussos, P.A.; Gasparatos, D.; Tsantili, E.; Pontikis, C.A. Mineral nutrition of jojoba explants in vitro under sodium chloride salinity. Sci. Hortic. 2007, 114, 59–66. [Google Scholar] [CrossRef]
- Nasrin, H.; Zakir, H.M.; Nipa, N.A.; Paul, N.R.; Quadir, Q.F. Effect of foliar application of chitosan on growth, yield and nutritional qualities of red amaranth (Amaranthus gangticus L.). J. Exp. Agric. Int. 2022, 44, 105–116. [Google Scholar] [CrossRef]



| Treatments | Macroelements (%) | Microelements (mg g−1 DW) | ||||
|---|---|---|---|---|---|---|
| N | P | K | Mg | Fe | Na | |
| Control | 2.13 a | 0.74 a | 2.49 a | 8.39 a | 0.89 a | 19.67 f |
| 25 mM NaCl | 1.73 c | 0.56 c | 2.08 c | 7.44 c | 0.85 b | 35.00 d |
| 25 mM NaCl + 1% chitosan | 2.0 ab | 0.63 b | 2.24 b | 7.72 b | 0.83 b | 26.67 e |
| 50 mM NaCl | 1.55 d | 0.37 e | 1.70 e | 6.77 e | 0.71 d | 54.67 b |
| 50 mM NaCl + 1% chitosan | 1.91 b | 0.52 d | 1.83 d | 7.13 d | 0.79 c | 44.67 c |
| 75 mM NaCl | 1.41 e | 0.23 g | 1.63 e | 4.19 g | 0.54 f | 74.67 a |
| 75 mM NaCl + 1% chitosan | 1.72 c | 0.31 f | 1.69 e | 5.77 f | 0.66 e | 48.67 c |
| LSD (p ≤ 0.05) | 0.122 | 0.066 | 0.126 | 0.159 | 0.0202 | 5.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkarmout, A.F.; Yang, M.; Hassan, F.A.S. Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis. Agronomy 2022, 12, 2513. https://doi.org/10.3390/agronomy12102513
Elkarmout AF, Yang M, Hassan FAS. Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis. Agronomy. 2022; 12(10):2513. https://doi.org/10.3390/agronomy12102513
Chicago/Turabian StyleElkarmout, Ahmed F., Mei Yang, and Fahmy A.S. Hassan. 2022. "Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis" Agronomy 12, no. 10: 2513. https://doi.org/10.3390/agronomy12102513
APA StyleElkarmout, A. F., Yang, M., & Hassan, F. A. S. (2022). Chitosan Treatment Effectively Alleviates the Adverse Effects of Salinity in Moringa oleifera Lam via Enhancing Antioxidant System and Nutrient Homeostasis. Agronomy, 12(10), 2513. https://doi.org/10.3390/agronomy12102513







