Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Sewage Sludge, and Sludge-Based Vermicompost
2.2. Experimental Design and Soil Sampling
2.3. Soil Physicochemical and Enzymatic Assays
2.4. DNA Extraction, Real-Time Quantitative PCR, and 16S rDNA Amplicon Sequencing
2.5. Bioinformatics Analysis and Statistical Analysis
3. Results
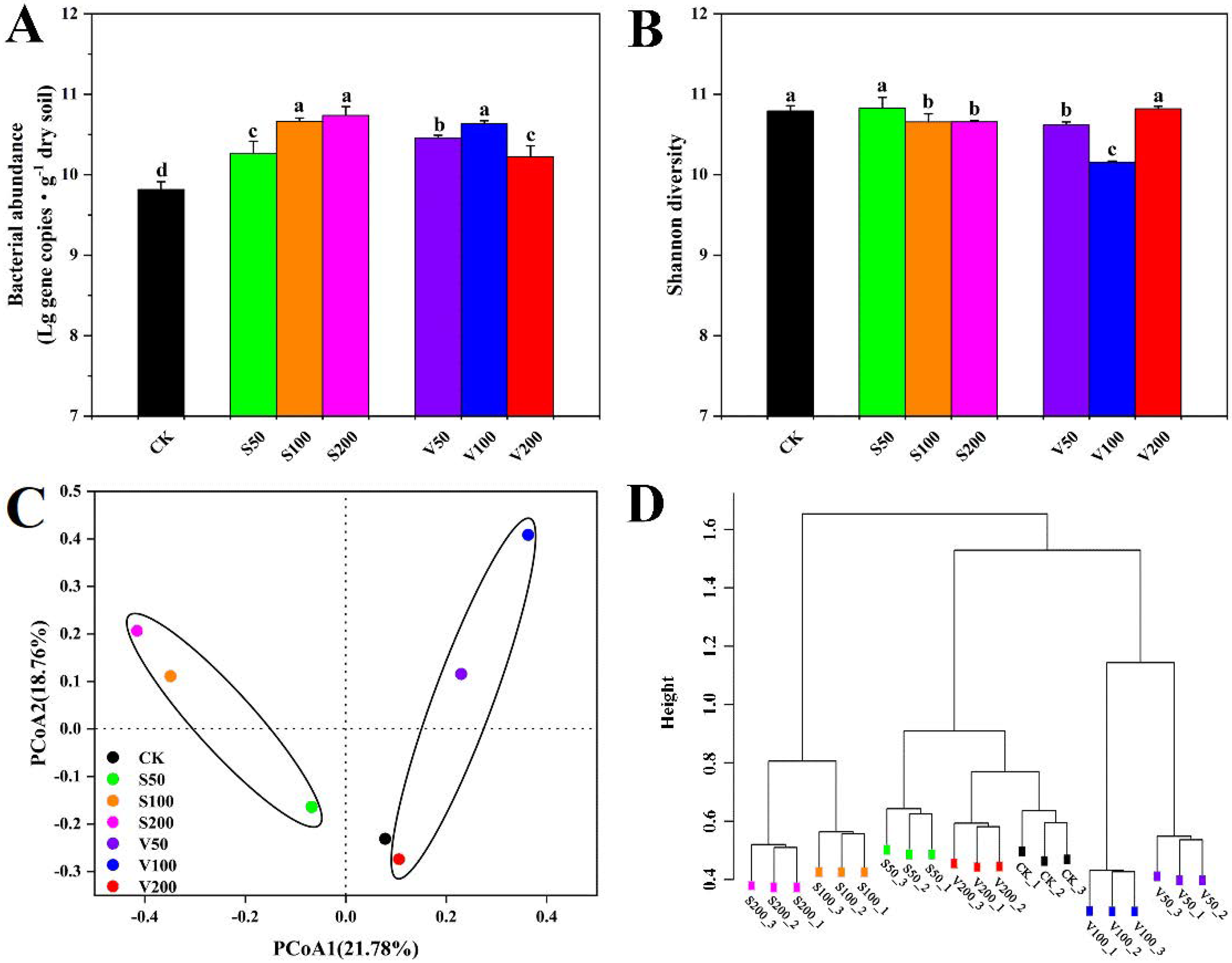
3.1. Compositional and Structural Diversities of Bacterial Communities
3.2. Taxonomic Compositions of Soil Bacterial Community
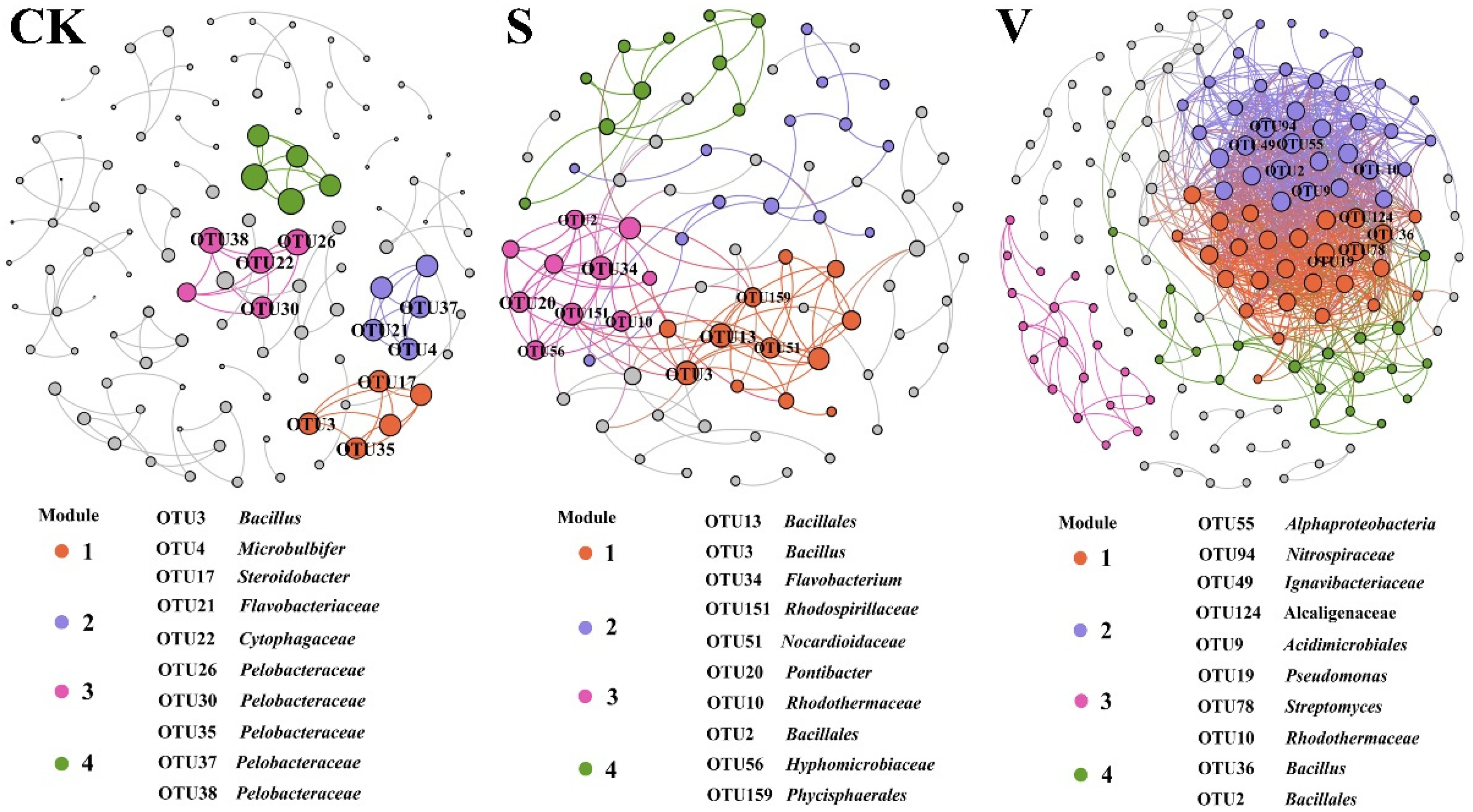
3.3. Co-Occurrence Networks of Core Microbiomes
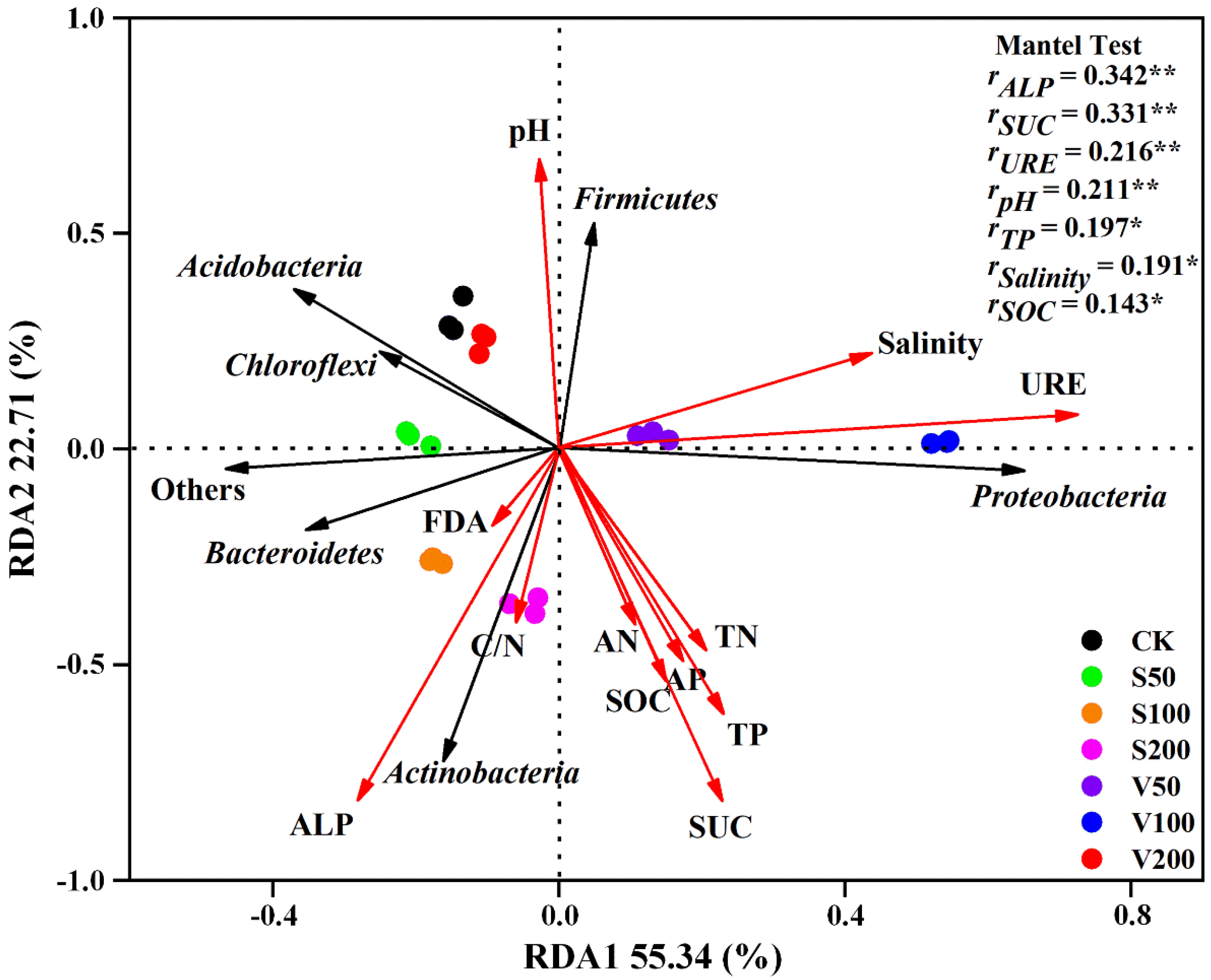
3.4. Driving Factors of Bacterial Community Differentiation in Coastal Mudflat Salt-Affected Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Z.Y.; Yu, Z.X.; Xu, L.; Zhao, Y.L.; Yi, S.Q.; Shen, C.; Wang, Y.M.; Li, Y.L.; Zuo, W.G.; Gu, C.H.; et al. Effects of vermicompost application on growth and heavy metal uptake of barley grown in mudflat salt-affected soils. Agronomy 2022, 12, 1007. [Google Scholar] [CrossRef]
- Cao, W.Z.; Wong, M.H. Current status of coastal zone issues and management in China: A review. Environ. Int. 2007, 33, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.F.; Pu, L.J.; Zhu, M.; Meadows, M.; Sun, L.C.; Wu, T.; Bu, X.G.; Xu, Y. Differential effects of various reclamation treatments on soil characteristics: An experimental study of newly reclaimed tidal mudflats on the east China coast. Sci. Total Environ. 2021, 768, 144996. [Google Scholar] [CrossRef] [PubMed]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Q.; Yu, Y.N.; Gao, R.W.; Wang, H.; Zhang, j.; Li, R.; Long, X.H.; Shen, Q.R.; Chen, W.; Cai, F. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Goldford, J.E.; Lu, N.; Bajić, D.; Estrela, S.; Tikhonov, M.; Sanchez-Gorostiaga, A.; Segrè, D.; Mehta, P.; Sanchez, A. Emergent simplicity in microbial community assembly. Science 2018, 361, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.A.; Falk, D.A.; Zedler, J.B. Ecological theory and restoration ecology. In Foundations of Restoration Ecology; Palmer, M.A., Zedler, J.B., Falk, D.A., Eds.; Island Press/Center for Resource Economics: Washington, DC, USA, 2016; pp. 3–26. [Google Scholar] [CrossRef]
- EI Azhari, N.; Laine, S.; Sappin-Didier, V.; Beguet, J.; Rouard, N.; Philippot, L.; Martin-Laurent, F. Long-term impact of 19 years’ farmyard manure or sewage sludge application on the structure, diversity and density of the protocatechuate-degrading bacterial community. Agric. Ecosyst. Environ. 2012, 158, 72–82. [Google Scholar] [CrossRef]
- Liu, X.; Guo, K.L.; Huang, L.; Ji, Z.; Jiang, H.M.; Li, H.; Zhang, J.F. Responses of absolute and specific enzyme activity to consecutive application of composted sewage sludge in a Fluventic Ustochrept. PLoS ONE 2017, 12, e0177796. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.T.; Li, Y.N.; Wang, C.Y.; Kim, K.S.; Wang, T.Y.; Liu, S.X. Characteristics of the rhizosphere bacterial community across different cultivation years in saline–alkaline paddy soils of Songnen Plain of China. Can. J. Microbiol. 2018, 64, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, Z.F.; Wang, X.L.; Zhou, Z.W.; Chen, D.; Zeng, H.; Zhao, S.M.; Chen, L.L.; Hu, Y.L.; Zhang, C.Y.; et al. Diversity and contributions to nitrogen cycling and carbon fixation of soil salinity shaped microbial communities in Tarim Basin. Front. Microbiol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.P.; Shi, Y.; Cui, X.Q.; Yue, P.; Li, K.H.; Liu, X.J.; Tripathi, B.M.; Chu, H.Y. Salinity is a key determinant for soil microbial communities in a desert ecosystem. mSystems 2019, 4, e00225-18. [Google Scholar] [CrossRef]
- Bai, Y.C.; Mei, L.J.; Zuo, W.G.; Zhang, Y.; Gu, C.H.; Shan, Y.H.; Hu, J.; Dai, Q.G. Response of bacterial communities in coastal mudflat saline soil to sewage sludge amendment. Appl. Soil Ecol. 2019, 144, 107–111. [Google Scholar] [CrossRef]
- Mallol, C. What’s in a beach? Soil micromorphology of sediments from the Lower Paleolithic site of ‘Ubeidiya, Israel. J. Hum. Evol. 2006, 51, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Wang, Y.M.; Shen, C.; Xu, L.; Yi, S.Q.; Zhao, Y.L.; Zuo, W.G.; Gu, C.H.; Shan, Y.H.; Bai, Y.C. structural and predicted functional diversities of bacterial microbiome in response to sewage sludge amendment in coastal mudflat soil. Biology 2021, 10, 1302. [Google Scholar] [CrossRef]
- Bai, Y.C.; Tao, T.Y.; Gu, C.H.; Wang, L.; Feng, K.; Shan, Y.H. Mudflat soil amendment by sewage sludge: Soil physicochemical properties, perennial ryegrass growth, and metal uptake. Soil Sci. Plant Nutr. 2013, 59, 942–952. [Google Scholar] [CrossRef]
- Wu, L.P.; Wang, Y.D.; Zhang, S.R.; Wei, W.L.; Kuzyakov, Y.; Ding, X.D. Fertilization effects on microbial community composition and aggregate formation in saline-alkaline soil. Plant Soil 2021, 463, 523–535. [Google Scholar] [CrossRef]
- Yao, R.J.; Yang, J.S.; Wang, X.P.; Xie, W.P.; Zheng, F.L.; Li, H.Q.; Tang, C.; Zhu, H. Response of soil characteristics and bacterial communities to nitrogen fertilization gradients in a coastal salt-affected agroecosystem. Land Degrad. Dev. 2021, 32, 338–353. [Google Scholar] [CrossRef]
- Bai, Y.C.; Zuo, W.G.; Shao, H.B.; Mei, L.J.; Tang, B.P.; Gu, C.H.; Wang, X.K.; Guan, Y.X. Eastern China coastal mudflats: Salt-soil amendment with sewage sludge. Land Degrad. Dev. 2018, 29, 3803–3811. [Google Scholar] [CrossRef]
- Tian, X.M.; Fan, H.; Wang, J.Q.; Ippolito, J.; Li, Y.B.; Feng, S.S.; An, A.J.; Zhang, F.H.; Wang, K.Y. Effect of polymer materials on soil structure and organic carbon under drip irrigation. Geoderma 2019, 340, 94–103. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, J.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Liu, H.; Liu, X.X.; Chen, Y.; Lu, Y.; Shen, M.C.; Dang, K.K.; Zhao, Y.; Dong, Y.H.; Li, Q.Y.; et al. Organic fertilizer enhances rice growth in severe saline–alkali soil by increasing soil bacterial diversity. Soil Use Manag. 2021, 38, 964–977. [Google Scholar] [CrossRef]
- Lu, P.N.; Bainard, L.D.; Ma, B.; Li, J.H. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline-alkaline environment. Sci. Rep. 2020, 10, 19896. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Xu, W.L.; Zhang, Y.; Tang, X.Y.; Bai, Y.C.; Hu, J. Bloom of tetracycline resistance genes in mudflats following fertilization is attributed to the increases in the shared potential hosts between soil and organic fertilizers. Environ. Sci. Pollut. Res. 2022, 29, 13292–13304. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.G.; Gu, C.H.; Zhang, W.J.; Xu, K.D.; Wang, Y.; Bai, Y.C.; Shan, Y.H.; Dai, Q.G. Sewage sludge amendment improved soil properties and sweet sorghum yield and quality in a newly reclaimed mudflat land. Sci. Total Environ. 2019, 654, 541–549. [Google Scholar] [CrossRef]
- Zuo, W.G.; Xu, K.D.; Zhang, W.J.; Wang, Y.; Gu, C.H.; Bai, Y.C.; Shan, Y.H.; Dai, Q.G. Heavy metal distribution and uptake by maize in a mudflat soil amended by vermicompost derived from sewage sludge. Environ. Sci. Pollut. Res. Int. 2019, 26, 30154–30166. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Wang, F.Y.; Xie, Y.J. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl. Soil Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Li, Y.L.; Dai, S.Y.; Wang, B.Y.; Jiang, Y.T.; Ma, Y.Y.; Pan, L.L.; Wu, K.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; et al. Autotoxic ginsenoside disrupts soil fungal microbiomes by stimulating potentially pathogenic microbes. Appl. Environ. Microb. 2020, 86, e00130-20. [Google Scholar] [CrossRef]
- Abis, L.; Loubet, B.; Ciuraru, R.; Lafouge, F.; Houot, S.; Nowak, V.; Tripied, J.; Dequiedt, S.; Maron, P.A.; Sadet-Bourgeteau, S. Reduced microbial diversity induces larger volatile organic compound emissions from soils. Sci. Rep. 2020, 10, 6104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W.; Shen, B.; Shen, Q.; Zhang, R.F. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 2014, 9, e85301. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiao, Y.; Wang, Y.F.; Liu, Z.H.; Zhang, Y.F.; Yang, K.J. Trichoderma asperellum alters fungal community composition in saline–alkaline soil maize rhizospheres. Soil Sci. Soc. Am. J. 2021, 85, 1091–1104. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Latare, A.M.; Singh, S.K.; Kumar, O. Impact of sewage sludge application on soil fertility, microbial population and enzyme activities in soil under rice-wheat system. J. Indian Soc. Soil Sci. 2018, 66, 300–309. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Earthworms as organic waste managers and biofertilizer producers. Waste Biomass Valorization 2018, 9, 1073–1086. [Google Scholar] [CrossRef]
- Huang, X.Q.; Zhao, J.; Zhou, X.; Zhang, J.B.; Cai, Z.C. Differential responses of soil bacterial community and functional diversity to reductive soil disinfestation and chemical soil disinfestation. Geoderma 2019, 348, 124–134. [Google Scholar] [CrossRef]
- Zhao, H.L.; Tian, X.H.; Jiang, Y.H.; Zhao, Y.; Si, B.C. Effect of combining straw-derived materials and wood ash on alkaline soil carbon content and the microbial community. Soil Sci. 2021, 72, 1863–1878. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.L.; Sun, J.W.; He, P.; Tang, S.H.; Yang, S.H.; Zhou, W.; Wang, X.B. The alleviation of acid soil stress in rice by inorganic or organic ameliorants is associated with changes in soil enzyme activity and microbial community composition. Biol. Fertil. Soils 2015, 51, 465–477. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Bei, S.K.; Zhang, Y.L.; Li, T.T.; Christie, P.; Li, X.L.; Zhang, J.L. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric. Ecosyst. Environ. 2018, 260, 58–69. [Google Scholar] [CrossRef]
- Shi, S.J.; Nuccio, E.E.; Shi, Z.J.; He, Z.L.; Zhou, J.Z.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Deng, Y.; Luo, F.; He, Z.L.; Yang, Y.F. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2, e00122-11. [Google Scholar] [CrossRef]
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Girardin, C.; Edwards, R.J.; Rumpel, C.; Fornasier, F.; et al. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy 2017, 9, 591–612. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Liu, T.; Chen, H.Y.; Verma, S.; Duan, Y.M.; Awasthi, K.A.; Wang, Q.; Ren, X.N.; Zhao, J.C.; Zhang, Z.Q. The behavior of antibiotic resistance genes and their associations with bacterial community during poultry manure composting. Bioresour. Technol. 2019, 280, 70–78. [Google Scholar] [CrossRef]
- Yang, D.Q.; Liu, Y.; Wang, Y.; Gao, F.; Zhao, J.H.; Li, Y.; Li, X.D. Effects of soil tillage, management practices, and mulching film application on soil health and peanut yield in a continuous cropping system. Front. Microbiol. 2020, 11, 570924. [Google Scholar] [CrossRef]
- Pasternak, T.; Potters, G.; Caubergs, R.; Jansen, M.A.K. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef]
- Su, C.; Zhang, M.L.; Lin, L.Y.; Yu, G.W.; Zhong, H.T.; Chong, Y.X. Reduction of iron oxides and microbial community composition in iron-rich soils with different organic carbon as electron donors. Int. Biodeter. Biodegr. 2020, 148, 104881. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Cleveland, C.C.; Wieder, W.R.; Washenberger, C.L.; Townsend, A.R. Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol. Biochem. 2010, 42, 2153–2160. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- Marcos, M.S.; Bertiller, M.B.; Olivera, N.L. Microbial community composition and network analyses in arid soils of the Patagonian Monte under grazing disturbance reveal an important response of the community to soil particle size. Appl. Soil Ecol. 2019, 138, 223–232. [Google Scholar] [CrossRef]
- Zhao, Q.; Chu, S.S.; He, D.; Wu, D.M.; Mo, Q.F.; Zeng, S.C. Sewage sludge application alters the composition and co-occurrence pattern of the soil bacterial community in southern China forestlands. Appl. Soil Ecol. 2021, 157, 103744. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Chandra, T.S. Do earthworms affect dynamics of functional response and genetic structure of microbial community in a lab-scale composting system? Bioresour. Technol. 2009, 100, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Wang, Y.Y.; Yang, J.; Xing, M.Y.; Li, X.W.; Yi, D.H.; Deng, D.H. Earthworm–microorganism interactions: A strategy to stabilize domestic wastewater sludge. Water Res. 2010, 44, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalezc, J.L.; Hernandezb, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Manasa, M.R.K.; Katukuri, N.R.; Darveekaran Nair, S.S.; Haojie, Y.; Yang, Z.; Guo, R.B. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737. [Google Scholar] [CrossRef] [PubMed]
- Arancon, N.Q.; Edwards, C.A.; Babenko, A.; Cannon, J.; Galvis, P.; Metzger, J.D. Influences of vermicomposts, produced by earthworms and microorganisms from cattle manure, food waste and paper waste, on the germination, growth and flowering of petunias in the greenhouse. Appl. Soil Ecol. 2008, 39, 91–99. [Google Scholar] [CrossRef]
- Liu, M.L.; Wang, C.; Wang, F.Y.; Xie, Y.J. Vermicompost and humic fertilizer improve coastal saline soil by regulating soil aggregates and the bacterial community. Arch. Agron. Soil Sci. 2019, 65, 281–293. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.G.; Yang, R.P.; Zhou, C.L.; Tang, B.P. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.C.; Li, Y.C.; Gao, B.; Tang, Y.F.; Xie, J.Z.; Zhao, H.C. Remediation of saline-sodic soil using organic and inorganic amendments: Physical, chemical, and enzyme activity properties. J. Soils Sediments 2020, 20, 1454–1467. [Google Scholar] [CrossRef]
- Jorenush, M.H.; Sepaskhah, A.R. Modelling capillary rise and soil salinity for shallow saline water table under irrigated and non-irrigated conditions. Agric. Water Manag. 2003, 61, 125–141. [Google Scholar] [CrossRef]
- Wu, W.; Huang, H.L.; Biber, P.; Bethel, M. Litter decomposition of Spartina alterniflora and Juncus roemerianus: Implications of climate change in salt marshes. J. Coast. Res. 2017, 33, 372–384. [Google Scholar] [CrossRef]
- Shi, S.H.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.S.; Yang, F.; Wang, Z.C.; Tian, C.J. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Singh, K. Microbial and enzyme activities of saline and sodic soils. Land Degrad. Dev. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Luo, N.Y.; Ji, C.L.; Li, J.; Zhang, L.; Xiao, L.; She, X.L.; Liu, Z.; Li, Y.L.; Liu, C.S.; et al. Liquid organic fertilizer amendment alters rhizosphere microbial community structure and co-occurrence patterns and improves sunflower yield under salinity-alkalinity stress. Microb. Ecol. 2021, 84, 423–438. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Shi, S.H.; Xu, S.Q.; Yang, F.; Li, X.J.; Wang, Z.C.; Tian, C.J. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 2018, 329, 108–117. [Google Scholar] [CrossRef]

| Treatments | CK | S50 | S100 | S200 | V50 | V100 | V200 |
|---|---|---|---|---|---|---|---|
| CK | 3815 | ||||||
| S50 | 1923 | 2906 | |||||
| S100 | 1377 | 1323 | 2803 | ||||
| S200 | 1081 | 1186 | 1603 | 3144 | |||
| V50 | 1489 | 1104 | 898 | 761 | 3047 | ||
| V100 | 884 | 615 | 495 | 437 | 1057 | 2904 | |
| V200 | 2254 | 1501 | 1044 | 782 | 1182 | 704 | 2840 |
| Core OTUs | 193 | 193 | 193 | 193 | 193 | 193 | 193 |
| Total OTUs | 8916 | 5850 | 5504 | 5555 | 5571 | 4479 | 5783 |
| Topological Characteristics | CK | S | V |
|---|---|---|---|
| Number of nodes | 113 | 84 | 153 |
| Number of edges | 110 | 142 | 1118 |
| Average degree | 1.947 | 3.381 | 14.614 |
| Network diameter | 1 | 10 | 8 |
| Modularity | 0.952 | 0.643 | 0.257 |
| Average clustering coefficient | 1 | 0.427 | 0.586 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Y.; Gu, C.; Shen, C.; Xu, L.; Zhao, Y.; Yi, S.; Zuo, W.; Shan, Y.; Zhang, Z.; et al. Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil. Agronomy 2022, 12, 2525. https://doi.org/10.3390/agronomy12102525
Li Y, Wang Y, Gu C, Shen C, Xu L, Zhao Y, Yi S, Zuo W, Shan Y, Zhang Z, et al. Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil. Agronomy. 2022; 12(10):2525. https://doi.org/10.3390/agronomy12102525
Chicago/Turabian StyleLi, Yunlong, Yimin Wang, Chuanhui Gu, Chao Shen, Lu Xu, Yilin Zhao, Siqiang Yi, Wengang Zuo, Yuhua Shan, Zhuqing Zhang, and et al. 2022. "Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil" Agronomy 12, no. 10: 2525. https://doi.org/10.3390/agronomy12102525
APA StyleLi, Y., Wang, Y., Gu, C., Shen, C., Xu, L., Zhao, Y., Yi, S., Zuo, W., Shan, Y., Zhang, Z., & Bai, Y. (2022). Differential Effects of Organic Ameliorants on the Reassembly of Bacterial Communities in Newly Amended Coastal Mudflat Salt-Affected Soil. Agronomy, 12(10), 2525. https://doi.org/10.3390/agronomy12102525







