Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Root Length and Shoot Length Measurement
2.3. Conserved Domain Analysis
2.4. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Analysis
2.5. Organ-Specific Expression Analysis
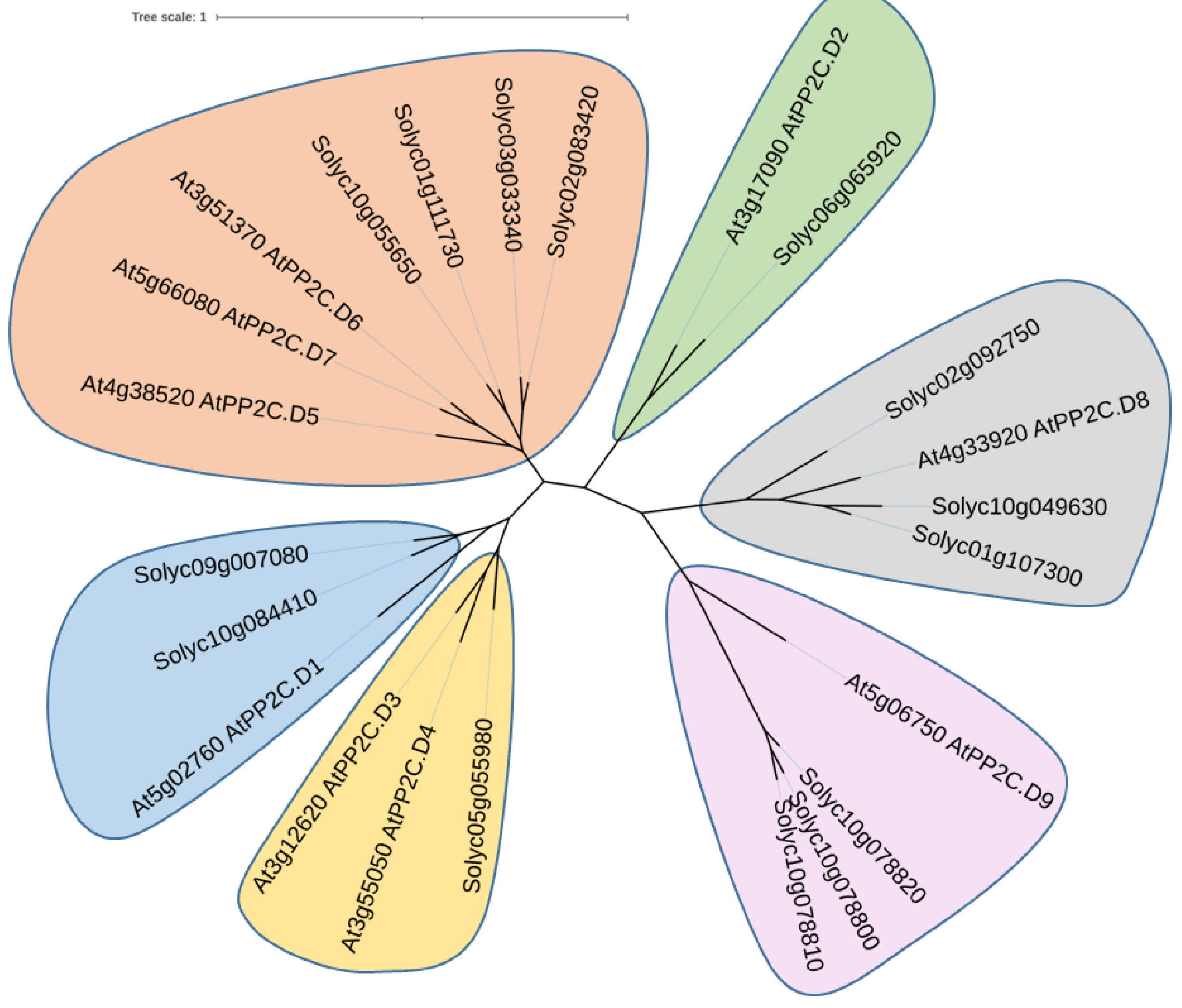
2.6. Polygenetic Tree by Maximum-Likelihood Method
2.7. Y2H Assays
3. Results
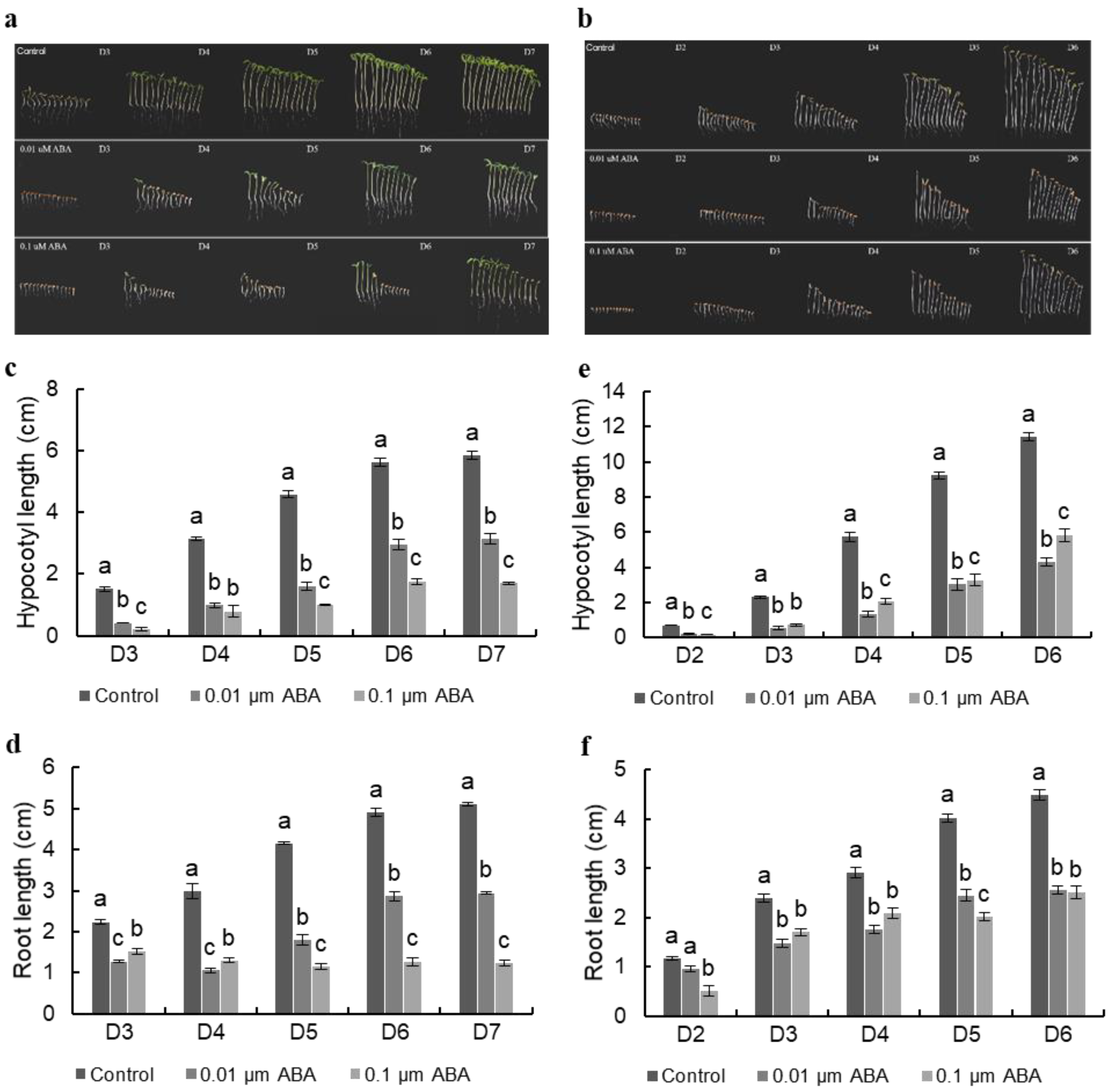
3.1. ABA Suppresses Hypocotyl Elongation in Both Light-Grown and Dark-Grown Seedlings in a Dose-Dependent Manner
3.2. Characterization of D-Clade PP2C (SlPP2C.D) Proteins in Tomato
3.3. Expression of SlPP2C.D Genes in Response to ABA
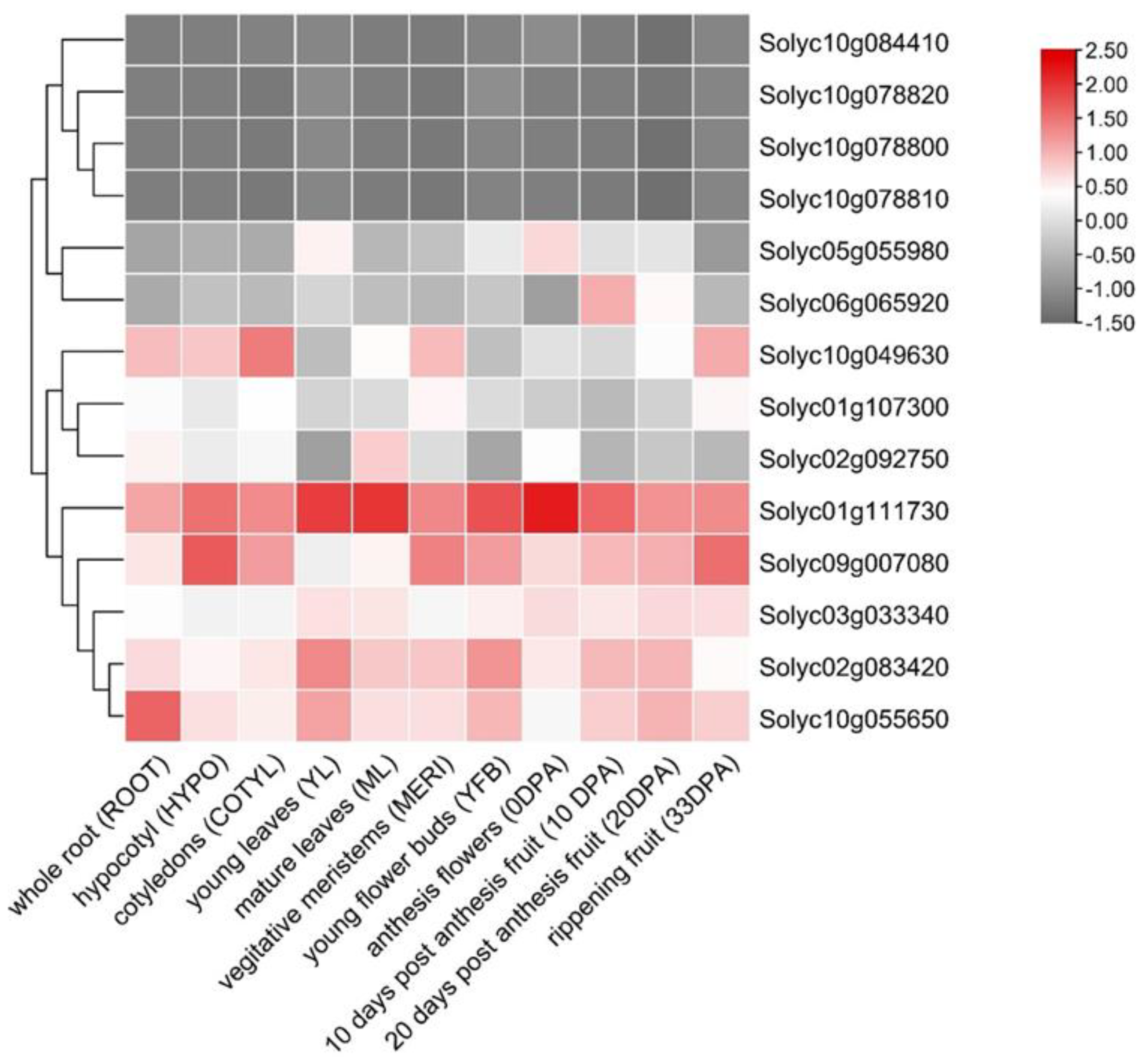
3.4. Organ-Specific Expression of SlPP2C.D Genes
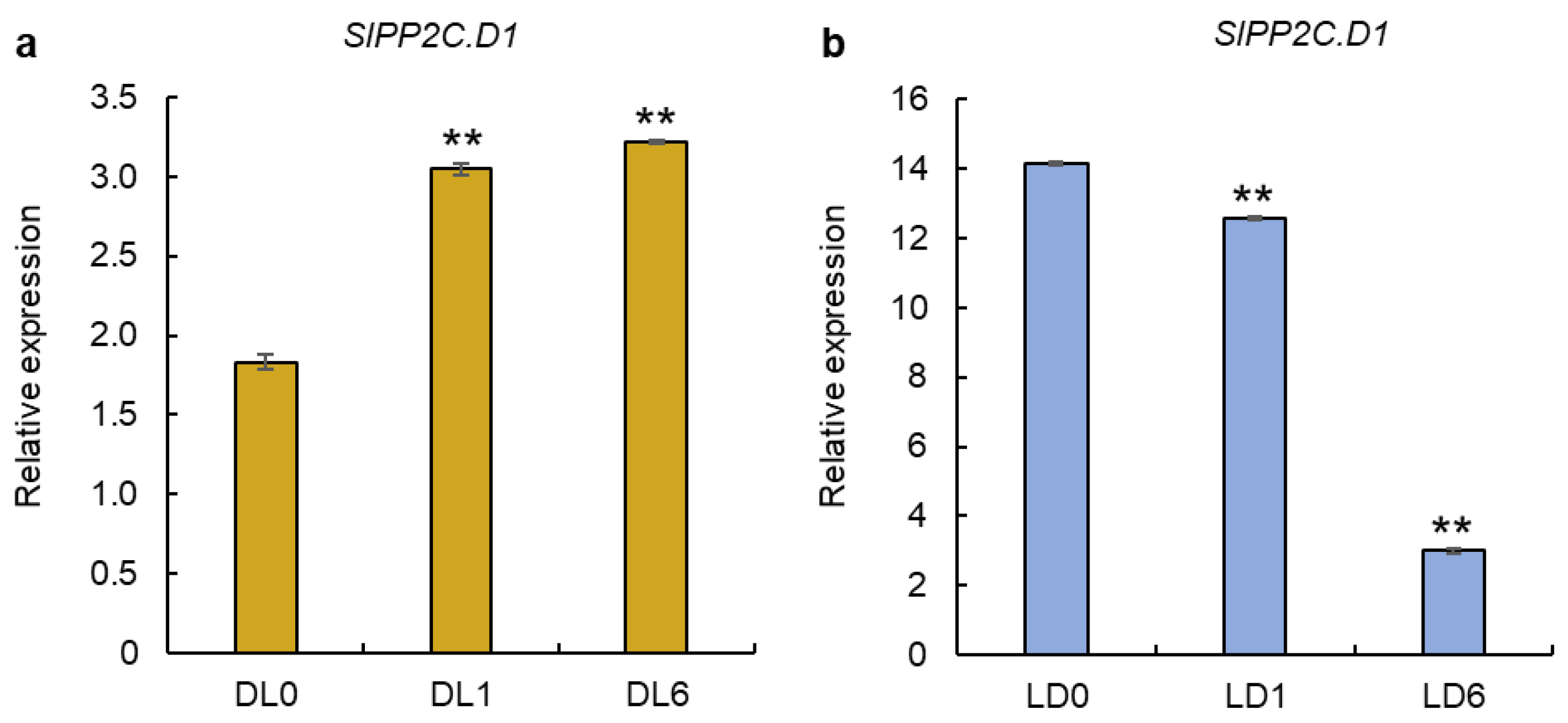
3.5. Expression of SlPP2C.D1 Gene in Light-Grown and Dark-Grown Seedlings
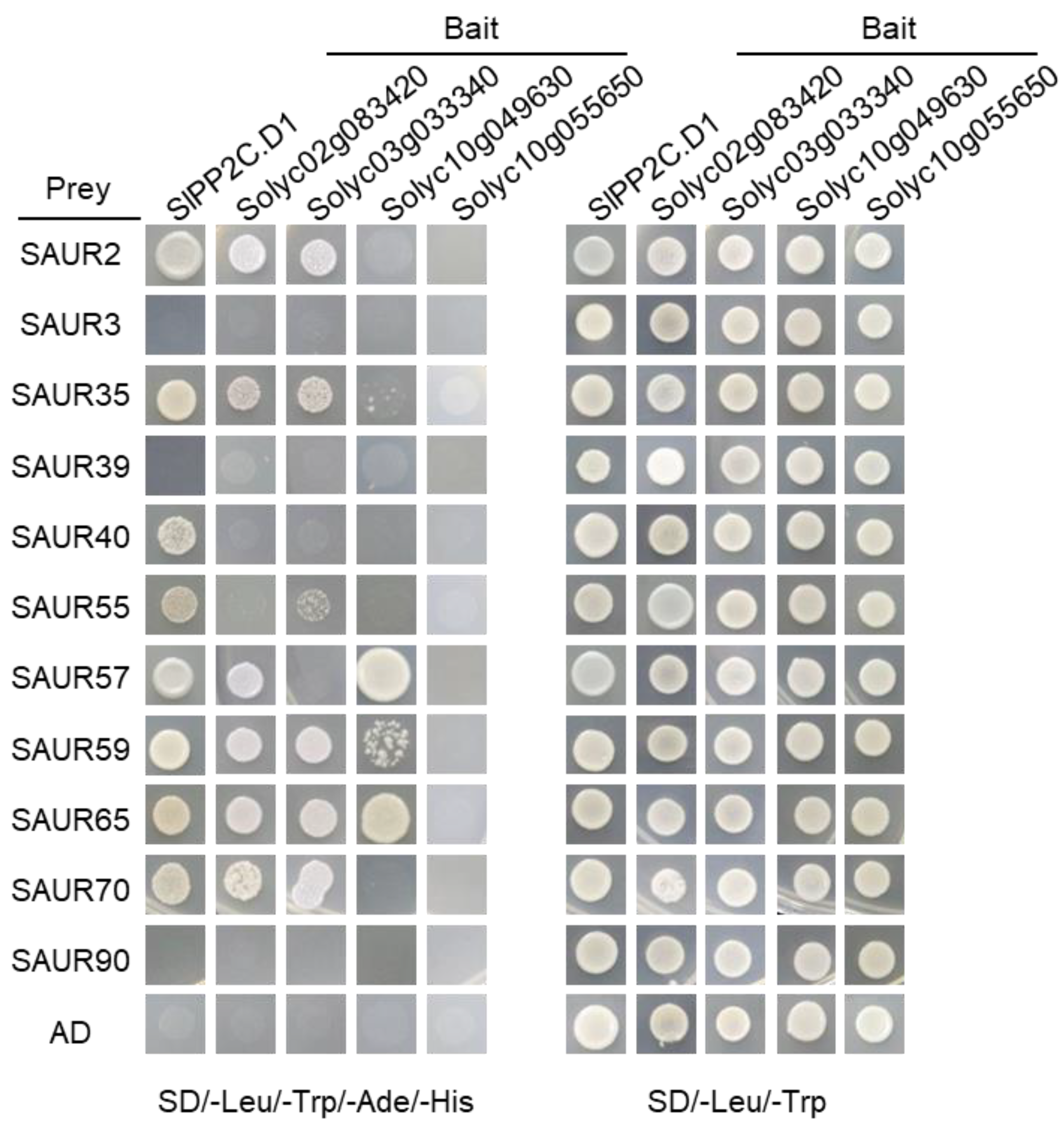
3.6. Characterization of the Interaction between SlPP2C.Ds and SlSAUR Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamm, P.; Kumar, P.P. The phytohormone signal network regulating elongation growth during shade avoidance. J. Exp. Bot. 2010, 61, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayle, D.L.; Cleland, R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970, 46, 250–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [PubMed] [Green Version]
- Nishitani, K.; Vissenberg, K. Roles of the XTH Protein Family in the Expanding Cell. In The Expanding Cell Plant Cell Monographs; Verbelen, J.P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6, pp. 89–116. [Google Scholar]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef]
- Katou, K.; Okamoto, H. Symplast as a Functional Unit in Plant Growth. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 142, pp. 263–304. [Google Scholar]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Fuglsang, A.T.; Visconti, S.; Drumm, K.; Jahn, T.; Stensballe, A.; Mattei, B.; Jensen, O.N.; Aducci, P.; Palmgren, M.G. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J. Biol. Chem. 1999, 274, 36774–36780. [Google Scholar] [CrossRef] [Green Version]
- Svennelid, F.; Olsson, A.; Piotrowski, M.; Rosenquist, M.; Ottman, C.; Larsson, C.; Oecking, C.; Sommarin, M. Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 1999, 11, 2379–2391. [Google Scholar]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Shimazaki, K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999, 18, 5548–5558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, M.; Takahashi, K.; Inoue, S.; Kinoshita, T. Evolutionary appearance of the plasma membrane H +-ATPase containing a penultimate threonine in the bryophyte. Plant Signal. Behav. 2012, 7, 979–982. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Inoue, S.; Takahashi, K.; Ishizaki, K.; Kohchi, T.; Kinoshita, T. Characterization of the plasma membrane H+-ATPase in the liverwort Marchantia polymorpha. Plant Physiol. 2012, 159, 826–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuhse, T.S.; Bottrill, A.R.; Jones, A.M.; Peck, S.C. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 2007, 51, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Cleland, R.E. Fusicoccin-induced growth and hydrogen ion excretion of Avena coleoptiles: Relation to auxin responses. Planta 1976, 128, 201–206. [Google Scholar] [CrossRef]
- Marre, E. Fusicoccin: A Tool in Plant Physiology. Annu. Rev. Plant Physiol. 1979, 30, 273–288. [Google Scholar] [CrossRef]
- Chen, Y.; Hoehenwarter, W.; Weckwerth, W. Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. Plant J. 2010, 63, 1–17. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takahashi, K.; Inoue, S.; Kinoshita, T. Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Duby, G.; Poreba, W.; Piotrowiak, D.; Bobik, K.; Derua, R.; Waelkens, E.; Boutry, M. Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J. Biol. Chem. 2009, 284, 4213–4221. [Google Scholar] [CrossRef] [Green Version]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Borch, J.; Bych, K.; Jahn, T.P.; Roepstorff, P.; Palmgren, M.G. The binding site for regulatory 14-3-3 protein in plant plasma membrane H+-ATPase: Involvement of a region promoting phosphorylation-independent interaction in addition to the phosphorylation-dependent C-terminal end. J. Biol. Chem. 2003, 278, 42266–42272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudashevskaya, E.L.; Ye, J.; Jensen, O.N.; Fuglsang, A.T.; Palmgren, M.G. Phosphosite mapping of P-type plasma membrane H+-ATPase in homologous and heterologous environments. J. Biol. Chem. 2012, 287, 4904–4913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef] [Green Version]
- Fuglsang, A.T.; Kristensen, A.; Cuin, T.A.; Schulze, W.X.; Persson, J.; Thuesen, K.H.; Ytting, C.K.; Oehlenschlaeger, C.B.; Mahmood, K.; Sondergaard, T.E.; et al. Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J. 2014, 80, 951–964. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, X.; Tang, W.; Takahashi, K.; Pan, X.; Dai, J.; Ren, H.; Zhu, X.; Pan, S.; Zheng, H.; et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 2021, 599, 278–282. [Google Scholar] [CrossRef]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the plasma membrane H+-ATPase AHA2 by BAK1 is required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef]
- Kerk, D.; Templeton, G.; Moorhead, G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008, 146, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989, 58, 453–508. [Google Scholar]
- Cohen, P.T. Novel protein serine.threonine phosphatases: Variety is the spice of life. Trends Biochem. Sci. 1997, 22, 245–251. [Google Scholar] [CrossRef]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Sakurai, N.; Kuraishi, S. Role of the outer tissue in abscisic acid-mediated growth suppression of etiolated squash hypocotyl segments. Physiol. Plant. 1989, 75, 151–156. [Google Scholar] [CrossRef]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.L.; Li, Y.; Zhang, J.H.; Zhu, Y.Y.; Rodriguez, P.L.; Xu, W.F. Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H+-ATPase 2. Sci. Adv. 2021, 7, eabd4113. [Google Scholar] [CrossRef] [PubMed]
- Merlot, S.; Gosti, F.; Guerrier, D.; Vavasseur, A.; Giraudat, J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001, 25, 295–303. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-Wide Analysis of the Protein Phosphatase 2C Genes in Tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Boccaccini, A.; Ruta, V.; Possenti, M.; Costantino, P.; Vittorioso, P. Abscisic acid inhibits hypocotyl elongation acting on gibberellins, DELLA proteins and auxin. AoB Plants 2018, 10, ply061. [Google Scholar] [PubMed]
- Humplik, J.F.; Bergougnoux, V.; Jandova, M.; Simura, J.; Pencik, A.; Tomanec, O.; Rolcik, J.; Novak, O.; Fellner, M. Endogenous abscisic acid promotes hypocotyl growth and affects endoreduplication during dark-induced growth in tomato (Solanum lycopersicum L.). PLoS ONE 2015, 10, e0117793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Wang, J.; Guo, X.N.; Shang, B.S.; Yan, H.R.; Zhang, X.; Zhao, X. A high concentration of abscisic acid inhibits hypocotyl phototropism in Gossypium arboreum by reducing accumulation and asymmetric distribution of auxin. J. Exp. Bot. 2021, 72, 6365–6381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hettenhausen, C.; Sun, G.; Zhuang, H.; Li, J.H.; Wu, J. The parasitic plant Cuscuta australis is highly insensitive to abscisic acid-induced suppression of hypocotyl elongation and seed germination. PLoS ONE 2015, 10, e0135197. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakamura, S.; Takemiya, A.; Takahashi, Y.; Shimazaki, K.; Kinoshita, T. Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol. 2010, 51, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Humplik, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Cramer, G.R.; Quarrie, S.A. Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct. Plant Biol. 2002, 29, 111–115. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W.J. Does ABA in the Xylem Control the Rate of Leaf Growth in Soil-Dried Maize and Sunflower Plants? J. Exp. Bot. 1990, 41, 1125–1132. [Google Scholar] [CrossRef]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of transport processes in plants. roles of the plasma membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Inoue, S.; Takahashi, K.; Kinoshita, T. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 2011, 52, 1238–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witthoft, J.; Caesar, K.; Elgass, K.; Huppenberger, P.; Kilian, J.; Schleifenbaum, F.; Oecking, C.; Harter, K. The activation of the Arabidopsis P-ATPase 1 by the brassinosteroid receptor BRI1 is independent of threonine 948 phosphorylation. Plant Signal. Behav. 2011, 6, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Bou Daher, F.; Liu, Y.; Steward, A.; Tillmann, M.; Zhang, X.; Wong, J.H.; Ren, H.; Cohen, J.D.; Li, C.; et al. Biphasic control of cell expansion by auxin coordinates etiolated seedling development. Sci. Adv. 2022, 8, eabj1570. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, N.; Zhang, F.; Yu, R.; Chen, H.; Deng, X.W.; Wei, N. SAUR17 and SAUR50 Differentially Regulate PP2C-D1 during Apical Hook Development and Cotyledon Opening in Arabidopsis. Plant Cell 2020, 32, 3792–3811. [Google Scholar] [CrossRef]
- Kodaira, K.S.; Qin, F.; Tran, L.S.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011, 157, 742–756. [Google Scholar] [CrossRef] [Green Version]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012, 71, 684–697. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; He, Y.; Guan, X.; Zhu, X.; Cheng, L.; Wang, J.; Lu, G. Genome-wide analysis of SAUR gene family in Solanaceae species. Gene 2012, 509, 38–50. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]

| Query | PL 1 (aa 2) | From | To | E-Value | Accession | Short Name |
|---|---|---|---|---|---|---|
| Solyc01g107300 | 376 | 42 | 337 | 4.67 × 10−62 | cd00143 | PP2Cc 3 |
| Solyc01g111730 | 388 | 49 | 356 | 2.06 × 10−64 | cd00143 | PP2Cc |
| Solyc02g083420 | 390 | 48 | 355 | 3.33 × 10−64 | cd00143 | PP2Cc |
| Solyc02g092750 | 366 | 32 | 336 | 4.59 × 10−58 | cd00143 | PP2Cc |
| Solyc03g033340 | 397 | 48 | 355 | 9.29 × 10−64 | cd00143 | PP2Cc |
| Solyc05g055980 | 384 | 53 | 360 | 2.43 × 10−68 | cd00143 | PP2Cc |
| Solyc06g065920 | 374 | 51 | 351 | 2.28 × 10−55 | cd00143 | PP2Cc |
| Solyc09g007080 | 378 | 43 | 350 | 1.25 × 10−72 | cd00143 | PP2Cc |
| Solyc10g049630 | 380 | 34 | 335 | 1.20 × 10−59 | cd00143 | PP2Cc |
| Solyc10g055650 | 339 | 1 | 306 | 1.44 × 10−64 | cd00143 | PP2Cc |
| Solyc10g078800 | 1312 | 51 | 342 | 3.46 × 10−55 | cd00143 | PP2Cc |
| Solyc10g078800 | 551 | 844 | 1.28 × 10−45 | cd00143 | PP2Cc | |
| Solyc10g078800 | 974 | 1266 | 1.36 × 10−54 | cd00143 | PP2Cc | |
| Solyc10g078810 | 438 | 63 | 339 | 2.40 × 10−53 | cd00143 | PP2Cc |
| Solyc10g078820 | 460 | 63 | 341 | 1.23 × 10−57 | cd00143 | PP2Cc |
| Solyc10g084410 | 376 | 42 | 349 | 1.38 × 10−72 | cd00143 | PP2Cc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Fei, S.; Wang, S.; He, Y.; Zhu, Z.; Liu, Y. Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy 2022, 12, 2542. https://doi.org/10.3390/agronomy12102542
Zheng X, Fei S, Wang S, He Y, Zhu Z, Liu Y. Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy. 2022; 12(10):2542. https://doi.org/10.3390/agronomy12102542
Chicago/Turabian StyleZheng, Xiaolin, Shihong Fei, Shajun Wang, Yong He, Zhujun Zhu, and Yuanyuan Liu. 2022. "Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation" Agronomy 12, no. 10: 2542. https://doi.org/10.3390/agronomy12102542
APA StyleZheng, X., Fei, S., Wang, S., He, Y., Zhu, Z., & Liu, Y. (2022). Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy, 12(10), 2542. https://doi.org/10.3390/agronomy12102542







