Comparative Analysis of Pedigree-Based BLUP and Phenotypic Mass Selection for Developing Elite Inbred Lines, Based on Field and Simulated Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Populations and Trials
2.2. Efficacy of Pedigree-Based BLUP and Statistical Analysis
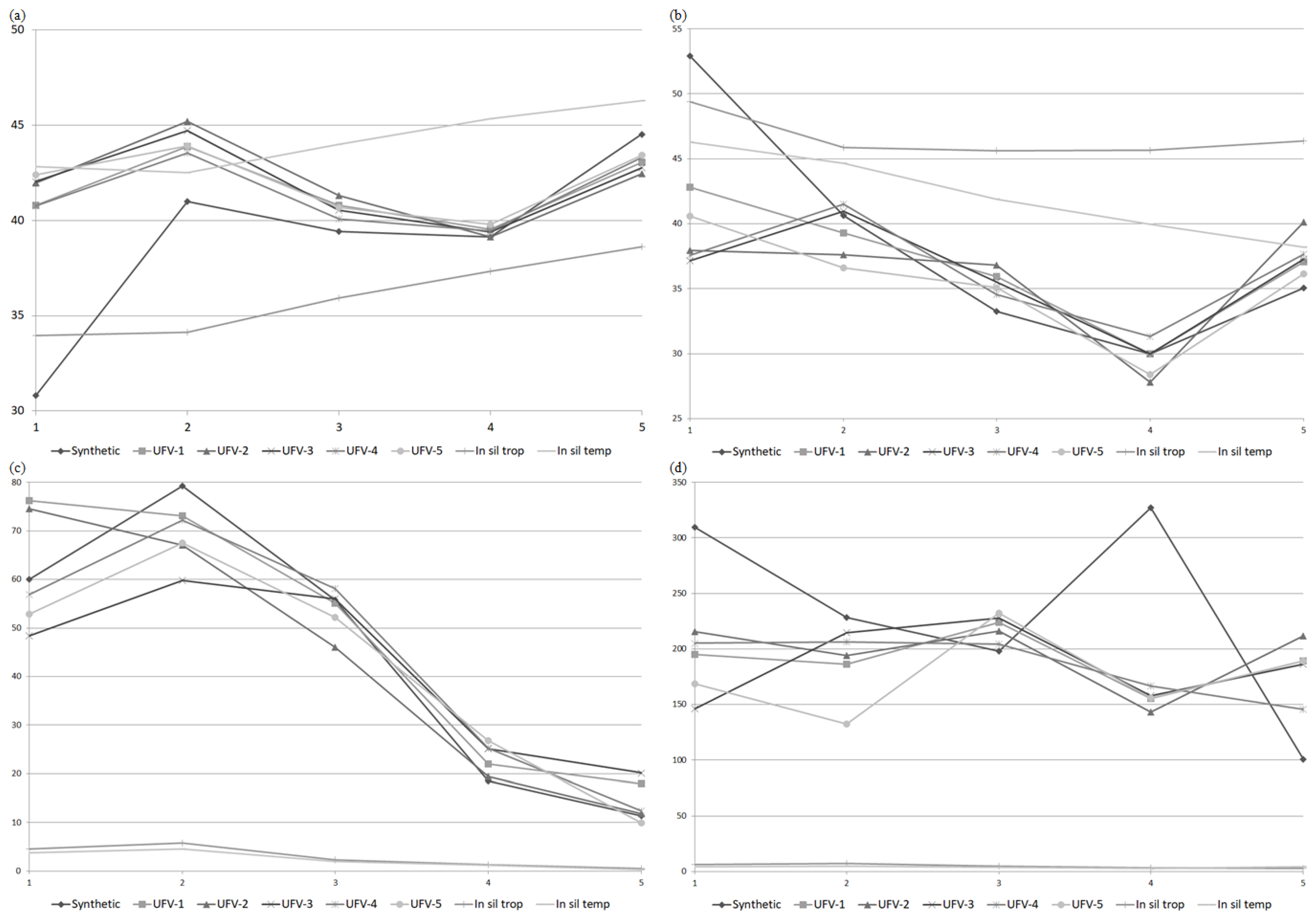
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, C.R. Sire evaluation and genetic trends. J. Anim. Sci. 1973, 1973, 10–41. [Google Scholar] [CrossRef]
- Henderson, C.R. General flexibility of linear model techniques for sire evaluation. J. Dairy Sci. 1974, 57, 963–972. [Google Scholar] [CrossRef]
- Blasco, A. The Bayesian controversy in animal breeding. J. Anim. Sci. 2001, 79, 2023–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, R. Prediction of Maize Single-Cross Performance Using RFLPs and Information from Related Hybrids. Crop Sci. 1994, 34, 20–25. [Google Scholar] [CrossRef]
- Gianola, D.; Rosa, G.J. One hundred years of statistical developments in animal breeding. Annu. Rev. Anim. Biosci. 2015, 3, 19–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viana, J.M.S.; Faria, V.R.; Fonseca e Silva, F.; Vilela de Resende, M.D. Best Linear Unbiased Prediction and Family Selection in Crop Species. Crop Sci. 2011, 51, 2371–2381. [Google Scholar] [CrossRef]
- Viana, J.M.S.; de Almeida, I.F.; Vilela de Resende, M.D.; Faria, V.R.; Fonseca e Silva, F. BLUP for genetic evaluation of plants in non-inbred families of annual crops. Euphytica 2010, 174, 31–39. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Weigel, K.A.; Young, A.E.; Cleveland, M.A.; Dekkers, J.C.M. Applied Animal Genomics: Results from the Field. Annu. Rev. Anim. Biosci. 2014, 2, 105–139. [Google Scholar] [CrossRef] [Green Version]
- Meuwissen, T.; Hayes, B.; Goddard, M. Accelerating Improvement of Livestock with Genomic Selection. Annu. Rev. Anim. Biosci. 2013, 1, 221–237. [Google Scholar] [CrossRef]
- Gianola, D.; Cecchinato, A.; Naya, H.; Schon, C.C. Prediction of Complex Traits: Robust Alternatives to Best Linear Unbiased Prediction. Front. Genet. 2018, 9, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vela-Avitua, S.; Meuwissen, T.H.E.; Luan, T.; Odegard, J. Accuracy of genomic selection for a sib-evaluated trait using identity-by-state and identity-by-descent relationships. Genet. Sel. Evol. 2015, 47, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholtens, M.; Lopez-Villalobos, N.; Lehnert, K.; Snell, R.; Garrick, D.; Blair, H.T. Advantage of including Genomic Information to Predict Breeding Values for Lactation Yields of Milk, Fat, and Protein or Somatic Cell Score in a New Zealand Dairy Goat Herd. Animals 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Velazco, J.G.; Malosetti, M.; Hunt, C.H.; Mace, E.S.; Jordan, D.R.; van Eeuwijk, F.A. Combining pedigree and genomic information to improve prediction quality: An example in sorghum. Theor. Appl. Genet. 2019, 132, 2055–2067. [Google Scholar] [CrossRef] [Green Version]
- Suontama, M.; Klapste, J.; Telfer, E.; Graham, N.; Stovold, T.; Low, C.; McKinley, R.; Dungey, H. Efficiency of genomic prediction across two Eucalyptus nitens seed orchards with different selection histories. Heredity 2019, 122, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Kainer, D.; Stone, E.A.; Padovan, A.; Foley, W.J.; Kulheim, C. Accuracy of Genomic Prediction for Foliar Terpene Traits in Eucalyptus polybractea. G3-Genes Genomes Genet. 2018, 8, 2573–2583. [Google Scholar] [CrossRef] [Green Version]
- Seno, L.D.; Guidolin, D.G.F.; Aspilcueta-Borquis, R.R.; do Nascimento, G.B.; da Silva, T.B.R.; de Oliveira, H.N.; Munari, D.P. Genomic selection in dairy cattle simulated populations. J. Dairy Res. 2018, 85, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Viana, J.M.S.; Garcia, A.A.F. Significance of linkage disequilibrium and epistasis on genetic variances in noninbred and inbred populations. BMC Genom. 2022, 23, 286. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: London, UK, 1996. [Google Scholar]
- Viana, J.M.S.; Faria, V.R.; Fonseca e Silva, F.; Vilela de Resende, M.D. Combined selection of progeny in crop breeding using best linear unbiased prediction. Can. J. Plant Sci. 2012, 92, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Cockerham, C.C. Covariances of relatives from self-fertilization. Crop Sci. 1983, 23, 1177–1180. [Google Scholar] [CrossRef]
- Butler, D.G.; Cullis, B.R.; Gilmour, A.R.; Gogel, B.G.; Thompson, R. ASReml-R Reference Manual Version 4; VSN International Ltd.: Hemel Hempstead, UK, 2017. [Google Scholar]
- Patterson, H.D.; Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Mrode, R.A. Linear Models for the Prediction of Animal Breeding Values, 2nd ed.; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Mehrban, H.; Naserkheil, M.; Lee, D.; Ibanez-Escriche, N. Multi-Trait Single-Step GBLUP Improves Accuracy of Genomic Prediction for Carcass Traits Using Yearling Weight and Ultrasound Traits in Hanwoo. Front. Genet. 2021, 12, 692356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Li, Q.H.; Wang, Q.; Wen, J.; Zhao, G.P. Comparison of the Efficiency of BLUP and GBLUP in Genomic Prediction of Immune Traits in Chickens. Animals 2020, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Fernandez, E.N.; Blasco, A.; Ravagnolo, O.; Legarra, A. Effects of ignoring inbreeding in model-based accuracy for BLUP and SSGBLUP. J. Anim. Breed. Genet. 2020, 137, 356–364. [Google Scholar] [CrossRef]
- Dunne, F.L.; Kelleher, M.M.; Walsh, S.W.; Berry, D.P. Characterization of best linear unbiased estimates generated from national genetic evaluations of reproductive performance, survival, and milk yield in dairy cows. J. Dairy Sci. 2018, 101, 7625–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viana, J.M.S.; Pereira, H.D.; Mundim, G.B.; Piepho, H.P.; Silva, F.F.E. Efficiency of genomic prediction of non-assessed single crosses. Heredity 2018, 120, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Viana, J.M.S.; Pereira, H.D.; Piepho, H.P.; Silva, F.F.E. Efficiency of Genomic Prediction of Nonassessed Testcrosses. Crop Sci. 2019, 59, 2020–2027. [Google Scholar] [CrossRef]
- Jibrila, I.; ten Napel, J.; Vandenplas, J.; Veerkamp, R.F.; Calus, M.P.L. Investigating the impact of preselection on subsequent single-step genomic BLUP evaluation of preselected animals. Genet. Sel. Evol. 2020, 52, 42. [Google Scholar] [CrossRef]
- El-Attrouny, M.M.; Manaa, E.A.; Ramadan, S.I. Genetic evaluation and selection correlated response of growth traits in Japanese quail. S. Afr. J. Anim. Sci. 2020, 50, 325–333. [Google Scholar] [CrossRef]
- D’Ambrosio, J.; Morvezen, R.; Brard-Fudulea, S.; Bestin, A.; Perez, A.A.; Guemen, D.; Poncet, C.; Haffray, P.; Dupont-Nivet, M.; Phocas, F. Genetic architecture and genomic selection of female reproduction traits in rainbow trout. BMC Genom. 2020, 21, 558. [Google Scholar] [CrossRef]
- Cobo, E.; Raoul, J.; Bodin, L. Genetic parameters of litter weight, an alternative criterion to prolificacy and pre-weaning weight for selection of French meat sheep. Livest. Sci. 2021, 250, 104596. [Google Scholar] [CrossRef]

| Pop. | Gen. | Prog. | Size | EV | Grain Yield | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/MF | M/MF | |||||||||||||||
| Synthetic | S0 | - | 417 | 30.8 | 60.1 | 44.25 | - | - | 11.34 | 52.9 | 309.5 | 155.13 | - | - | 148.16 | −0.05 |
| S1 | 32 | 165 | 41.0 | 79.3 | 97.42 | - | - | 11.34 | 40.6 | 228.2 | 50.93 | - | - | 148.16 | −0.21 | |
| S2 | 59 | 290 | 39.3 | 51.0 | 43.91 | - | - | 11.34 | 32.3 | 209.8 | 36.10 | - | - | 148.16 | 0.57 | |
| S3 | 75 | 360 | 39.0 | 23.8 | 10.82 | - | - | 11.34 | 30.4 | 201.3 | 39.29 | - | - | 148.16 | −0.11 | |
| S4 | 76 | 367 | 43.3 | 11.8 | 0.50 | - | - | 11.34 | 36.9 | 168.2 | 11.86 | - | - | 148.16 | 0.11 | |
| UFV-1 | S0 | - | 378 | 40.8 | 76.2 | 32.11 | - | - | 17.90 | 42.8 | 195.2 | 52.92 | - | - | 141.26 | −0.25 |
| S1 | 215 | 804 | 43.9 | 73.0 | 69.59 | - | - | 17.90 | 39.3 | 186.0 | 30.61 | - | - | 141.26 | −0.40 | |
| S2 | 317 | 1130 | 40.7 | 54.0 | 37.82 | - | - | 17.90 | 35.9 | 225.7 | 46.58 | - | - | 141.26 | 0.02 | |
| S3 | 276 | 1134 | 39.6 | 25.4 | 3.85 | - | - | 17.90 | 29.8 | 166.6 | 15.68 | - | - | 141.26 | −0.58 | |
| S4 | 427 | 1981 | 43.1 | 17.3 | 0.26 | - | - | 17.90 | 36.2 | 177.0 | 17.62 | - | - | 141.26 | −1.00 | |
| UFV-2 | S0 | - | 391 | 42.0 | 74.5 | 34.96 | - | - | 20.21 | 37.9 | 215.8 | 44.68 | - | - | 142.84 | −0.17 |
| S1 | 168 | 490 | 45.2 | 67.0 | 47.00 | - | - | 20.21 | 37.6 | 194.0 | 29.96 | - | - | 142.84 | −0.13 | |
| S2 | 169 | 617 | 41.2 | 48.2 | 24.72 | - | - | 20.21 | 36.6 | 219.6 | 30.72 | - | - | 142.84 | −0.16 | |
| S3 | 183 | 706 | 39.2 | 24.9 | 2.30 | - | - | 20.21 | 29.5 | 177.1 | 17.08 | - | - | 142.84 | −0.20 | |
| S4 | 315 | 1468 | 42.7 | 20.3 | 0.70 | - | - | 20.21 | 36.7 | 173.8 | 17.23 | - | - | 142.84 | 0.03 | |
| UFV-3 | S0 | - | 530 | 42.0 | 48.4 | 26.06 | - | - | 16.82 | 37.1 | 145.9 | 0.00 | - | - | 146.75 | - |
| S1 | 310 | 946 | 44.7 | 59.8 | 46.83 | - | - | 16.82 | 41.0 | 214.8 | 42.22 | - | - | 146.75 | −0.23 | |
| S2 | 357 | 1330 | 40.4 | 55.9 | 36.47 | - | - | 16.82 | 35.4 | 225.7 | 28.09 | - | - | 146.75 | 0.13 | |
| S3 | 337 | 1421 | 39.3 | 27.7 | 7.18 | - | - | 16.82 | 29.9 | 167.1 | 14.45 | - | - | 146.75 | −0.44 | |
| S4 | 502 | 2433 | 42.9 | 17.1 | 0.80 | - | - | 16.82 | 36.4 | 183.5 | 19.15 | - | - | 146.75 | −0.02 | |
| UFV-4 | S0 | - | 252 | 40.7 | 56.8 | 24.13 | - | - | 20.84 | 37.5 | 205.1 | 71.60 | - | - | 133.33 | −0.10 |
| S1 | 101 | 369 | 43.5 | 72.2 | 53.12 | - | - | 20.84 | 41.5 | 206.3 | 47.47 | - | - | 133.33 | −0.03 | |
| S2 | 116 | 517 | 40.6 | 61.6 | 38.44 | - | - | 20.84 | 35.5 | 218.1 | 32.94 | - | - | 133.33 | 0.12 | |
| S3 | 144 | 603 | 39.0 | 30.3 | 5.32 | - | - | 20.84 | 30.6 | 170.0 | 17.74 | - | - | 133.33 | −0.29 | |
| S4 | 218 | 1107 | 42.6 | 20.2 | 0.50 | - | - | 20.84 | 36.2 | 154.5 | 12.18 | - | - | 133.33 | −0.41 | |
| UFV-5 | S0 | - | 753 | 42.4 | 52.8 | 35.73 | - | - | 12.11 | 40.6 | 168.4 | 39.58 | - | - | 126.92 | −0.53 |
| S1 | 546 | 2037 | 43.9 | 67.5 | 78.68 | - | - | 12.11 | 36.6 | 132.6 | 9.63 | - | - | 126.92 | −1.00 | |
| S2 | 600 | 1913 | 40.7 | 51.7 | 41.12 | - | - | 12.11 | 35.1 | 231.3 | 50.00 | - | - | 126.92 | −0.26 | |
| S3 | 533 | 2163 | 39.8 | 29.8 | 13.21 | - | - | 12.11 | 28.8 | 151.6 | 15.92 | - | - | 126.92 | −0.63 | |
| S4 | 840 | 3914 | 43.2 | 12.6 | 0.59 | - | - | 12.11 | 37.0 | 191.3 | 46.52 | - | - | 126.92 | −1.00 | |
| In silico | S0 | 1000 | 42.9 | 14.0 | 4.04 | 0.17 | 0.00 | 9.84 | 48.2 | 26.5 | 4.40 | 0.30 | 0.00 | 18.82 | −0.65 | |
| temperate | 42.8 | 13.4 | 1.11 | - | - | 12.32 | 48.4 | 23.2 | 0.09 | - | - | 23.16 | −1.00 | |||
| S1 | 247 | 1000 | 42.5 | 18.4 | 5.38 | 0.16 | −0.01 | 12.87 | 44.9 | 30.2 | 6.01 | 0.54 | −0.28 | 23.93 | −0.61 | |
| 42.5 | 16.3 | 3.13 | - | - | 12.32 | 44.5 | 16.74 | 3.44 | - | - | 23.16 | −0.74 | ||||
| S2 | 250 | 1000 | 42.3 | 20.0 | 5.91 | 0.12 | −0.02 | 13.96 | 43.2 | 31.8 | 6.69 | 0.44 | −0.43 | 25.09 | −0.65 | |
| 42.1 | 16.6 | 4.05 | - | - | 12.32 | 43.3 | 16.32 | 3.60 | - | - | 23.16 | −0.60 | ||||
| S3 | 220 | 1000 | 42.2 | 20.6 | 6.13 | 0.09 | −0.03 | 14.40 | 42.4 | 32.2 | 7.00 | 0.35 | −0.50 | 25.39 | −0.65 | |
| 42.0 | 16.5 | 3.36 | - | - | 12.32 | 42.1 | 17.37 | 5.16 | - | - | 23.16 | −0.59 | ||||
| S4 | 243 | 1000 | 42.1 | 20.9 | 6.23 | 0.08 | −0.03 | 14.58 | 42.0 | 32.4 | 7.14 | 0.30 | −0.53 | 25.49 | −0.52 | |
| 41.6 | 16.4 | 3.57 | - | - | 12.32 | 42.1 | 16.88 | 4.00 | - | - | 23.16 | −0.36 | ||||
| In silico | S0 | - | 1000 | 33.9 | 14.8 | 4.31 | 0.12 | 0.00 | 10.33 | 49.2 | 32.2 | 6.08 | 0.36 | 0.00 | 25.76 | 0.78 |
| tropical | 34.0 | 15.3 | 2.77 | - | - | 12.49 | 49.0 | 32.9 | 2.04 | - | - | 30.87 | 1.00 | |||
| S1 | 243 | 1000 | 33.8 | 19.3 | 5.71 | 0.11 | −0.01 | 13.51 | 45.5 | 41.6 | 8.19 | 0.64 | −0.28 | 33.06 | 0.75 | |
| 34.1 | 20.4 | 5.34 | - | - | 12.49 | 45.9 | 41.0 | 6.28 | - | - | 30.87 | 1.00 | ||||
| S2 | 227 | 1000 | 33.7 | 21.0 | 6.25 | 0.08 | −0.02 | 14.68 | 43.6 | 43.9 | 9.06 | 0.49 | −0.43 | 34.76 | 0.80 | |
| 33.9 | 21.0 | 4.87 | - | - | 12.49 | 43.9 | 44.9 | 8.29 | - | - | 30.87 | 0.75 | ||||
| S3 | 237 | 1000 | 33.9 | 21.7 | 6.48 | 0.06 | −0.02 | 15.15 | 42.7 | 44.5 | 9.43 | 0.37 | −0.50 | 35.20 | 0.81 | |
| 33.9 | 22.5 | 5.15 | - | - | 12.49 | 42.6 | 43.3 | 6.74 | - | - | 30.87 | 0.67 | ||||
| S4 | 217 | 1000 | 33.6 | 22.0 | 6.58 | 0.05 | −0.02 | 15.36 | 42.2 | 44.7 | 9.60 | 0.30 | −0.53 | 35.34 | 0.82 | |
| 34.0 | 23.7 | 5.45 | - | - | 12.49 | 42.9 | 42.4 | 6.11 | - | - | 30.87 | 0.62 | ||||
| Pop. | Gen. | Ac1 | Ac2 | Ac3 | Ac4 | Ac5 | %S | iDgd | iDgi |
|---|---|---|---|---|---|---|---|---|---|
| Synthetic | S0 | - | 0.89 | - | - | 0.88 | 0 | - | - |
| S1 | - | 0.95 | - | - | 0.92 | 50 | 0.00 | 0.00 | |
| S2 | - | 0.89 | - | - | 0.85 | 45 | −0.58 | 0.16 | |
| S3 | - | 0.70 | - | - | 0.55 | 40 | 0.45 | −0.17 | |
| S4 | - | 0.20 | - | - | - 2 | - | - | - | |
| UFV-1 | S0 | - | 0.80 | - | - | - 2 | 0 | - | - |
| S1 | - | 0.89 | - | - | 0.62 | 50 | 0.13 | −0.08 | |
| S2 | - | 0.82 | - | - | - 2 | 45 | −0.20 | −0.38 | |
| S3 | - | 0.42 | - | - | - 2 | 40 | 0.06 | 0.10 | |
| S4 | - | 0.12 | - | - | - 2 | - | - | - | |
| UFV-2 | S0 | - | 0.80 | - | - | 0.75 | 0 | - | - |
| S1 | - | 0.84 | - | - | 0.85 | 50 | −0.01 | 0.03 | |
| S2 | - | 0.74 | - | - | 0.69 | 45 | −0.04 | −0.05 | |
| S3 | - | 0.32 | - | - | - 2 | 40 | 0.02 | 0.46 | |
| S4 | - | 0.18 | - | - | - 2 | - | - | - | |
| UFV-3 | S0 | - | 0.78 | - | - | 0.76 | 0 | - | - |
| S1 | - | 0.86 | - | - | 0.86 | 50 | 0.00 | −0.01 | |
| S2 | - | 0.83 | - | - | 0.83 | 45 | 0.05 | −0.01 | |
| S3 | - | 0.55 | - | - | - 2 | 40 | 0.00 | 0.18 | |
| S4 | - | 0.21 | - | - | - 2 | - | - | - | |
| UFV-4 | S0 | - | 0.73 | - | - | 0.68 | 0 | - | - |
| S1 | - | 0.85 | - | - | 0.86 | 50 | −0.25 | −0.10 | |
| S2 | - | 0.80 | - | - | 0.80 | 45 | 0.06 | −0.07 | |
| S3 | - | 0.45 | - | - | - 2 | 40 | 0.05 | −0.04 | |
| S4 | - | 0.15 | - | - | - 2 | - | - | - | |
| UFV-5 | S0 | - | 0.86 | - | - | 0.84 | 0 | - | - |
| S1 | - | 0.93 | - | - | 0.94 | 50 | −0.01 | 0.00 | |
| S2 | - | 0.88 | - | - | 0.87 | 45 | −0.04 | 0.00 | |
| S3 | - | 0.72 | - | - | 0.49 | 40 | 0.15 | −0.01 | |
| S4 | - | 0.21 | - | - | - 2 | - | - | - | |
| In silico | S0 | 0.54 | 0.29 | 0.52 | 0.52 | 0.28 | 0 | - | - |
| temperate | S1 | 0.54 | 0.45 | 0.52 | 0.67 | 0.42 | 50 | 0.07 | −0.04 |
| 0.46 3 | −0.31 3 | ||||||||
| 1.16 4 | −0.68 4 | ||||||||
| S2 | 0.54 | 0.50 | 0.49 | 0.72 | 0.45 | 45 | 0.14 | −0.04 | |
| 0.41 3 | −0.16 3 | ||||||||
| 0.32 4 | −0.27 4 | ||||||||
| S3 | 0.54 | 0.46 | 0.51 | 0.78 | 0.34 | 40 | 0.29 | −0.20 | |
| 0.83 3 | −0.96 3 | ||||||||
| 2.05 4 | −1.74 4 | ||||||||
| S4 | 0.55 | 0.47 | 0.48 | 0.78 | 0.34 | - | - | - | |
| In silico | S0 | 0.54 | 0.43 | 0.56 | 0.56 | 0.42 | 0 | - | - |
| tropical | S1 | 0.54 | 0.55 | 0.53 | 0.71 | 0.58 | 50 | 0.02 | 0.02 |
| 0.15 3 | 0.23 3 | ||||||||
| 0.75 4 | 0.48 4 | ||||||||
| S2 | 0.55 | 0.53 | 0.54 | 0.79 | 0.49 | 45 | 0.03 | −0.01 | |
| 0.15 3 | −0.06 3 | ||||||||
| 0.54 4 | 0.30 4 | ||||||||
| S3 | 0.55 | 0.54 | 0.56 | 0.82 | 0.48 | 40 | 0.26 | 0.31 | |
| 0.96 3 | 0.86 3 | ||||||||
| 1.25 4 | 0.81 4 | ||||||||
| S4 | 0.55 | 0.55 | 0.57 | 0.83 | 0.48 | - | - | - |
| Gen. | Ac | %S | iDgd | iDgi | ||
|---|---|---|---|---|---|---|
| S0 | 1.99 [0.83, 3.42] | 11.80 [10.71, 12.94] | 0.54 [0.52, 0.58] | - | - | - |
| S1 | 4.29 [3.06, 5.37] | 11.80 [10.71, 12.94] | 0.71 [0.63, 0.75] | 50 | −0.48 [−0.93, 0.59] 2 | 0.25 [−0.37, 0.74] |
| 0.08 [−0.28, 1.16] 3 | −0.03 [−0.68, 0.35] | |||||
| S2 | 4.36 [3.16, 5.35] | 11.80 [10.71, 12.94] | 0.75 [0.71, 0.81] | 45 | 0.32 [−0.04, 1.03] 2 | −0.23 [−0.77, 0.11] |
| 0.33 [0.06, 0.66] 3 | −0.28 [−0.71, 0.04] | |||||
| S3 | 4.57 [3.15, 6.76] | 11.80 [10.71, 12.94] | 0.78 [0.72, 0.82] | 40 | 0.50 [−0.40, 0.93] 2 | −0.33 [−1.12, 0.59] |
| 1.33 [0.80, 2.21] 3 | −0.76 [−1.74, 0.18] | |||||
| S4 | 4.32 [3.03, 5.87] | 11.80 [10.71, 12.94] | 0.80 [0.73, 0.83] | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, J.M.S.; Dias, K.O.d.G.; Silva, J.P.A.d. Comparative Analysis of Pedigree-Based BLUP and Phenotypic Mass Selection for Developing Elite Inbred Lines, Based on Field and Simulated Data. Agronomy 2022, 12, 2560. https://doi.org/10.3390/agronomy12102560
Viana JMS, Dias KOdG, Silva JPAd. Comparative Analysis of Pedigree-Based BLUP and Phenotypic Mass Selection for Developing Elite Inbred Lines, Based on Field and Simulated Data. Agronomy. 2022; 12(10):2560. https://doi.org/10.3390/agronomy12102560
Chicago/Turabian StyleViana, José Marcelo Soriano, Kaio Olimpio das Graças Dias, and Jean Paulo Aparecido da Silva. 2022. "Comparative Analysis of Pedigree-Based BLUP and Phenotypic Mass Selection for Developing Elite Inbred Lines, Based on Field and Simulated Data" Agronomy 12, no. 10: 2560. https://doi.org/10.3390/agronomy12102560






