Antifungal Efficacy and Convenience of Krameria lappacea for the Development of Botanical Fungicides and New Alternatives of Antifungal Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Fungal Strains
2.3. Experimental Design Used for Determination of Inhibitory Effect
2.4. Statistical Analysis
2.5. Purification and Preparation of K. lappacea Extract Fractions
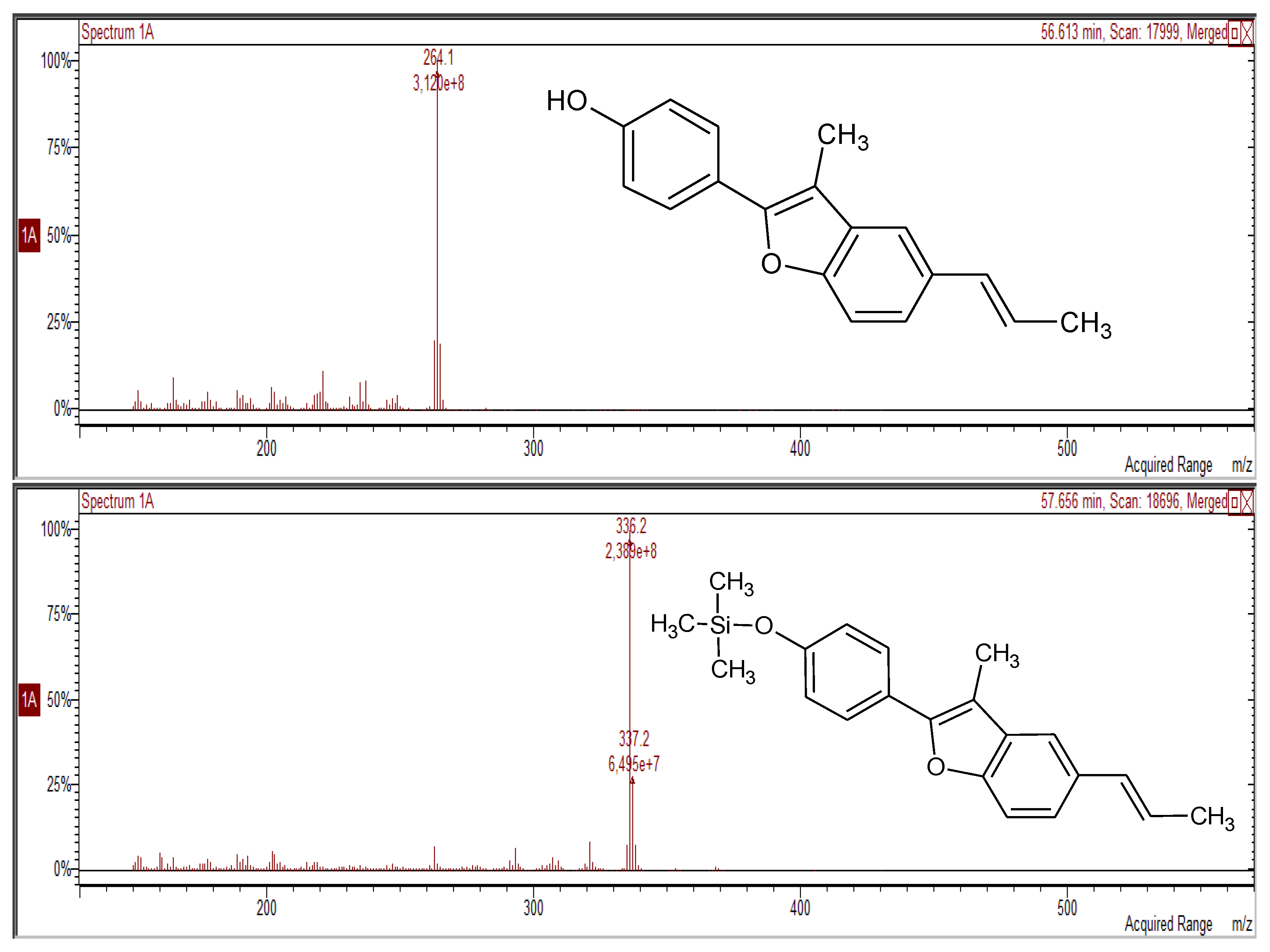
2.6. Derivatization
2.7. GC/MS Analysis
2.8. UHPLC-ToFMS Analysis
3. Results
4. Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- González Pereyra, M.L.; Chiacchiera, S.M.; Rosa, C.A.; Sager, R.; Dalcero, A.M.; Cavaglieri, L. Comparative analysis of the mycobiota and mycotoxins contaminating corn trench silos and silo bags. J. Sci. Food Agric. 2011, 91, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus species and mycotoxins: Occurrence and importance in major food commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Review chapter: Fusarium genus and essential oils. In Natural Antimicrobial Agents; Mérillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 95–120. [Google Scholar]
- Cutuli, M.T.; Gibello, A.; Rodriguez-Bertos, A.; Blanco, M.M.; Villarroel, M.; Giraldo, A.; Guarro, J. Skin and subcutaneous mycoses in tilapia (Oreochromis niloticus) caused by Fusarium oxysporum in coinfection with Aeromonas hydrophila. Med. Mycol. Case Rep. 2015, 9, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lorf, T.; Braun, F.; Rüche, l.R.; Müller, A.; Sattler, B.; Ringe, B. Systemic mycoses during prophylactical use of liposomal amphotericin B (Ambisome®) after liver transplantation. Mycoses 1999, 42, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Suarez, M.; Sutton, D.A.; Cano-Lira, J.F.; García, D.; Martin-Vicente, A.; Wiederhold, N.; Gené, J. Identification and antifungal susceptibility of penicillium-like fungi from clinical samples in the United States. J. Clin. Microbiol. 2016, 54, 2155–2161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glynn, M.; Jo, W.; Minowa, K.; Sanada, H.; Nejishima, H.; Matsuuchi, H.; Mutter, L. Efinaconazole: Developmental and reproductive toxicity potential of a novel antifungal azole. Reprod. Toxicol. 2015, 52, 18–25. [Google Scholar] [CrossRef]
- Heise, T.; Schmidt, F.; Knebel, C.; Rieke, S.; Haider, W.; Pfeil, R.; Marx-Stoelting, P. Hepatotoxic effects of (tri) azole fungicides in a broad dose range. Arch. Toxicol. 2015, 89, 2105–2117. [Google Scholar] [CrossRef]
- Cao, S.; Ye, L.; Wu, Y.; Mao, B.; Chen, L.; Wang, X.; Ge, R.S. The effects of fungicides on human 3β-hydroxysteroid dehydrogenase 1 and aromatase in human placental cell line JEG-3. Pharmacology 2017, 100, 139–147. [Google Scholar] [CrossRef]
- Gupta, P.K. Herbicides and fungicides. In Reproductive and Developmental Toxicology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 657–679. [Google Scholar]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Levine, M.T.; Chandrasekar, P.H. Adverse effects of voriconazole: Over a decade of use. Clin. Transplant. 2016, 30, 1377–1386. [Google Scholar] [CrossRef]
- Steffens, J.J.; Pell, E.J.; Tien, M. Mechanisms of fungicide resistance in phytopathogenic fungi. Curr. Opin. Biotechnol. 1996, 7, 348–355. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Mavridou, E.; Mortensen, K.L.; Snelders, E.; Frimodt-Møller, N.; Khan, H.; Verweij, P.E. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS ONE 2010, 5, e10080. [Google Scholar] [CrossRef] [PubMed]
- Becher, R.; Hettwer, U.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G. Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 2010, 100, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Prokinova, E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 2014, 112, 443–448. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, B.; Kumar, A.; Kumar, R.; Dubey, N.K.; Gupta, R. Assessment of Pelargonium graveolens oil as plant-based antimicrobial and aflatoxin suppressor in food preservation. J. Sci. Food Agric. 2008, 88, 2421–2425. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Brokamp, G. Now, where did all the Rhatanies go? Abundance, seed ecology, and regeneration of Krameria lappacea from the Peruvian Andes. In Relevance and Sustainability of Wild Plant Collection in NW South America; Springer Spektrum: Wiesbaden, Germany, 2015; pp. 103–122. [Google Scholar]
- Zabka, M.; Pavela, R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: New York, NY, USA, 1971. [Google Scholar]
- Zabka, M.; Drastichova, K.; Jegorov, A.; Soukupova, J.; Nedbal, L. Direct evidence of plant-pathogenic activity of fungal metabolites of Trichothecium roseum on apple. Mycopathologia 2006, 162, 65–68. [Google Scholar] [CrossRef]
- Pavela, R.; Zabka, M.; Tylova, T.; Kresinova, Z. Insecticidal activity of compounds from Ailanthus altissima against Spodoptera littoralis larvae. Pak. J. Agric. Sci. 2014, 51, 101–112. [Google Scholar]
- Fecik, R.A.; Frank, K.E.; Gentry, E.J.; Mitscher, L.A.; Shibata, M. Use of combinatorial and multiple parallel synthesis methodologies for the development of anti-infective natural products. Pure Appl. Chem. 1999, 71, 559–564. [Google Scholar] [CrossRef]
- Oakley, K.L.; Moore, C.B.; Denning, D.W. Comparison of In Vitro Activity of Liposomal Nystatin against Aspergillus Species with Those of Nystatin, Amphotericin B (AB) Deoxycholate, AB Colloidal Dispersion, Liposomal AB, AB Lipid Complex, and Itraconazole. Antimicrob. Agents Chemother. 1999, 43, 1264–1266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Uno, B.E. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Apers, S.; Vlietinck, A.; Pieters, L. Lignans and neolignans as lead compounds. Phytochem. Rev. 2003, 2, 201–217. [Google Scholar] [CrossRef]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef]
- Abreu, P.M.; Branco, P.S. Natural product-like combinatorial libraries. J. Braz. Chem. Soc. 2003, 14, 675–712. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Basha, A.; Basha, F.; Kashif Ali, S.R.; Hanson, P.A.; Mitscher, L.R.; Oakley, B. Recent progress in the chemotherapy of human fungal diseases. Emphasis on 1, 3-β-glucan synthase and chitin synthase inhibitors. Curr. Med. Chem. 2013, 20, 4859–4887. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Kovaříková, K.; Tříska, J.; Vrchotová, N.; Bednář, J. Antifungal and Insecticidal Potential of the Essential Oil from Ocimum sanctum L. against Dangerous Fungal and Insect Species and Its Safety for Non-Target Useful Soil Species Eisenia fetida (Savigny, 1826). Plants 2021, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Pasqualotto, A.C.; Warn, P.A.; Bowyer, P.; Denning, D.W. Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology 2007, 153, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, A.D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Simpson, B.B. The past and present uses of rhatany (Krameria, Krameriaceae). Econ. Bot. 1991, 45, 397–409. [Google Scholar] [CrossRef]
- Roth, I.; Lindorf, H. South American Medicinal Plants: Botany, Remedial Properties and General Use; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Neto, C.C.; Owens, C.W.; Langfield, R.D.; Comeau, A.B.; Onge, J.S.; Vaisberg, A.J.; Hammond, G.B. Antibacterial activity of some Peruvian medicinal plants from the Callejon de Huaylas. J. Ethnopharmacol. 2002, 79, 133–138. [Google Scholar] [CrossRef]
- Moreno-Salazar, S.F.; Robles-Zepeda, R.E.; Johnson, D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia 2008, 79, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Moreno De-la-Cruz, H.; Vilcapoma, G.; Zevallos, P.A. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. J. Ethnopharmacol. 2007, 111, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, L.; Sosa, S.; Atanasov, A.G.; Bodensieck, A.; Fakhrudin, N.; Bauer, J.; Ladurner, A. Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J. Nat. Prod. 2011, 74, 1779–1786. [Google Scholar] [CrossRef]
- Meinke, M.C.; Schanzer, S.; Haag, S.F.; Casetti, F.; Müller, M.L.; Wölfle, U.; Schempp, C.M. In vivo photoprotective and anti-inflammatory effect of hyperforin is associated with high antioxidant activity in vitro and ex vivo. Eur. J. Pharm. Biopharm. 2012, 81, 346–350. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities–Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]

| Species | Family | Plant Part Assayed | Yield (%) | Origin |
|---|---|---|---|---|
| Acanthopanax senticosus (Rupr. & Maxim.) Harms | Araliaceae | Roots | 5.4 | Cicenice, Czech Republic |
| Acer campestre L. | Aceraceae | Leaves | 9.8 | Prague, Czech Republic |
| Acer capillipes Maxim. | Aceraceae | Leaves | 12.5 | Prague, Czech Republic |
| Acer platanoides L. | Aceraceae | Leaves | 9.3 | Prague, Czech Republic |
| Achillea ageratum L. | Asteraceae | Stem | 11.8 | Prague, Czech Republic |
| Achillea collina Heimerl | Asteraceae | Stem | 7.0 | Prague, Czech Republic |
| Achillea nobilis L. | Asteraceae | Stem | 10.9 | Prague, Czech Republic |
| Aegopodium podagraria L. | Apiaceae | Stem | 5.6 | Dobre, Czech Republic |
| Ajuga chamaepitys (L.) Schreber | Lamiaceae | Stem | 14.8 | Prague, Czech Republic |
| Alpinia purpurata K. Schum. | Zingiberaceae | Roots | 7.0 | Cicenice, Czech Republic |
| Anethum graveolens L. | Apiaceae | Stem | 12.4 | Prague, Czech Republic |
| Angelica archangelica L. | Apiaceae | Fruits | 6.1 | Cicenice, Czech Republic |
| Angostura trifoliata (Willd.) T.S.Elias | Rutaceae | Bark | 11.7 | Cicenice, Czech Republic |
| Asarum europaeum L. | Arisrolochiaceae | Stem | 7.5 | Vranov, Czech Republic |
| Astragalus glycyphyllos L. | Fabaceae | Stem | 9.1 | Prague, Czech Republic |
| Bistorta officinalis Delarbre | Polygonaceae | Roots | 7.2 | Cicenice, Czech Republic |
| Borago officinalis L. | Boraginaceae | Stem | 4.5 | Prague, Czech Republic |
| Buddleja davidii Franch. | Buddlejaceae | Stem | 21.0 | Prague, Czech Republic |
| Cinchona officinalis L. | Rubiaceae | Bark | 10.4 | Cicenice, Czech Republic |
| Citrus × sinensis (L.) Osbeck | Rutaceae | Pericarp | 8.3 | Cicenice, Czech Republic |
| Citrus aurantium L. | Rutaceae | Pericarp | 5.6 | Cicenice, Czech Republic |
| Citrus bergamia (Risso) Wright & Arn | Rutaceae | Pericarp | 9.1 | Cicenice, Czech Republic |
| Daucus carota L. | Apiaceae | Stem | 8.1 | Prague, Czech Republic |
| Dracocephalum moldavica L. | Lamiaceae | Stem | 12.7 | Prague, Czech Republic |
| Foeniculum vulgare Mill. | Apiaceae | Seeds | 5.9 | Prague, Czech Republic |
| Galega officinalis L. | Fabaceae | Stem | 14.8 | Prague, Czech Republic |
| Galium sylvaticum L. | Rubiaceae | Stem | 5.2 | Vranov, Czech Republic |
| Geum urbanum L. | Rosaceae | Roots | 13.6 | Cicenice, Czech Republic |
| Gonolobus condurango Decne. | Apocynaceae | Bark | 12.6 | Cicenice, Czech Republic |
| Guaiacum officinale L. | Zygophyllaceae | Xylem | 5.3 | Cicenice, Czech Republic |
| Harpagophytum procumbens (Burch.) DC ex Meissn. | Pedaliaceae | Roots | 5.2 | Cicenice, Czech Republic |
| Hyssopus seravschanicus (Dub.) Pazij | Lamiaceae | Stem | 11.0 | Prague, Czech Republic |
| Inula magnifica Lipsky | Asteraceae | Stem | 11.8 | Prague, Czech Republic |
| Krameria lappacea (Dombey) Burdet & B.B.Simpson | Krameriaceae | Roots | 17.6 | Cicenice, Czech Republic |
| Lamium argentatum(Smejkal) Henker ex G. H. Loos | Lamiaceae | Stem | 15.1 | Vranov, Czech Republic |
| Lathyrus tuberosus L. | Fabaceae | Stem | 13.5 | Znojmo, Czech Republic |
| Lavandula angustifolia Mill. | Lamiaceae | Stem | 9.4 | Prague, Czech Republic |
| Lavandula canariensis Mill. | Lamiaceae | Stem | 6.2 | Prague, Czech Republic |
| Leuzea carthamoides DC. | Asteraceae | Roots | 2.3 | Cicenice, Czech Republic |
| Lotus corniculatus L. | Fabaceae | Stem | 7.3 | Dobre, Czech Republic |
| Lythrum virgatum L. | Lythraceae | Stem | 9.6 | Prague, Czech Republic |
| Melilotus albus Medik. | Fabaceae | Stem | 10.7 | Dobre, Czech Republic |
| Mentha arvensis L. | Lamiaceae | Stem | 6.2 | Prague, Czech Republic |
| Mentha longifolia (L.) L. | Lamiaceae | Stem | 9.2 | Prague, Czech Republic |
| Mentha suaveolens Ehrh. | Lamiaceae | Stem | 16.9 | Prague, Czech Republic |
| Nepeta pannonica L. | Lamiaceae | Stem | 8.5 | Prague, Czech Republic |
| Ononis arvensis L. | Fabaceae | Stem | 12.6 | Prague, Czech Republic |
| Orlaya grandiflora (L.) Hoffm. | Apiaceae | Stem | 11.4 | Prague, Czech Republic |
| Picramnia excelsa (Swartz) Planch. | Simaroubaceae | Xylem | 10.7 | Cicenice, Czech Republic |
| Plantago lanceolata L. | Plantaginaceae | Stem | 18.6 | Prague, Czech Republic |
| Potentilla anserina L. | Rosaceae | Stem | 7.3 | Prague, Czech Republic |
| Potentilla fruticosa L. | Rosaceae | Stem | 21.6 | Prague, Czech Republic |
| Potentilla hirta L. | Rosaceae | Stem | 2.1 | Prague, Czech Republic |
| Potentilla reptans L. | Rosaceae | Stem | 12.2 | Prague, Czech Republic |
| Quercus robur L. | Fagaceae | Bark | 4.6 | Cicenice, Czech Republic |
| Rhamnus frangula L. | Rhamnaceae | Bark | 9.7 | Cicenice, Czech Republic |
| Rheum officinale L. | Polygonaceae | Roots | 8.0 | Cicenice, Czech Republic |
| Salix alba L. | Salicaceae | Bark | 3.5 | Cicenice, Czech Republic |
| Salvia officinalis L. | Lamiaceae | Stem | 14.0 | Prague, Czech Republic |
| Stachys palustris L. | Lamiaceae | Stem | 8.0 | Prague, Czech Republic |
| Stachys recta L. | Lamiaceae | Stem | 8.3 | Prague, Czech Republic |
| Symphytum officinale L. | Boraginaceae | Roots | 7.5 | Cicenice, Czech Republic |
| Tabebuia impetiginosa (Mart. ex DC.) Standl. | Bignoniaceae | bark | 11.2 | Cicenice, Czech Republic |
| Tanacetumparthenium (L.) Schlutz-Bip. | Asteraceae | Stem | 12.3 | Prague, Czech Republic |
| Teucrium botrys L. | Lamiaceae | Stem | 4.6 | Prague, Czech Republic |
| Teucrium capitatum L. | Lamiaceae | Stem | 9.7 | Prague, Czech Republic |
| Uncaria tomentosa (Willd. ex Schult.) DC | Rubiaceae | Bark | 8.3 | Cicenice, Czech Republic |
| Urtica dioica L. | Urticaceae | Roots | 3.6 | Cicenice, Czech Republic |
| Valeriana officinalis L. | Valerianaceae | Roots | 11.7 | Prague, Czech Republic |
| Plant Extract | % Inhibition of Target Fungi (Mean ± SE) | |||||
|---|---|---|---|---|---|---|
| Fusarium oxysporum | Fusarium verticillioides | Penicillium brevicompactum | Penicillium expansum | Aspergillus flavus | Aspergillus fumigatus | |
| Acanthopanax senticosus | 31.22 ± 0.05 | 33.33 ± 0.08 | 12.20 ± 0.08 | 22.64 ± 0.09 | 13.54 ± 0.05 | 44.44 ± 0.05 |
| Acer campestre | 13.82 ± 0.09 | 17.43 ± 0.00 | −8.16 ± 0.05 | 5.66 ± 0.05 | 4.71 ± 0.14 | 5.26 ± 0.00 |
| Acer capillipes | 24.39 ± 0.00 | 18.35 ± 0.05 | −4.08 ± 0.00 | 9.43 ± 0.00 | 8.24 ± 0.00 | 28.07 ± 0.05 |
| Acer platanoides | 12.20 ± 0.00 | 14.68 ± 0.00 | −4.08 ± 0.00 | 9.43 ± 0.00 | 0.00 ± 0.05 | 5.26 ± 0.00 |
| Achillea ageratum | 35.34 ± 0.00 | 27.59 ± 0.00 | 20.00 ± 0.00 | 32.08 ± 0.00 | 32.22 ± 0.05 | 51.72 ± 0.05 |
| Achillea collina | 19.83 ± 0.00 | 20.69 ± 0.00 | 11.11 ± 0.05 | 35.85 ± 0.05 | 23.33 ± 0.00 | 27.59 ± 0.00 |
| Achillea nobilis | 22.41 ± 0.00 | 17.24 ± 0.00 | 6.67 ± 0.00 | 26.42 ± 0.00 | 26.67 ± 0.00 | 41.38 ± 0.05 |
| Aegopodium podagraria | 0.00 ± 0.05 | −5.75 ± 0.05 | −2.22 ± 0.05 | 13.21 ± 0.05 | 15.56 ± 0.05 | 31.03 ± 0.05 |
| Ajuga chamaepitys | −1.72 ± 0.05 | −12.64 ± 0.09 | 15.56 ± 0.05 | 18.87 ± 0.05 | 17.78 ± 0.05 | 18.97 ± 0.05 |
| Alpinia purpurata | 76.19 ± 0.00 | 65.93 ± 0.17 | 21.95 ± 0.09 | 52.83 ± 0.05 | 48.96 ± 0.05 | 72.73 ± 0.00 |
| Anethum graveolens | 22.41 ± 0.00 | 27.59 ± 0.00 | 20.00 ± 0.00 | 26.42 ± 0.00 | 16.67 ± 0.08 | 15.52 ± 0.05 |
| Angelica archangelica | 82.01 ± 0.05 | 73.33 ± 0.00 | 48.78 ± 0.00 | 64.71 ± 0.09 | 50.00 ± 0.00 | 88.89 ± 0.05 |
| Angostura trifoliata | 58.20 ± 0.05 | 46.67 ± 0.00 | 48.78 ± 0.00 | 37.74 ± 0.00 | 16.67 ± 0.19 | 63.54 ± 0.05 |
| Asarum europaeum | 63.43 ± 0.05 | 60.75 ± 0.00 | 83.33 ± 0.05 | 55.10 ± 0.05 | 42.86 ± 0.00 | 57.81 ± 0.00 |
| Astragalus glycyphyllos | 18.70 ± 0.24 | 22.94 ± 0.00 | 0.00 ± 0.05 | 7.55 ± 0.05 | 18.82 ± 0.00 | 10.53 ± 0.00 |
| Bistorta officinalis | 23.81 ± 0.00 | 11.85 ± 0.05 | −9.76 ± 0.00 | 11.32 ± 0.05 | −33.33 ± 0.05 | 2.02 ± 0.24 |
| Borago officinalis | 3.25 ± 0.09 | 14.68 ± 0.00 | 18.37 ± 0.12 | 5.66 ± 0.05 | −3.53 ± 0.05 | 8.77 ± 0.05 |
| Buddleja davidii | 12.20 ± 0.00 | 21.10 ± 0.05 | −6.12 ± 0.05 | 9.43 ± 0.00 | 8.24 ± 0.00 | 12.28 ± 0.09 |
| Cinchona officinalis | 23.28 ± 0.05 | 11.11 ± 0.14 | 24.39 ± 0.05 | 15.09 ± 0.00 | 2.08 ± 0.33 | −26.26 ± 0.05 |
| Citrus × sinensis | 17.99 ± 0.09 | 12.59 ± 0.12 | −17.07 ± 0.00 | 3.77 ± 0.00 | 8.33 ± 0.05 | 21.21 ± 0.00 |
| Citrus aurantium | 12.17 ± 0.05 | 14.07 ± 0.05 | −14.63 ± 0.05 | 15.09 ± 0.00 | 2.08 ± 0.05 | 6.06 ± 0.00 |
| Citrus bergamia | 14.81 ± 0.09 | 16.30 ± 0.09 | −14.63 ± 0.05 | 20.75 ± 0.00 | −1.04 ± 0.05 | 26.26 ± 0.05 |
| Daucus carota | 21.55 ± 0.05 | 13.79 ± 0.14 | 8.89 ± 0.05 | 24.53 ± 0.05 | 33.33 ± 0.00 | 34.48 ± 0.09 |
| Dracocephalum moldavica | 21.95 ± 0.00 | 16.51 ± 0.05 | 2.04 ± 0.00 | 9.43 ± 0.00 | 10.59 ± 0.05 | 3.51 ± 0.05 |
| Foeniculum vulgare | 65.85 ± 0.00 | 60.55 ± 0.05 | 97.96 ± 0.05 | 69.81 ± 0.05 | 78.95 ± 0.00 | 94.74 ± 0.00 |
| Galega officinalis | 24.39 ± 0.00 | 22.94 ± 0.00 | −4.08 ± 0.00 | 5.66 ± 0.05 | 8.24 ± 0.00 | −7.02 ± 0.05 |
| Galium sylvaticum | 10.45 ± 0.00 | 22.43 ± 0.05 | 14.58 ± 0.09 | 20.41 ± 0.00 | 23.81 ± 0.09 | 23.44 ± 0.05 |
| Geum urbanum | 20.63 ± 0.14 | 9.63 ± 0.09 | 17.07 ± 0.05 | 18.87 ± 0.09 | −10.42 ± 0.05 | 8.08 ± 0.05 |
| Gonolobus condurango | 26.98 ± 0.00 | 22.22 ± 0.14 | 2.44 ± 0.05 | 28.30 ± 0.05 | −10.42 ± 0.05 | 24.24 ± 0.00 |
| Guaiacum officinale | 64.02 ± 0.05 | 47.41 ± 0.05 | 19.51 ± 0.00 | 20.75 ± 0.00 | 3.13 ± 0.00 | 51.52 ± 0.00 |
| Harpagophytum procumbens | 21.69 ± 0.05 | 13.33 ± 0.08 | 0.00 ± 0.09 | 24.53 ± 0.05 | 1.04 ± 0.05 | 23.23 ± 0.09 |
| Hyssopus seravschanicus | 21.55 ± 0.05 | 18.39 ± 0.09 | 13.33 ± 0.00 | 26.42 ± 0.00 | 26.67 ± 0.00 | 24.14 ± 0.05 |
| Inula magnifica | 35.77 ± 0.05 | 32.11 ± 0.05 | 16.33 ± 0.05 | 16.98 ± 0.05 | 24.71 ± 0.05 | 49.93 ± 0.00 |
| Krameria lappacea | 87.30 ± 0.00 | 90.37 ± 0.05 | 63.41 ± 0.00 | 52.83 ± 0.05 | 69.38 ± 0.00 | 91.92 ± 0.00 |
| Lamium argentatum | 12.07 ± 0.08 | 2.30 ± 0.09 | 6.67 ± 0.00 | 20.75 ± 0.00 | 13.33 ± 0.00 | 17.24 ± 0.00 |
| Lathyrus tuberosus | −2.99 ± 0.00 | 6.54 ± 0.09 | 12.50 ± 0.08 | 20.41 ± 0.08 | 10.71 ± 0.00 | 20.31 ± 0.00 |
| Lavandula angustifolia | 19.51 ± 0.14 | 31.19 ± 0.00 | 8.16 ± 0.00 | −9.43 ± 0.09 | 18.82 ± 0.00 | 7.02 ± 0.05 |
| Lavandula canariensis | 14.66 ± 0.00 | −6.90 ± 0.00 | −13.33 ± 0.00 | 32.08 ± 0.00 | 23.33 ± 0.00 | 17.24 ± 0.00 |
| Leuzea carthamoides | 55.03 ± 0.09 | 37.78 ± 0.14 | 78.05 ± 0.00 | 69.81 ± 0.05 | 18.75 ± 0.00 | 37.37 ± 0.05 |
| Lotus corniculatus | 8.62 ± 0.05 | −2.30 ± 0.12 | −13.33 ± 0.00 | 9.43 ± 0.00 | 12.22 ± 0.05 | 25.86 ± 0.05 |
| Lythrum virgatum | 6.50 ± 0.05 | 12.84 ± 0.05 | −4.08 ± 0.00 | 0.00 ± 0.05 | 8.24 ± 0.00 | −5.26 ± 0.14 |
| Melilotus albus | 6.90 ± 0.00 | −2.30 ± 0.09 | −4.44 ± 0.05 | 18.87 ± 0.05 | 23.33 ± 0.00 | 37.93 ± 0.00 |
| Mentha arvensis | 51.72 ± 0.09 | 51.72 ± 0.00 | 57.78 ± 0.05 | 66.04 ± 0.00 | 32.22 ± 0.05 | 55.17 ± 0.09 |
| Mentha longifolia | 32.76 ± 0.00 | 13.79 ± 0.00 | 46.67 ± 0.00 | 58.49 ± 0.05 | 30.00 ± 0.00 | 72.41 ± 0.05 |
| Mentha suaveolens | 25.86 ± 0.09 | 18.39 ± 0.09 | 46.67 ± 0.00 | 54.72 ± 0.00 | 32.22 ± 0.05 | 56.90 ± 0.05 |
| Nepeta pannonica | 31.90 ± 0.09 | 10.34 ± 0.00 | 26.67 ± 0.00 | 45.28 ± 0.05 | 33.33 ± 0.00 | 32.76 ± 0.00 |
| Ononis arvensis | 15.52 ± 0.05 | 12.64 ± 0.05 | 6.67 ± 0.00 | 41.51 ± 0.05 | 27.78 ± 0.05 | 51.72 ± 0.12 |
| Orlaya grandiflora | 29.27 ± 0.08 | 28.44 ± 0.00 | 8.16 ± 0.00 | 18.87 ± 0.05 | 17.65 ± 0.05 | 31.58 ± 0.00 |
| Picramnia excelsa | 20.11 ± 0.25 | 5.93 ± 0.09 | −2.44 ± 0.00 | 5.66 ± 0.09 | −12.50 ± 0.14 | 18.18 ± 0.08 |
| Plantago lanceolata | −6.90 ± 0.05 | −14.94 ± 0.05 | 0.00 ± 0.00 | 20.75 ± 0.00 | 21.11 ± 0.47 | 17.24 ± 0.00 |
| Potentilla anserina | −8.94 ± 0.17 | 5.50 ± 0.24 | −2.04 ± 0.05 | 16.98 ± 0.09 | 18.82 ± 0.00 | 7.02 ± 0.05 |
| Potentilla fruticosa | −3.25 ± 0.05 | 11.01 ± 0.09 | 0.00 ± 0.05 | −1.89 ± 0.00 | 21.18 ± 0.05 | 12.28 ± 0.09 |
| Potentilla hirta | −3.25 ± 0.05 | 12.84 ± 0.05 | −4.08 ± 0.00 | 9.43 ± 0.00 | 18.82 ± 0.00 | 8.77 ± 0.19 |
| Potentilla reptans | 13.82 ± 0.05 | 7.34 ± 0.09 | 6.12 ± 0.05 | 5.66 ± 0.09 | 16.47 ± 0.19 | 12.28 ± 0.09 |
| Quercus robur | 17.99 ± 0.09 | 2.22 ± 0.08 | 12.20 ± 0.00 | 5.66 ± 0.05 | 0.00 ± 0.00 | 15.15 ± 0.00 |
| Rhamnus frangula | 36.51 ± 0.00 | 4.44 ± 0.00 | 19.51 ± 0.00 | 30.19 ± 0.05 | −13.54 ± 0.05 | 9.09 ± 0.00 |
| Rheum officinale | 46.56 ± 0.05 | 30.37 ± 0.05 | 2.44 ± 0.12 | 37.74 ± 0.00 | 12.50 ± 0.00 | 27.27 ± 0.14 |
| Salix alba | 6.35 ± 0.08 | −0.74 ± 0.09 | −12.20 ± 0.05 | 7.55 ± 0.05 | −18.75 ± 0.14 | 2.02 ± 0.09 |
| Salvia officinalis | 60.34 ± 0.05 | 47.13 ± 0.05 | 46.67 ± 0.00 | 33.96 ± 0.05 | 35.56 ± 0.05 | 82.76 ± 0.05 |
| Stachys palustris | 13.82 ± 0.05 | 12.84 ± 0.09 | −2.04 ± 0.05 | 9.43 ± 0.00 | 1.18 ± 0.14 | 7.02 ± 0.09 |
| Stachys recta | 10.34 ± 0.09 | 1.15 ± 0.05 | −6.67 ± 0.00 | 18.87 ± 0.09 | 16.67 ± 0.00 | 8.62 ± 0.09 |
| Symphytum officinale | 0.00 ± 0.00 | −4.44 ± 0.00 | 2.44 ± 0.05 | 24.53 ± 0.05 | −14.58 ± 0.09 | −24.24 ± 0.36 |
| Tabebuia impetiginosa | 50.79 ± 0.00 | 34.81 ± 0.09 | 26.83 ± 0.00 | 37.74 ± 0.00 | −3.12 ± 0.00 | 36.36 ± 0.00 |
| Tanacethum parthenium | 30.17 ± 0.00 | 33.33 ± 0.05 | 20.00 ± 0.00 | 35.85 ± 0.05 | 30.00 ± 0.00 | 36.21 ± 0.05 |
| Teucrium botrys | 28.46 ± 0.05 | 28.44 ± 0.00 | −8.16 ± 0.52 | 13.21 ± 0.05 | 7.06 ± 0.05 | 19.30 ± 0.09 |
| Teucrium capitatum | 25.20 ± 0.05 | 30.28 ± 0.05 | 4.08 ± 0.05 | 11.32 ± 0.05 | 11.76 ± 0.00 | 15.79 ± 0.00 |
| Uncaria tomentosa | 11.64 ± 0.05 | 5.19 ± 0.05 | −4.88 ± 0.05 | 13.21 ± 0.05 | −26.04 ± 0.09 | 6.06 ± 0.00 |
| Urtica dioica | 22.75 ± 0.09 | −2.22 ± 0.49 | 29.27 ± 0.09 | 35.85 ± 0.05 | −28.13 ± 0.00 | 13.13 ± 0.05 |
| Valeriana officinalis | 48.78 ± 0.00 | 28.44 ± 0.00 | 34.69 ± 0.05 | 37.74 ± 0.00 | 34.12 ± 0.09 | 43.86 ± 0.09 |
| Plant Species | Target Fungal Species | |||||
|---|---|---|---|---|---|---|
| Fusarium oxysporum | Fusarium verticillioides | Penicillium brevicompactum | Penicillium expansum | Aspergillus flavus | Aspergillus fumigatus | |
| MIC50 (CI95) a Chi b | MIC50 (CI95) a Chi b | MIC50 (CI95) a Chi b | MIC50 (CI95) a Chi b | MIC50 (CI95) a Chi b | MIC50 (CI95) a Chi b | |
| Achillea ageratum | >2 | >2 | >2 | >2 | >2 | 0.96 (0.75–1.50) |
| 2.226 | ||||||
| Alpinia purpurata | 0.30 (0.24–0.38) | 0.84 (0.61–1.07) | >2 | 0.74 (0.44–2.27) | >2 | 0.17 (0.13–0.20) |
| 3.177 | 5.019 | 1.615 | 0.962 | |||
| Angelica archangelica | 0.20 (0.15–0.29) | 0.17 (0.12–0.21) | >2 | 0.28 (0.19–0.44) | >2 | 0.39 (0.26–0.50) |
| 1.579 | 0.401 | 2.219 | 1.487 | |||
| Angostura trifoliata | 1.76 (1.36–2.98) | >2 | >2 | >2 | >2 | 0.29 (0.21–0.45) |
| 2.251 | 1.173 | |||||
| Asarum europaeum | 1.68 (1.01–5.05) | 1.87 (1.53–2.31) | 1.58 (1.03–1.73) | 1.78 (1.57–2.22) | >2 | 1.85 (1.73–2.05) |
| 2.078 | 1.790 | 0.612 | 1.337 | 0.868 | ||
| Foeniculum vulgare | 1.44 (1.27–1.67) | 1.22 (1.01–1.50) | 0.71 (0.63–0.79) | 1.14 (0.98–1.34) | 0.89 (0.49–1.10) | 0.27 (0.23–0.32) |
| 0.165 | 1.517 | 0.594 | 4.846 | 1.625 | 2.716 | |
| Guaiacum officinale | 0.84 (0.62–1.57) | >2 | >2 | >2 | >2 | 0.44 (0.34–0.72) |
| 2.743 | 0.842 | |||||
| Krameria lappacea | 0.14 (0.07–0.19) | 0.12 (0.07–0.16) | 0.38 (0.30–0.49) | 1.24 (0.74–1.61) | 0.25 (0.20–0.33) | 0.11 (0.08–0.14) |
| 2.190 | 0.506 | 1.317 | 3.019 | 2.175 | 0.190 | |
| Leuzea carthamoides | 1.98 (1.63–2.31) | >2 | 1.46 (1.22–1.86) | 1.32 (1.13–1.54) | >2 | >2 |
| 0.656 | 2.916 | 0.491 | 1.291 | |||
| Mentha arvensis | 1.99 (1.66–2.62) | 1.89 (1.60–2.82) | 1.85 (1.69–2.14) | 1.84 (1.66–2.14) | >2 | 1.86 (1.53–2.50) |
| 3.532 | 1.725 | 2.785 | 0.113 | 1.053 | ||
| Mentha longifolia | >2 | >2 | >2 | 0.82 (0.60–1.23) | >2 | 0.92 (0.76–1.22) |
| 0.468 | 3.472 | |||||
| Mentha suaveolens | >2 | >2 | >2 | 0.66 (0.44–1.74) | >2 | 1.27 (1.03–1.69) |
| 2.941 | 0.030 | |||||
| Tabebuia impetiginosa | 1.60 (1.13–1.85) | >2 | >2 | >2 | >2 | >2 |
| 3.150 | ||||||
| Propiconazole * | 0.69 (0.47–0.93) * | 0.52 (0.35–0.69) * | 0.75 (0.52–1.01) * | 0.53 (0.42–0.63) * | 3.16 (2.19–5.23) * | 0.49 (0.40–0.59) * |
| 0.534 | 0.361 | 3.339 | 1.001 | 1.531 | 1.827 | |
| Column Fraction MeOH (%) | % Inhibition of Target Fungi (Mean ± SE) | |||||
|---|---|---|---|---|---|---|
| Fusarium oxysporum | Fusarium verticillioides | Penicillium brevicompactum | Penicillium expansum | Aspergillus flavus | Aspergillus fumigatus | |
| 0.1% | NI | NI | NI | NI | NI | NI |
| 0.5% | NI | NI | NI | NI | NI | NI |
| 1% | 13.2 ± 0.05 | 18.7 ± 0.05 | 12.6 ± 0.01 | NI | NI | 23.9 ± 0.05 |
| 3% | 86.9 ± 0.10 | 84.2 ± 0.00 | 74.5 ± 0.00 | 69.3 ± 0.05 | 70.0 ± 0.05 | 95.5 ± 0.00 |
| 5% | 85.0 ± 0.05 | 95.2 ± 0.00 | 79.5 ± 0.00 | 63.1 ± 0.00 | 55.7 ± 0.10 | 93.0 ± 0.00 |
| 10% | 9.0 ± 0.00 | 7.9 ± 0.00 | NI | NI | NI | 16.8 ± 0.05 |
| 20% | NI | NI | NI | NI | NI | NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabka, M. Antifungal Efficacy and Convenience of Krameria lappacea for the Development of Botanical Fungicides and New Alternatives of Antifungal Treatment. Agronomy 2022, 12, 2599. https://doi.org/10.3390/agronomy12112599
Zabka M. Antifungal Efficacy and Convenience of Krameria lappacea for the Development of Botanical Fungicides and New Alternatives of Antifungal Treatment. Agronomy. 2022; 12(11):2599. https://doi.org/10.3390/agronomy12112599
Chicago/Turabian StyleZabka, Martin. 2022. "Antifungal Efficacy and Convenience of Krameria lappacea for the Development of Botanical Fungicides and New Alternatives of Antifungal Treatment" Agronomy 12, no. 11: 2599. https://doi.org/10.3390/agronomy12112599
APA StyleZabka, M. (2022). Antifungal Efficacy and Convenience of Krameria lappacea for the Development of Botanical Fungicides and New Alternatives of Antifungal Treatment. Agronomy, 12(11), 2599. https://doi.org/10.3390/agronomy12112599








