Low Concentration of Rotenone Impairs Membrane Function of Spodoptera litura Cells by Promoting Their Aggregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Insect, Cell Line, and Culture Conditions
2.2. Toxicity of Rotenone to S. litura Larvae and SL-1 Cells
2.3. Effects of Low Concentration of Rotenone on the Aggregation of SL-1 Cells
2.4. Effects of Rotenone on the Skeleton of SL-1 Cells
2.5. Effects of Rotenone on the Morphology of SL-1 Cell
2.6. Effects of Rotenone on the Membrane Function of SL-1 Cells
2.7. Data Analysis
3. Results
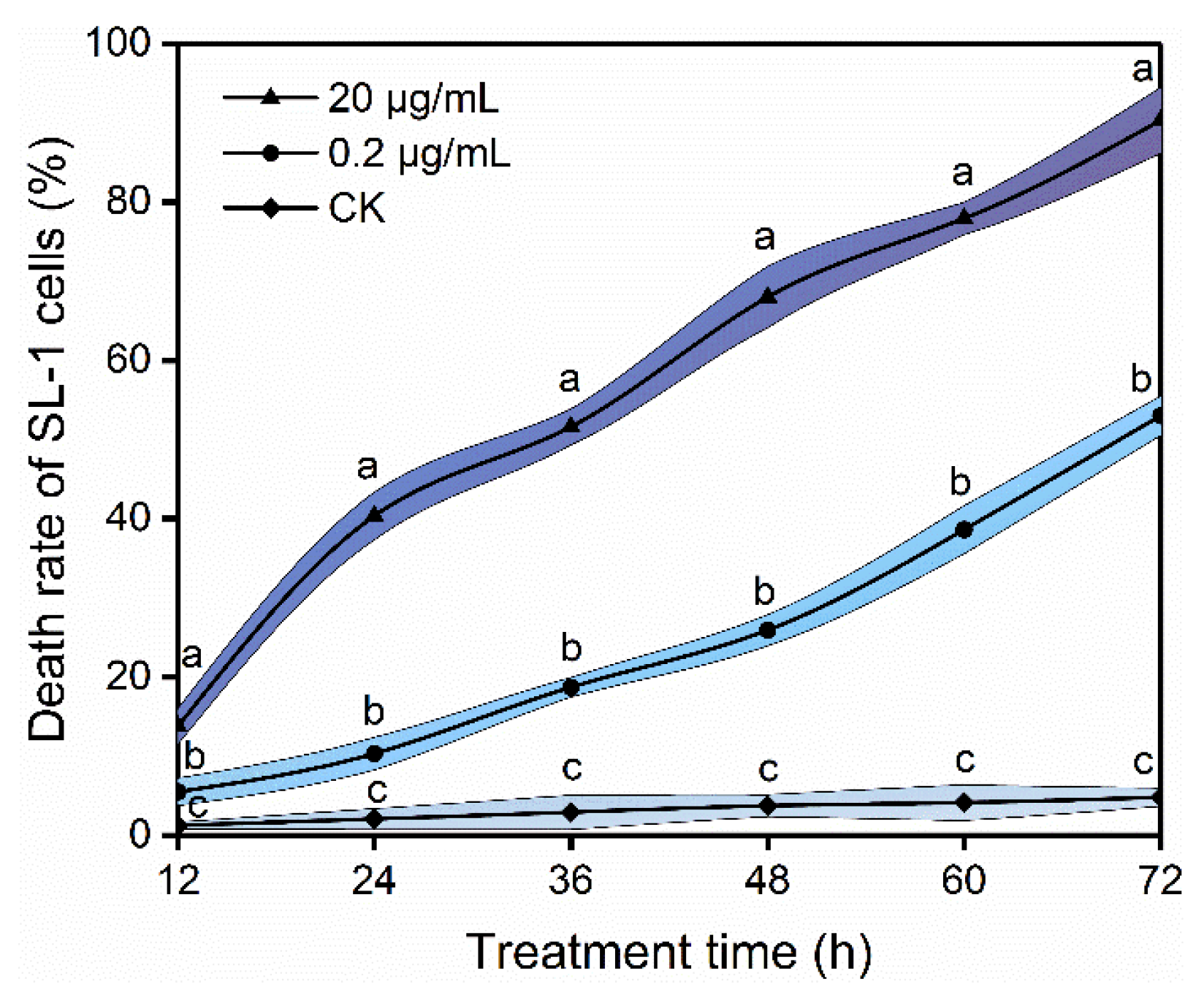
3.1. Toxicity of Rotenone to S. litura Larvae and SL-1 Cells
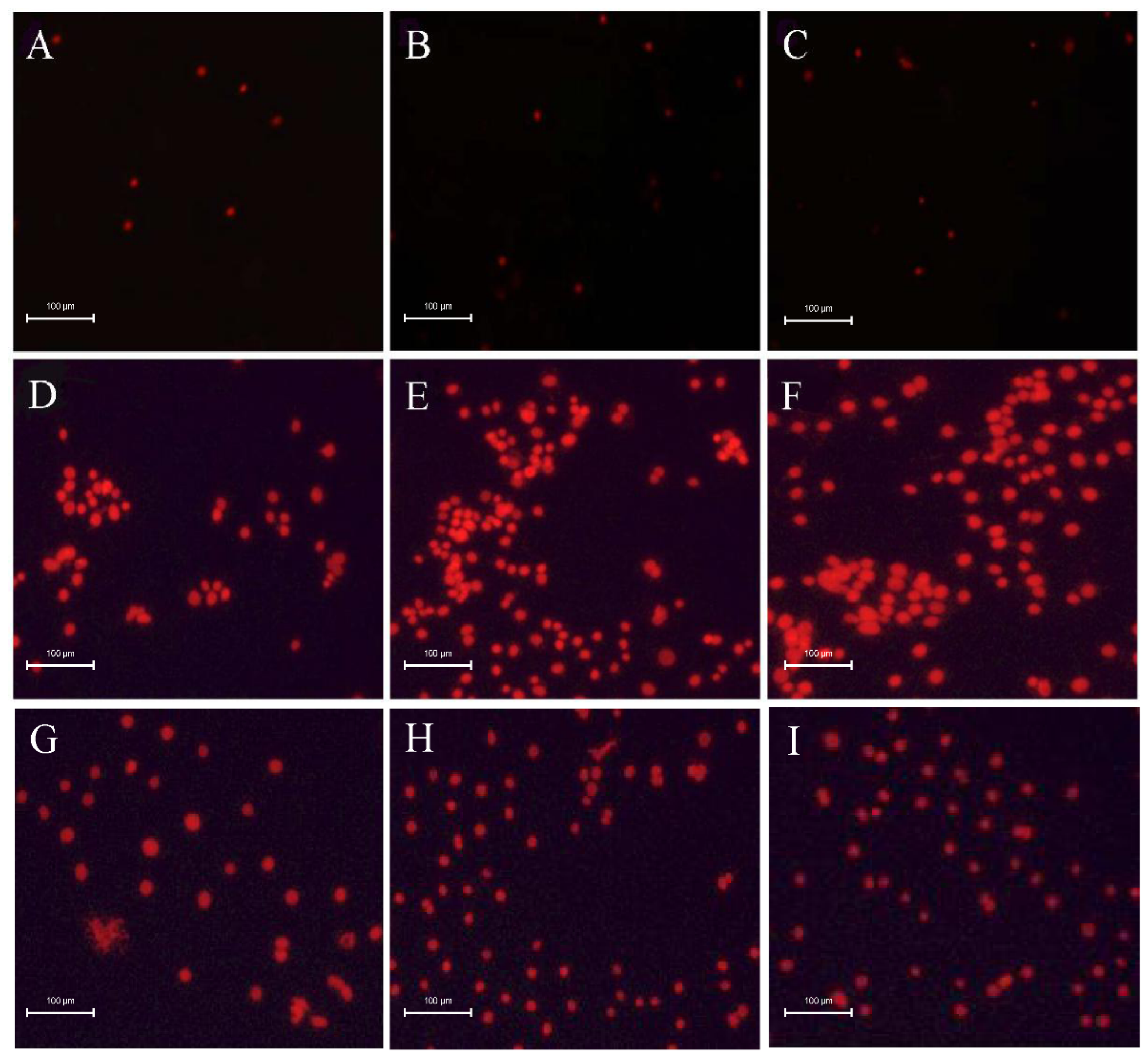
3.2. Effects of Low Concentration of Rotenone on the Aggregation of SL-1 Cells
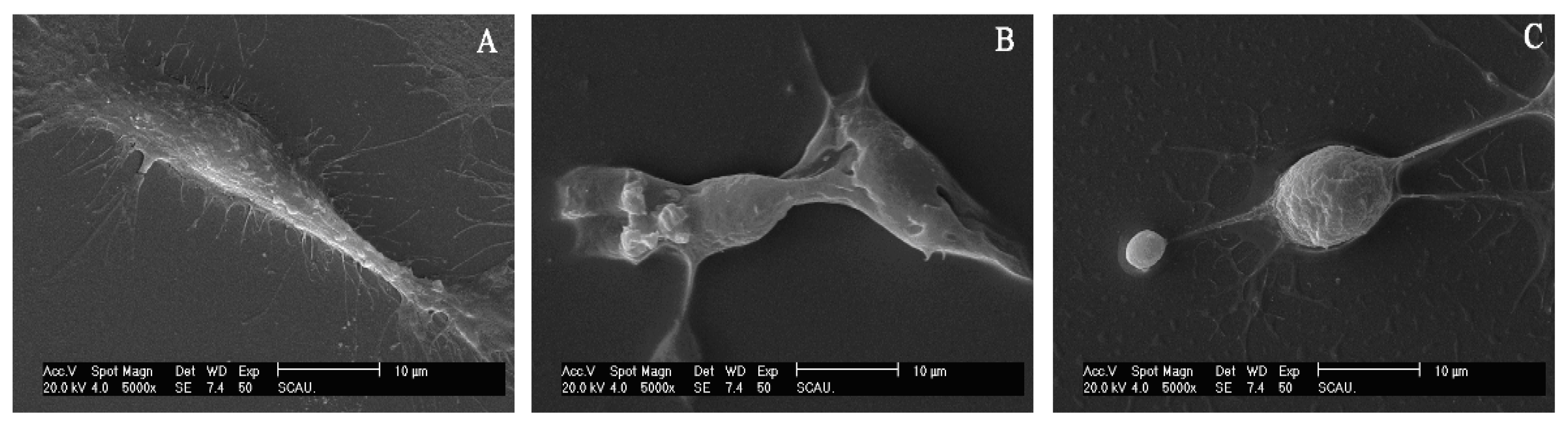
3.3. Effects of Rotenone on the Skeleton of SL-1 Cells
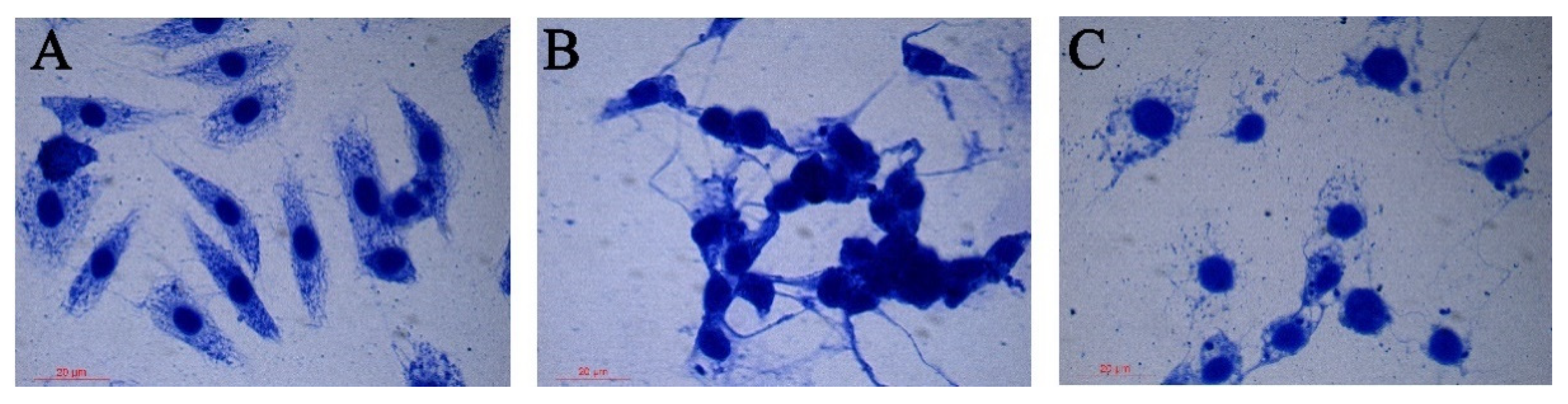
3.4. Effects of Rotenone on the Morphology of SL-1 Cell
3.5. Effects of Rotenone on Membrane Permeability of SL-1 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Kohl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest. Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Abbey, L.; Abbey, J.; Leke-Aladekoba, A.; Iheshiulo, E.M.; Ijenyo, M. Biopesticides and Biofertilizers: Types, Production, Benefits, and Utilization. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2020; pp. 479–500. [Google Scholar]
- Zhu, Y.; Blanco, C.A.; Portilla, M.; Adamczyk, J.; Luttrell, R.; Huang, F. Evidence of multiple/cross resistance to Bt and organophosphate insecticides in Puerto Rico population of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2015, 122, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Qin, D.; Zhang, Y.; Zheng, Q.; Yang, L.; Cheng, D.; Huang, S.; Chen, J.; Zhang, Z. Toxicity and sublethal effects of autumn crocus (Colchicum autumnale) bulb powder on red imported fire ants (Solenopsis invicta). Toxins 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.; Jumbo, L.; Rezende, S.M.; Haddi, K.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef]
- Hernández-Moreno, D.; Soffers, A.; Wiratno, W.; Falke, H.; Rietjens, I.; Murk, A. Consumer and farmer safety evaluation of application of botanical pesticides in black pepper crop protection. Food Chem. Toxicol. 2013, 56, 483–490. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Castagnoli, M.; Liguori, M.; Simoni, S.; Duso, C. Toxicity of some insecticides to Tetranychus urticae, Neoseiulus californicus and Tydeus californicus. BioControl 2005, 50, 611–622. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Pennell, K.D.; Miller, G.W. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 2008, 29, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Khairnar, A.; Rudakucerova, J.; Arab, A.; Hadjistyllis, C.; Sejnoha, M.A.; Shang, Q.; Chovsepain, A.; Drazanova, E.; Szabó, N.; Starcuk, Z.; et al. Diffusion kurtosis imaging detects the time-dependent progress of pathological changes in the oral rotenone mouse model of Parkinson’s disease. J. Neurochem. 2021, 158, 779–797. [Google Scholar] [CrossRef]
- Hollingworth, R.M.; Ahammadsahib, K.I.; Gedelhak, G.; Mclaughlin, J.L. New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem. Soc. Trans. 1994, 22, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, G.; Pires, A.; Martinez, G.; Rocha, M.; Cadena, S. The antioxidant effect of the mesoionic compound syd-1 in mitochondria. Chem. Biol. Interact. 2013, 205, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.; Belcastro, V.; Tozzi, A.; Di Filippo, M.; Tantucci, M.; Siliquini, S.; Autuori, A.; Picconi, B.; Spillantini, M.G.; Fedele, E.; et al. Electrophysiology and pharmacology of striatal neuronal dysfunction induced by mitochondrial complex I inhibition. J. Neurosci. 2008, 28, 8040–8052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, M.; Westerink, R. Chemically-induced oxidative stress increases the vulnerability of PC12 cells to rotenone-induced toxicity. Neurotoxicology 2014, 43, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Darrouzet, E.; Issartel, J.; Lunardi, J.; Dupuis, A. The 49-kDa subunit of NADH-ubiquinone oxidoreductase (complex I) is involved in the binding of piericidin and rotenone, two quinone-related inhibitors. FEBS Lett. 1998, 431, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Vani, C.; Brindhaa, U. Silica nanoparticles as nanocides against Corcyra cephalonica (S.), the stored grain pest. Int. J. Pharma Bio Sci. 2013, 4, B1108–B1118. [Google Scholar]
- Akhtar, Y.; Yeoung, Y.; Isman, M. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2008, 7, 77–88. [Google Scholar] [CrossRef]
- Esteve-Rudd, J.; Fernández-Sánchez, L.; Lax, P.; De Juan, E.; Martín-Nieto, J.; Cuenca, N. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol. Dis. 2011, 44, 102–115. [Google Scholar] [CrossRef]
- Hosamani, R.; Ramesh, S.R.; Muralidhara, M. Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem. Res. 2010, 35, 1402–1412. [Google Scholar] [CrossRef]
- Sun, Z.; Xue, L.; Li, Y.; Cui, G.; Sun, R.; Hu, M.; Zhong, G. Rotenone-induced necrosis in insect cells via the cytoplasmic membrane damage and mitochondrial dysfunction. Pestic. Biochem. Physiol. 2020, 173, 104801. [Google Scholar] [CrossRef]
- Qin, D.; Zheng, Q.; Zhang, P.; Lin, S.; Huang, S.; Cheng, D.; Zhang, Z. Azadirachtin directly or indirectly affects the abundance of intestinal flora of Spodoptera litura and the energy conversion of intestinal contents mediates the energy balance of intestine-brain axis, and along with decreased expression CREB in the brain neurons. Pestic. Biochem. Physiol. 2021, 173, 104778. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, S.; Liu, Z.; Zhang, L.; Wu, H.; Cheng, D.; Zhang, Z. Using Azadirachtin to Transform Spodoptera frugiperda from Pest to Natural Enemy. Toxins 2021, 13, 541. [Google Scholar] [CrossRef] [PubMed]
- Ronald, L.; Alfaro, A.C.; Merien, F.; Burdass, M.; Young, T.; Meyer, J.; Nguyen, T.V.; Trembath, C. Characterisation of Chinook salmon (Oncorhynchus tshawytscha) blood and validation of flow cytometry cell count and viability assay kit. Fish Shellfish Immunol. 2019, 88, 179–188. [Google Scholar] [CrossRef]
- Tominaga, M.; Nishihara, E.; Oogami, T.; Iwasaki, M.; Nakagawa, H. Neurite elongation from Drosophila neural BG2-c6 cells stimulated by 20-hydroxyecdysone. Neurosci. Lett. 2010, 482, 250–254. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeil, N. Botanical pesticides and their mode of action. Gesunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Goncalves, A.P.; Máximo, V.; Lima, J.; Singh, K.K.; Soares, P.; Videira, A. Involvement of p53 in cell death following cell cycle arrest and mitotic catastrophe induced by rotenone. BBA-Mol. Cell Res. 2011, 1813, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, P.; Panda, D. Rotenone inhibits mammalian cell proliferation by inhibiting microtubule assembly through tubulin binding. FEBS J. 2007, 274, 4788–4801. [Google Scholar] [CrossRef]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Liu, C.; Liu, W.; Zhang, H.; Zhang, R.; Liu, J.; Zhang, R.; Xu, C.; Liu, L.; Huang, S.; et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol. Sci. 2015, 143, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Estrella-Parra, E.A.; Gomez-Verjan, J.C.; González-Sánchez, I.; Vázquez-Martínez, E.R.; Vergara-Castañeda, E.; Cerbón, M.A.; Alavez-Solano, D.; Reyes-Chilpa, R. Rotenone isolated from Pachyrhizus erosus displays cytotoxicity and genotoxicity in K562 cells. Nat. Prod. Res. 2014, 28, 1780–1785. [Google Scholar] [CrossRef]
- Pal, R.; Monroe, T.O.; Palmieri, M.; Sardiello, M.; Rodney, G.G. Rotenone induces neurotoxicity through Rac1-dependent activation of NADPH oxidase in SHSY-5Y cells. Febs Lett. 2014, 588, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenner, P. Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci. 2001, 24, 245–246. [Google Scholar] [CrossRef]
- Zhong, G.; Cui, G.; Yi, X.; Sun, R.; Zhang, J. Insecticide cytotoxicology in China: Current status and challenges. Pestic. Biochem. Phys. 2016, 132, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.B.; Perez-Polo, J.R. Bax shuttling after rotenone treatment of neuronal primary cultures: Effects on cell death phenotypes. J. Neurosci. Res. 2009, 87, 2047–2065. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Guo, H.; Liang, Z.; Zheng, P.; Li, L.; Xian, J.; Zhu, X. Effects of nonylphenol exposure on histological changes, apoptosis and time-course transcriptome in gills of white shrimp Litopenaeus vannamei. Sci. Total Environ. 2021, 781, 146731. [Google Scholar] [CrossRef]
- Basili, D.; Lutfi, E.; Falcinelli, S.; Balbuena-Pecino, S.; Carnevali, O. Photoperiod manipulation affects transcriptional profile of genes related to lipid metabolism and apoptosis in zebrafish (Danio rerio) larvae: Potential roles of gut microbiota. Microb. Ecol. 2020, 79, 933–946. [Google Scholar] [CrossRef]
- Li, M.; Cao, J.; Zhao, Y.; Wu, P.; Li, X.; Khodaei, F.; Han, Y.; Wang, J. Fluoride impairs ovary development by affecting oogenesis and inducing oxidative stress and apoptosis in female zebrafish (Danio rerio). Chemosphere 2020, 256, 127105. [Google Scholar] [CrossRef]
- Kim, M.I.; Nina, H.; Chris, M.K.; Marion, W.; Fisher, J.S.; Sharpe, R.M. Abnormal leydig cell aggregation in the fetal testis of rats exposed to di (n-Butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology 2005, 146, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Iurisci, I.; Cumashi, A.; Sherman, A.A.; Tsvetkov, Y.E.; Tinari, N.; Piccolo, E.; Egidio, M.D.; Adamo, V.; Natoli, C.; Rabinovich, G.A.; et al. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009, 209, 403–410. [Google Scholar]
- Kajiwara, K.; Beharier, O.; Chng, C.P.; Goff, J.P.; Ouyang, Y.; St Croix, C.M.; Huang, C.J.; Kagan, V.E.; Hsia, K.J.; Sadovsky, Y. Ferroptosis induces membrane blebbing in placental trophoblasts. J. Cell Sci. 2022, 135, 255737. [Google Scholar] [CrossRef] [PubMed]
- Fahanikbabaei, J.; Rezaee, B.; Nazari, M.; Torabi, N.; Saghiri, R.; Sauvé, R.; Eliassi, A. A new brain mitochondrial sodium-sensitive potassium channel: Effect of sodium ions on respiratory chain activity. J. Cell Sci. 2020, 133, 242446. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Xu, K.; Zhang, Q.; Zhu, Q.; Khan, M.M.; Zhang, Z.; Cheng, D. Low Concentration of Rotenone Impairs Membrane Function of Spodoptera litura Cells by Promoting Their Aggregation. Agronomy 2022, 12, 2611. https://doi.org/10.3390/agronomy12112611
Lin S, Xu K, Zhang Q, Zhu Q, Khan MM, Zhang Z, Cheng D. Low Concentration of Rotenone Impairs Membrane Function of Spodoptera litura Cells by Promoting Their Aggregation. Agronomy. 2022; 12(11):2611. https://doi.org/10.3390/agronomy12112611
Chicago/Turabian StyleLin, Sukun, Kaijie Xu, Qingpeng Zhang, Qiuming Zhu, Muhammad Musa Khan, Zhixiang Zhang, and Dongmei Cheng. 2022. "Low Concentration of Rotenone Impairs Membrane Function of Spodoptera litura Cells by Promoting Their Aggregation" Agronomy 12, no. 11: 2611. https://doi.org/10.3390/agronomy12112611
APA StyleLin, S., Xu, K., Zhang, Q., Zhu, Q., Khan, M. M., Zhang, Z., & Cheng, D. (2022). Low Concentration of Rotenone Impairs Membrane Function of Spodoptera litura Cells by Promoting Their Aggregation. Agronomy, 12(11), 2611. https://doi.org/10.3390/agronomy12112611






