Pb4CL2 Inducing Lignin Accumulation in Superficial Scald ‘Chili’ (Pyrus bretschneideri) Pear Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Soluble Sugar Content
2.3. Weight Loss Rate
2.4. Respiratory Rate
2.5. Ethylene Production Rate
2.6. Firmness
2.7. Wiesner Staining
2.8. Lignin Content
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Vector Construction and Transient Expression of Pb4CL2 in Pear Fruit
2.11. Statistical Analyses
3. Results
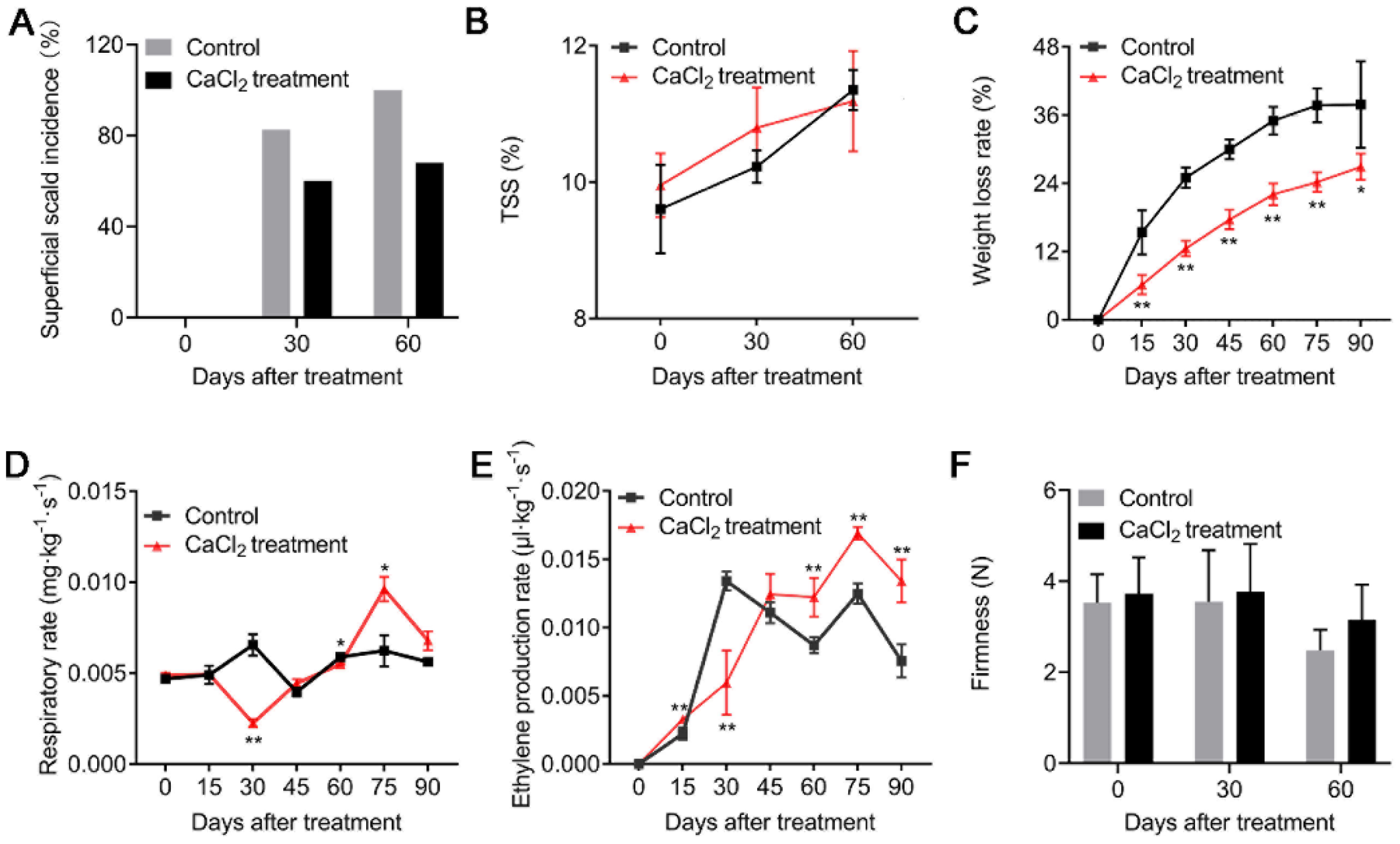
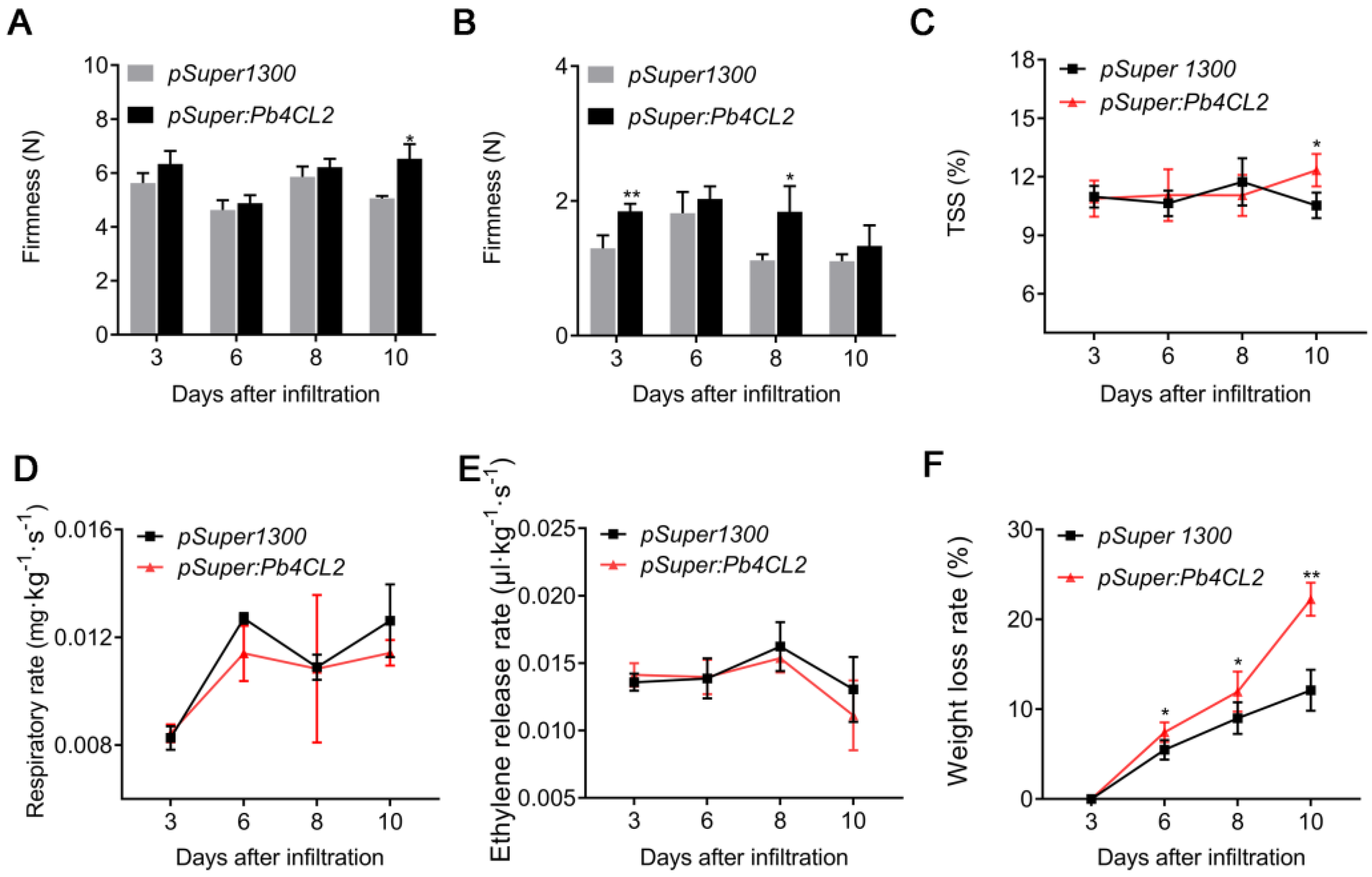
3.1. The Incidence of Superficial Scald, TSS, Weight Loss Rate, Respiratory Rate, Ethylene Production, and Firmness Rate in Pear Fruit
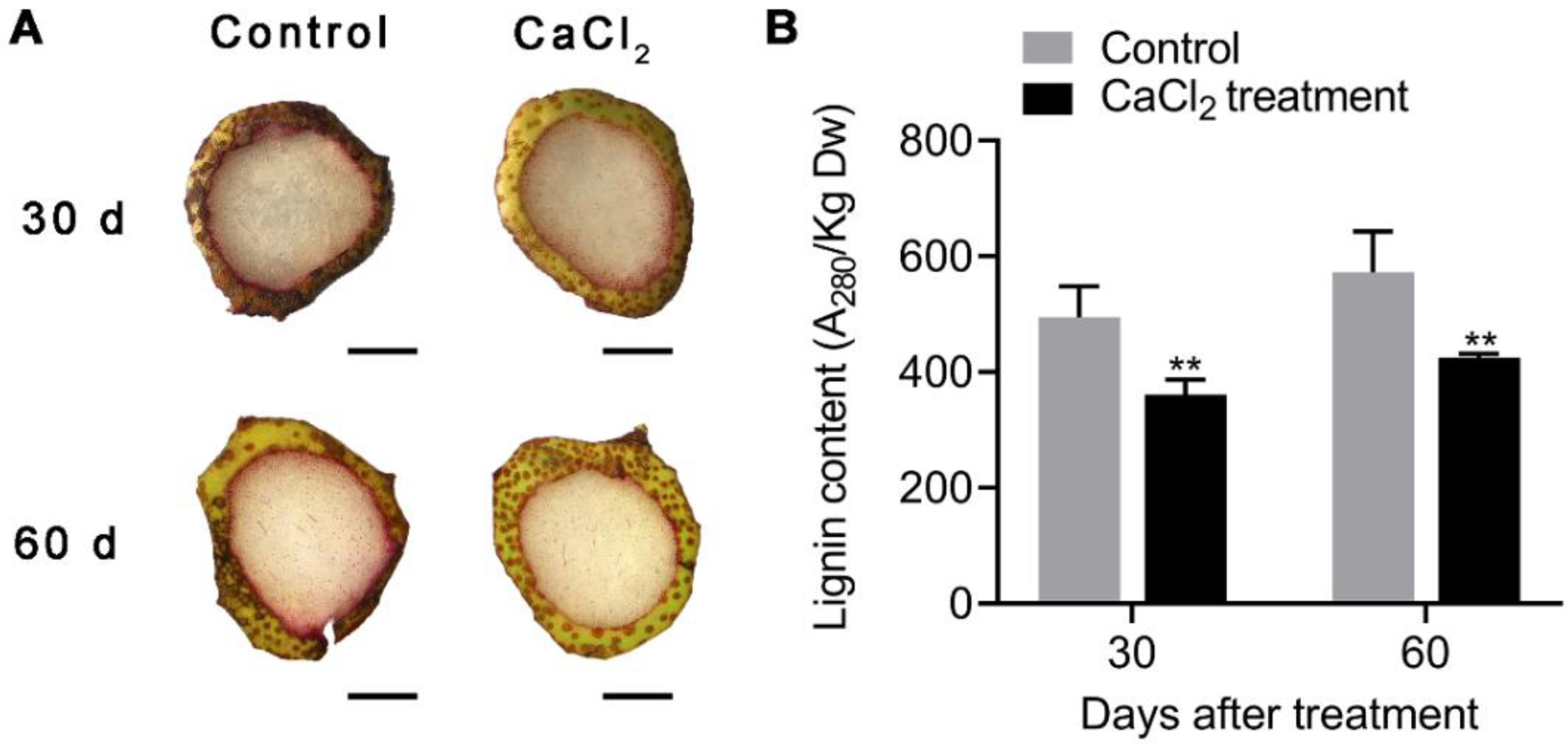
3.2. Lignin Staining in Pear Fruit
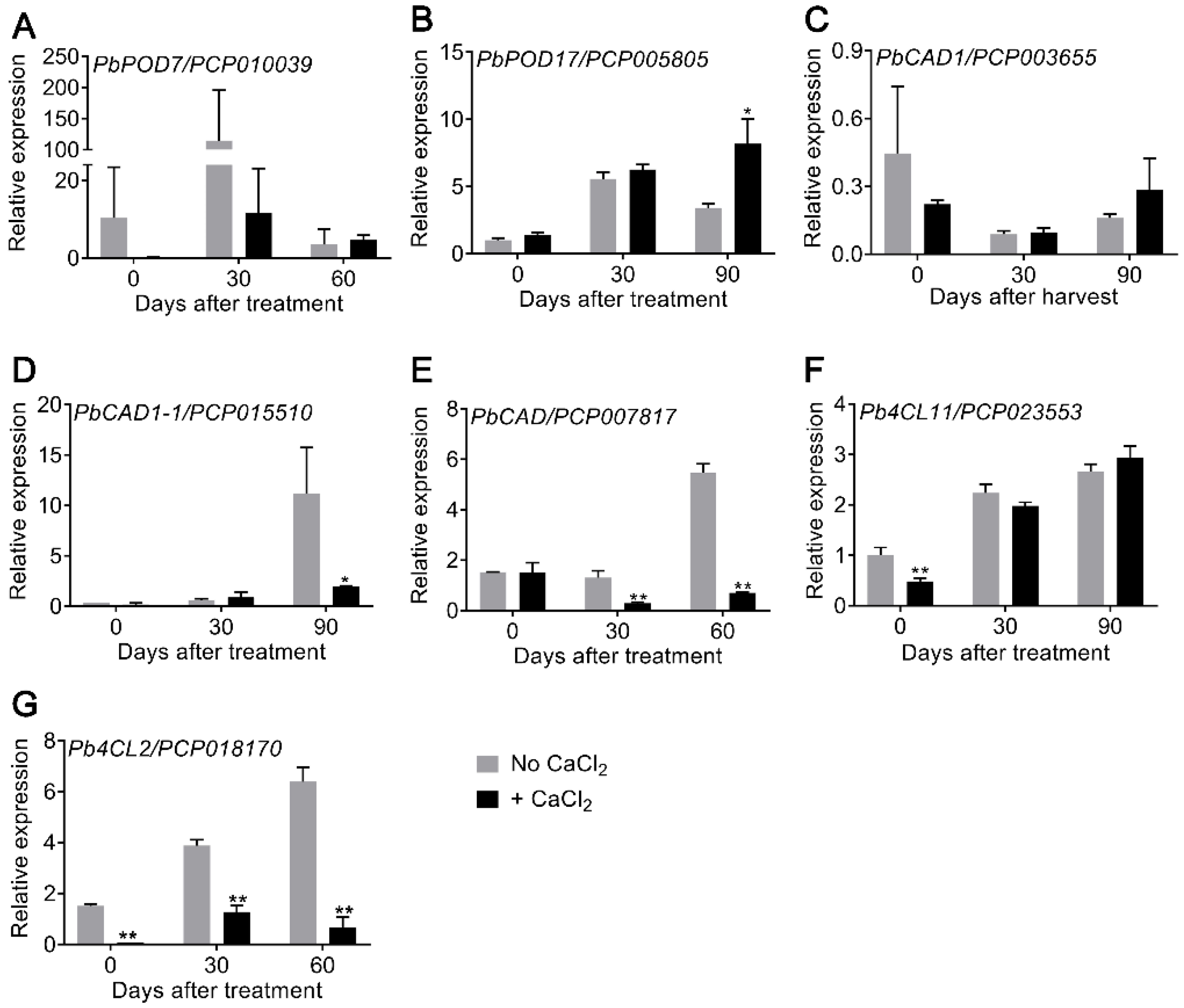
3.3. Expression of Lignin-Related Genes
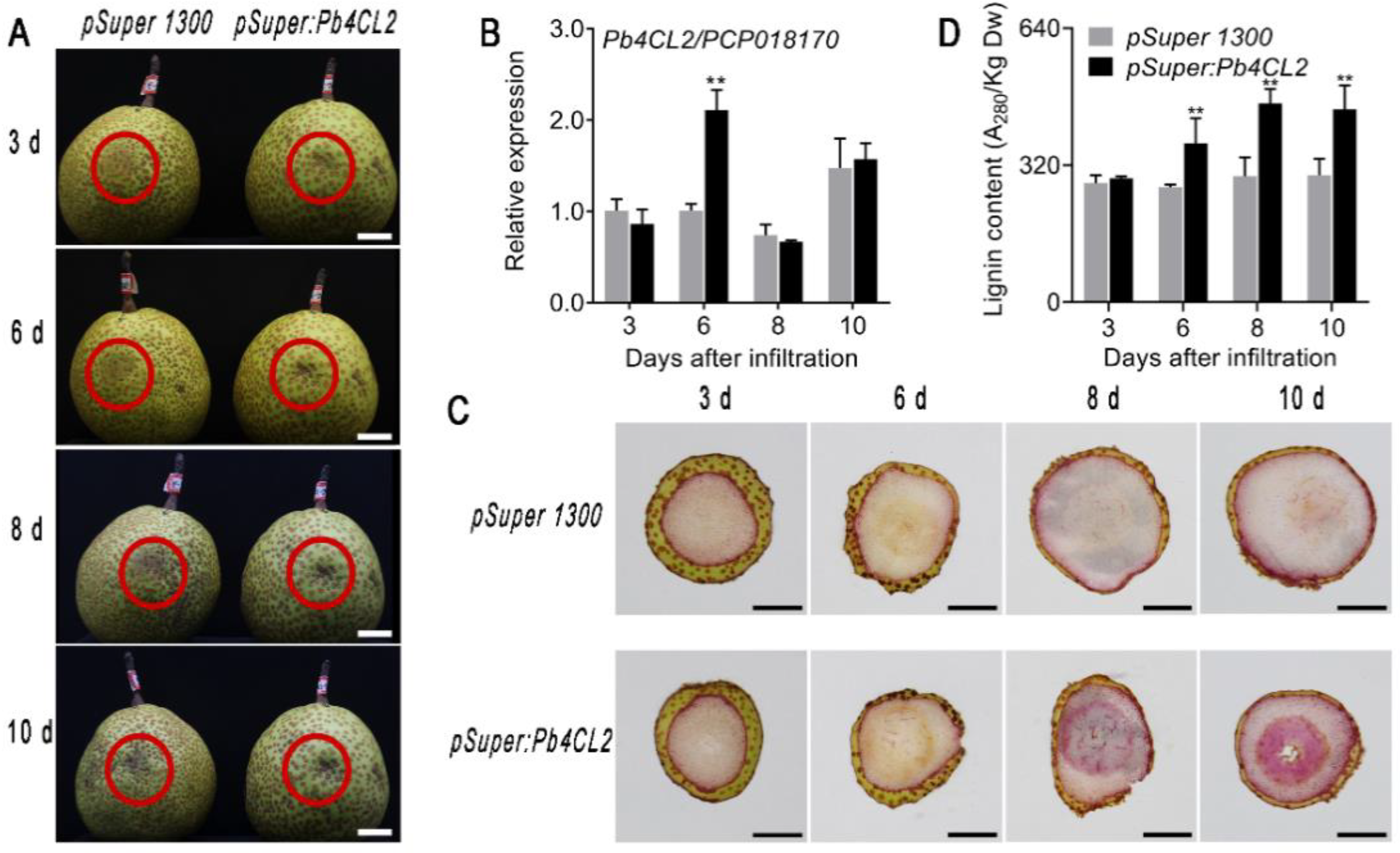
3.4. Overexpression of Pb4CL2-Induced Superficial Scald Disorder
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Busatto, N.; Farneti, B.; Commisso, M.; Bianconi, M.; Iadarola, B.; Zago, E.; Ruperti, B.; Spinelli, F.; Zanella, A.; Velasco, R.; et al. Apple fruit superficial scald resistance mediated by ethylene inhibition is associated with diverse metabolic processes. Plant J. 2018, 93, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Tsantili, E.; Gapper, N.E.; Arquiza, J.M.; Whitaker, B.D.; Watkins, C.B. Ethylene and alpha-farnesene metabolism in green and red skin of three apple cultivars in response to 1-methylcyclopropene (1-MCP) treatment. J. Agric. Food Chem. 2007, 55, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.B.; Bramlage, W.J.; Cregoe, B.A. Superficial Scald of ‘Granny Smith’ apples is expressed as a typical chilling injury. J. Am. Soc. Hortic. Sci. 1995, 120, 88–94. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1048. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signalling. Ann. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Kudla, J.R.; Batisti, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef]
- Raese, J.T.; Drake, S.R. Calcium sprays and timing affect fruit calcium concentrations, yield, fruit weight, and cork spot of ‘Anjou’ pears. Hort. Sci. 1995, 30, 1037–1039. [Google Scholar] [CrossRef]
- Dong, Y.; Guan, J.F.; Ma, S.J.; Liu, L.L.; Feng, Y.X.; Cheng, Y.D. Calcium content and its correlated distribution with skin browning spot in bagged huangguan pear. Protoplasma 2015, 252, 165–171. [Google Scholar] [CrossRef]
- Miqueloto, A.; Steffens, C.A.; Santos, A.D.; Mitcham, E. Relationship between xylem functionality, calcium content and the incidence of bitter pit in apple fruit. Sci. Hortic. 2014, 165, 319–323. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, C.X.; Zhang, X.F.; Wang, C.H.; Yang, S.L. Preharvest bagging and postharvest calcium treatment affects superficial scald incidence and calcium nutrition during storage of ‘Chili’ pear (Pyrus bretschneideri) fruit. Postharvest Biol. Technol. 2020, 163, 111149. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, R.; Morreel, K.; Goeminne, G.; Gielen, B.; Rohde, A.; Van Beeumen, J.; Ralph, J.; Boudet, A.M.; Kopka, J.; Rochange, S.F.; et al. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. Plant J. 2007, 52, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Peter, G.; Neale, D. Molecular basis for the evolution of xylem lignification. Curr. Opin. Plant Bio. 2004, 7, 737–742. [Google Scholar] [CrossRef]
- Yuan, G.P.; Bian, S.X.; Han, X.L.; He, S.S.; Liu, K.; Zhang, C.X.; Cong, P.H. An integrated transcriptome and proteome analysis reveals new insights into russeting of bagging and non-bagging ‘Golden Delicious’ apple. Int. J. Mol. Sci. 2019, 20, 4462. [Google Scholar] [CrossRef]
- Cui, Z.H.; Jiao, Q.N.; Wang, R.; Ma, C.H. Investigation and analysis of relationship between mineral elements alteration and cork spot physiological disorder of Chinese pear ‘Chili’ (Pyrus bretschneideri Rehd.). Sci. Hortic. 2020, 260, 108883. [Google Scholar] [CrossRef]
- Wang, B.G.; Wang, Y.X.; Li, W.S.; Zhou, J.H.; Chang, H.; Golding, J.B. Effect of 1-MCP and ethylene absorbent on the development of lenticel disorder of ‘Xinli No.7′ pear and possible mechanisms. J. Sci. Food Agric. 2020, 101, 2525–2533. [Google Scholar] [CrossRef]
- Reitz, N.F.; Mitcham, E.J. Lignification of tomato (Solanum lycopersicum) pericarp tissue during blossom-end rot development. Sci. Hortic. 2021, 276, 109759. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Yue, J.Q.; Yang, H.B.; Zhu, C.H.; Zhu, F.; Li, J.X.; Xu, R.W.; Gao, J.Y.; Zhou, D.G.; Deng, X.X.; et al. Integration of metabolome, histochemistry and transcriptome analysis provides insights into lignin accumulation in oleocellosis-damaged flavedo of citrus fruit. Postharvest Bio. Technol. 2021, 172, 111362. [Google Scholar] [CrossRef]
- Yao, S.X.; Wang, Z.M.; Cao, Q.; Xie, J.; Wang, X.R.; Zhang, R.; Deng, L.L.; Ming, J.; Zeng, K.F. Molecular basis of postharvest granulation in orange fruit revealed by metabolite, transcriptome and methylome profiling. Postharvest Biol. Technol. 2020, 166, 111205. [Google Scholar] [CrossRef]
- Kamdee, C.; Imsabai, W.; Kirk, R.; Allan, A.C.; Ferguson, I.B.; Ketsa, S. Regulation of lignin biosynthesis in fruit pericarp hardening of mangosteen (Garcinia mangostana L.) after impact. Postharvest Biol. Technol. 2014, 97, 68–76. [Google Scholar] [CrossRef]
- Zhang, J.; Ge, H.; Zang, C.; Li, X.; Grierson, D.; Chen, K.S.; Yin, X.R. EjODO1, a MYB transcription factor, regulating lignin biosynthesis in developing loquat (Eriobotrya japonica) fruit. Front. Plant Sci. 2016, 7, 1360. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.Q.; Tan, P.H.; Sun, Y.F.; Lv, Y.M.; Liu, J.M.; Sun, J.; Huang, Y.T.; Lu, J.; Jin, N.; et al. Citrus sinensis MYB transcription factor CsMYB85 induce fruit juice sac lignification through interaction with other CsMYB transcription factors. Front. Plant Sci. 2019, 10, 213. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.F.; Wang, R.; Bai, Y.X.; Li, C.L.; Yuan, Y.B.; Yang, Y.J.; Yang, S.L. Differential gene expression analysis of ‘Chili’ (Pyrus bretschneideri) fruit pericarp with two types of bagging treatments. Hortic. Res. 2017, 4, 17005. [Google Scholar] [CrossRef]
- Yang, S.L.; Zhang, X.N.; Lu, G.L.; Wang, C.R.; Wang, R. Regulation of gibberellin on gene expressions related with the lignin biosynthesis in ‘Wangkumbae’ pear (Pyrus pyrifolia Nakai) fruit. Plant Growth Regu. 2015, 76, 127–134. [Google Scholar] [CrossRef]
- Lu, G.L.; Li, Z.J.; Zhang, X.F.; Wang, R.; Yang, S.L. Expression analysis of lignin-associated genes in hard end pear (Pyrus pyrifolia Whangkeumbae) and its response to calcium chloride treatment conditions. J. Plant Growth Regu. 2015, 34, 251–262. [Google Scholar] [CrossRef]
- Blanco-Portales, R.; Medina-Escobar, N.; López-Ráez, J.A.; González-Reyes, J.A.; Villalba, J.M.; Moyano, E.; Caballero, J.L.; Muñoz-Blanco, J. Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria x ananassa cv. Chandler). J. Exp. Bot. 2002, 53, 1723–1734. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, Z.; Qin, H.; Tan, J.; Ding, G. Exogenous brassinosteroid facilitates xylem development in pinus massoniana seedlings. Int. J. Mol. Sci. 2021, 22, 7615. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef]
- Li, M.T.; Cheng, C.X.; Zhang, X.F.; Zhou, S.P.; Wang, C.H.; Ma, C.H.; Yang, S.L. PpNAC187 enhances lignin synthesis in ‘Whangkeumbae’ pear (Pyrus pyrifolia) ‘Hard-End’ fruit. Molecules 2019, 24, 4338. [Google Scholar] [CrossRef]
- Lata, D.; Aftab, M.A.; Homa, F.; Ahmad, M.S.; Siddiqui, M.W. Effect of eco-safe compounds on postharvest quality preservation of papaya (Carica papaya L.). Acta Physiol. Plant. 2018, 40, 8. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, M.T.M.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.F.; Wang, Y.Z.; Yang, S.L.; Qu, H.Y. The changes of intracellular calcium concentration and distribution in the hard end pear (Pyrus pyrifolia cv. ‘Whangkeumbae’) fruit. Cell Calcium 2018, 71, 15–23. [Google Scholar] [CrossRef]
- Cheng, X.; Li, M.L.; Li, D.H.; Zhang, J.Y.; Jin, Q.; Sheng, L.L.; Cai, Y.P.; Lin, Y. Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit. Biol. Open 2017, 6, 1602–1613. [Google Scholar] [CrossRef]
- Liu, J.Q.; Cheng, Y.S.; Zhang, S.L.; He, Z.S.; Zhang, H.P. Study on relationship between browning suberization fruit and mineral nutrition of early crisp pear. Shanxi Fruits 2018, 108, 5–8. (In Chinese) [Google Scholar]
- Aquino-Bolanos, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Heng, W.; Liu, L.; Wang, M.D.; Jia, B.; Liu, P.; Ye, Z.F.; Zhu, L.W. Differentially expressed genes related to the formation of russet fruit skin in a mutant of ‘Dangshansuli’ pear (Pyrus bretchnederi Rehd.) determined by suppression subtractive hybridization. Euphytica 2014, 196, 285–297. [Google Scholar] [CrossRef]
- Heng, W.; Wang, Z.T.; Jiang, X.H.; Jia, B.; Liu, P.; Liu, L.; Ye, Z.F.; Zhu, L.W. The role of polyamines during exocarp formation in a russet mutant of ‘Dangshansuli’ pear (Pyrus bretschneideri Rehd.). Plant Cell Rep. 2016, 35, 1841–1852. [Google Scholar] [CrossRef]
- Legay, S.; Guerriero, G.; Deleruelle, A.; Lateur, M.; Evers, D.; André, C.M.; Hausman, J.F. Apple russeting as seen through the RNA-seq lens: Strong alterations in the exocarp cell wall. Plant Mol. Biol. 2015, 88, 21–40. [Google Scholar] [CrossRef]
- Shi, C.H.; Qi, B.; Wang, X.Q.; Shen, L.Y.; Luo, J.; Zhang, Y.X. Proteomic analysis of the key mechanism of exocarp russet pigmentation of semi-russet pear under rainwater condition. Sci. Hortic. 2019, 254, 178–186. [Google Scholar] [CrossRef]
- Costa, M.A.; Bedgar, D.L.; Moinuddin, S.G.; Kim, K.W.; Cardenas, C.L.; Cochrane, F.C.; Shockey, J.M.; Helms, G.L.; Amakura, Y.; Takahashi, H.; et al. Characterization in vitro and in vivo of the putative multigene 4-coumarate: CoA ligase network in Arabidopsis: Syringyl lignin and sinapate/sinapyl alcohol derivative formation. Phytochemistry 2005, 66, 2072–2091. [Google Scholar] [CrossRef]
- Gui, J.S.; Shen, J.H.; Li, L.G. Functional characterization of evolutionarily divergent 4-coumarate: Coenzyme a ligases in rice. Plant Physiol. 2011, 157, 574–586. [Google Scholar] [CrossRef]
- Shi, C.H.; Wang, X.Q.; Zhang, X.Y.; Luo, J. Effects of bagging on rust formation and physiology of fruit exocarp in pear variety ‘Cuiguan’. Acta. Agr. JiangXi 2018, 30, 1–7. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cheng, C.; Zhang, C.; Xue, J.; Zhang, Y.; Wang, C.; Dang, R.; Yang, S. Pb4CL2 Inducing Lignin Accumulation in Superficial Scald ‘Chili’ (Pyrus bretschneideri) Pear Fruit. Agronomy 2022, 12, 2650. https://doi.org/10.3390/agronomy12112650
Li Q, Cheng C, Zhang C, Xue J, Zhang Y, Wang C, Dang R, Yang S. Pb4CL2 Inducing Lignin Accumulation in Superficial Scald ‘Chili’ (Pyrus bretschneideri) Pear Fruit. Agronomy. 2022; 12(11):2650. https://doi.org/10.3390/agronomy12112650
Chicago/Turabian StyleLi, Qian, Chenxia Cheng, Chunjian Zhang, Junxiu Xue, Yong Zhang, Caihong Wang, Ruihong Dang, and Shaolan Yang. 2022. "Pb4CL2 Inducing Lignin Accumulation in Superficial Scald ‘Chili’ (Pyrus bretschneideri) Pear Fruit" Agronomy 12, no. 11: 2650. https://doi.org/10.3390/agronomy12112650
APA StyleLi, Q., Cheng, C., Zhang, C., Xue, J., Zhang, Y., Wang, C., Dang, R., & Yang, S. (2022). Pb4CL2 Inducing Lignin Accumulation in Superficial Scald ‘Chili’ (Pyrus bretschneideri) Pear Fruit. Agronomy, 12(11), 2650. https://doi.org/10.3390/agronomy12112650




