Metabolism Reorganization in Kale (Brassica oleracea L. var acephala) Populations with Divergent Glucosinolate Content under Thermal Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Agronomic Parameters
2.3. Biochemical Analysis
2.4. Statistical Analysis
2.5. Compound Identification
3. Results
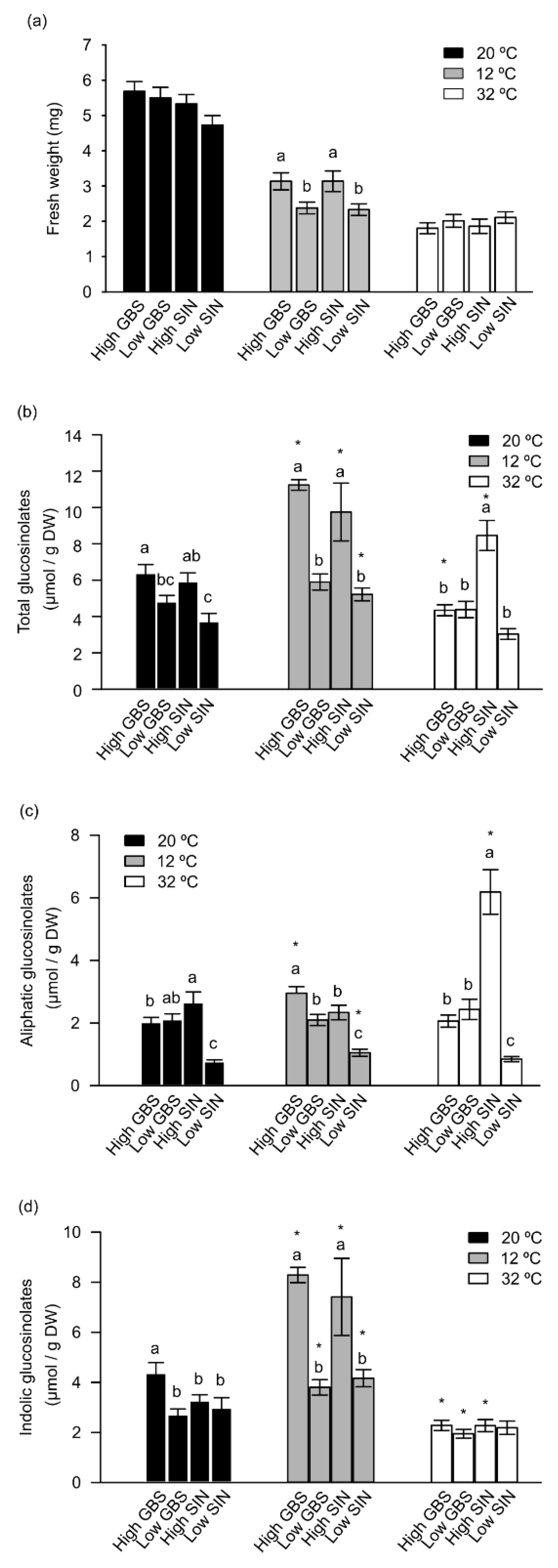
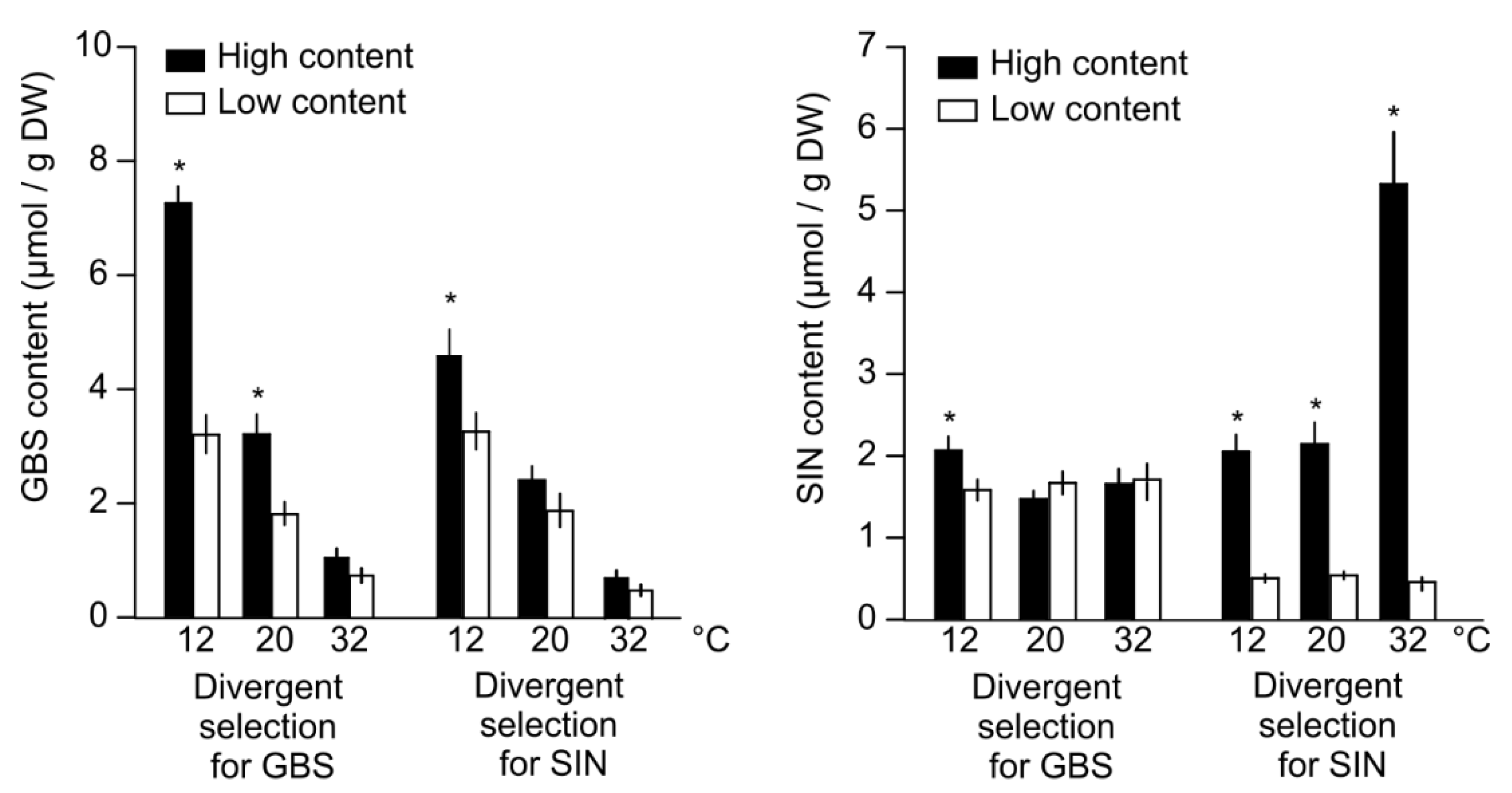
3.1. Populations with High GSLs Content Performed Better at Cold Conditions
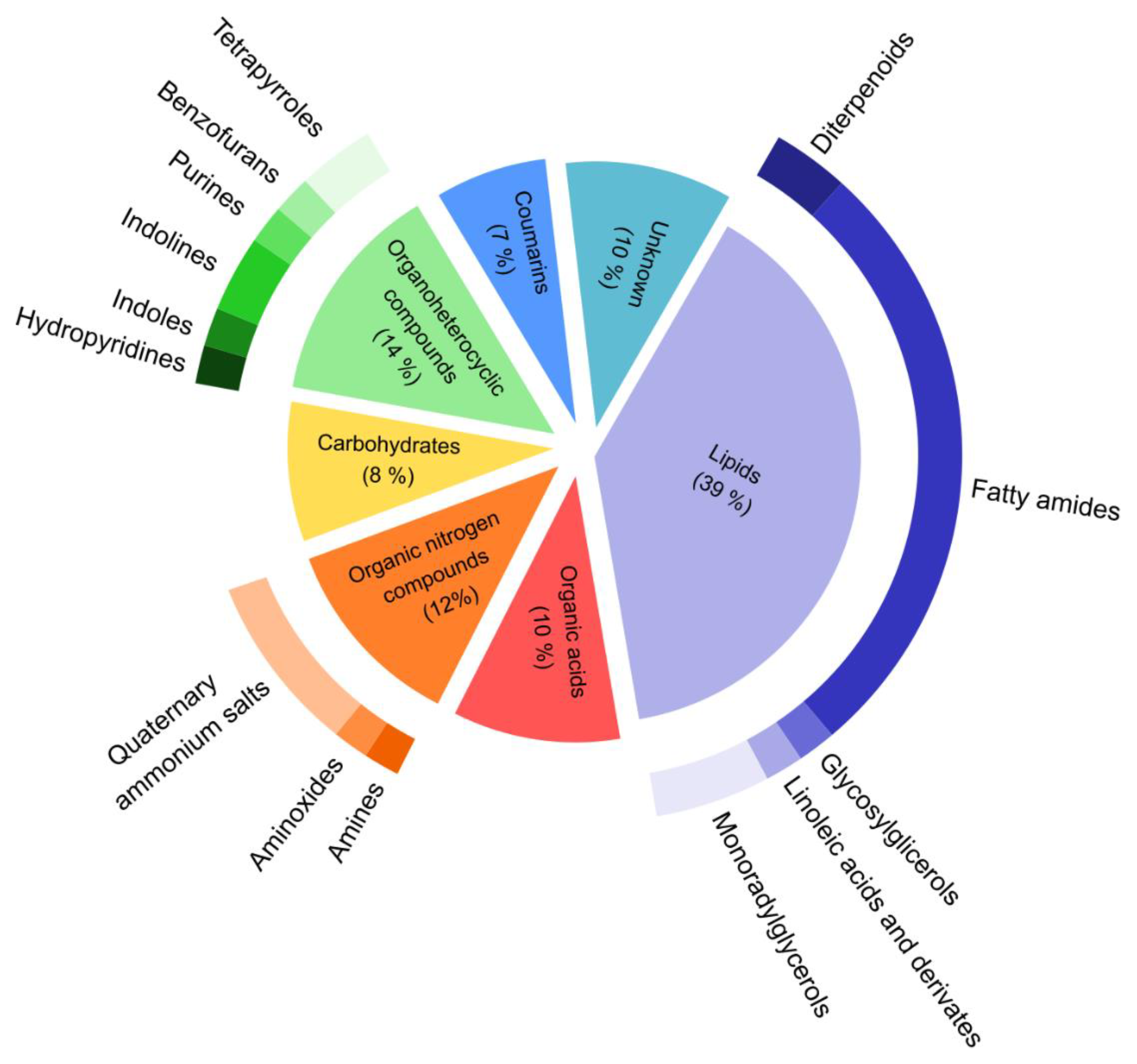
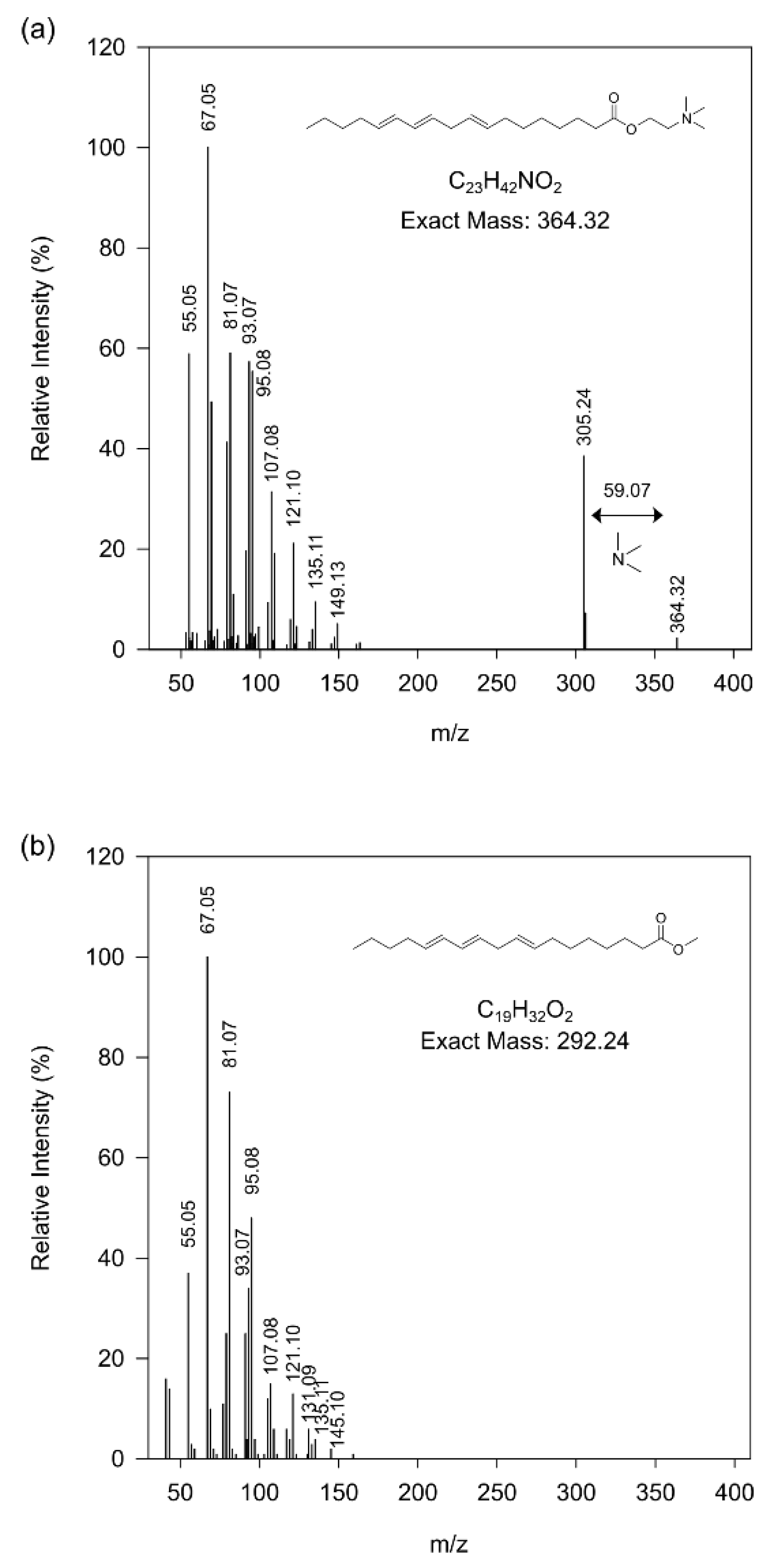
3.2. Profile of Metabolomics Reorganization of Plants with High GSLs Content at Low Temperatures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Significance of temperature in plant life. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 48–69. ISBN 1405131926. [Google Scholar]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Chen, M.; Thelen, J.J. ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis. Plant Cell 2013, 25, 1430–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, M.; James, D., Jr.; Dooner, H.; Browse, J. Arabidopsis requires polyunsaturated lipids for low-temperature survival. Proc. Natl. Acad. Sci. USA 1993, 90, 6208–6212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, Y.; Finka, A.; Goloubinoff, P. Heat perception and signalling in plants: A tortuous path to thermotolerance. New Phytol. 2011, 190, 556–565. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold Signaling and Cold Response in Plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lee, C.-M.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Gilmour, S.J.; Grumet, R.; Thomashow, M.F. CBF-dependent and CBF-independent regulatory pathways contribute to the differences in freezing tolerance and cold-regulated gene expression of two Arabidopsis ecotypes locally adapted to sites in Sweden and Italy. PLoS ONE 2018, 13, e0207723. [Google Scholar] [CrossRef] [Green Version]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D.; et al. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Hoermiller, I.I.; Naegele, T.; Augustin, H.; Stutz, S.; Weckwerth, W.; Heyer, A.G. Subcellular reprogramming of metabolism during cold acclimation in Arabidopsis thaliana. Plant. Cell Environ. 2017, 40, 602–610. [Google Scholar] [CrossRef]
- Olenichenko, N.A.; Zagoskina, N.V.; Astakhova, N.V.; Trunova, T.I.; Kuznetsov, Y. V Primary and secondary metabolism of winter wheat under cold hardening and treatment with antioxidants. Appl. Biochem. Microbiol. 2008, 44, 535. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassão, D.G. How glucosinolates affect generalist lepidopteran larvae: Growth, development and glucosinolate metabolism. Front. Plant Sci. 2017, 8, 1995. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.E.; Velasco, P. In vitro activity of glucosinolates and their degradation products against brassica-pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2015, 81, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Badenes-Pérez, F.R.; Cartea, M.E. Glucosinolate induction and resistance to the cabbage moth, Mamestra brassicae, differs among kale genotypes with high and low content of sinigrin and glucobrassicin. Plants 2021, 10, 1951. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antibiotic properties of the glucosinolates of Brassica oleracea var. acephala similarly affect generalist and specialist larvae of two lepidopteran pests. J. Pest Sci. 2016, 89, 195–206. [Google Scholar] [CrossRef]
- Bohinc, T.; Trdan, S. Environmental factors affecting the glucosinolate content in Brassicaceae. J. Food Agric. Environ. 2012, 10, 357–360. [Google Scholar]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Krishna, P.; Forreiter, C. A glucosinolate mutant of Arabidopsis is thermosensitive and defective in cytosolic Hsp90 expression after heat stress. Plant Physiol. 2000, 123, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J.; Velasco, P.; de Haro, A.; Johansen, T.J.; McAlvay, A.C.; Möllers, C.; Mølmann, J.A.B.; Ordiales, E.; Rodríguez, V.M. Agronomic and metabolomic side-effects of a divergent selection for indol-3-ylmethylglucosinolate content in kale (Brassica oleracea var. acephala). Metabolites 2021, 11, 684. [Google Scholar] [CrossRef]
- Sotelo, T.; Velasco, P.; Soengas, P.; Rodríguez, V.M.; Cartea, M.E. Modification of leaf glucosinolate contents in Brassica Oleracea by divergent selection and effect on expression of genes controlling glucosinolate pathway. Front. Plant Sci. 2016, 7, 1012. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Soengas, P.; Cartea, E.; Sotelo, T.; Velasco, P. Suitability of a European nuclear collection of Brassica oleracea L. landraces to grow at high temperatures. J. Agron. Crop Sci. 2014, 200, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Velasco, P.; Francisco, M.; Moreno, D.A.; Ferreres, F.; García-Viguera, C.; Cartea, M.E. Phytochemical fingerprinting of vegetable Brassica oleracea and Brassica napus by simultaneous identification of glucosinolates and phenolics. Phytochem. Anal. 2011, 22, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, R.; Li, R.; Kuwahara, A.; Nakabayashi, R.; Sotta, N.; Mori, T.; Ito, T.; Ohkama-Ohtsu, N.; Fujiwara, T.; Saito, K.; et al. Retrograde sulfur flow from glucosinolates to cysteine in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2017890118. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Chapter 12-Role of Glucosinolates in Plant Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 271–291. ISBN 978-0-12-800876-8. [Google Scholar]
- Ljubej, V.; Radojčić Redovniković, I.; Salopek-Sondi, B.; Smolko, A.; Roje, S.; Šamec, D. Chilling and freezing temperature stress differently influence glucosinolates content in Brassica oleracea var. acephala. Plants 2021, 10, 1305. [Google Scholar] [CrossRef]
- KeLing, H.; Zhujun, Z. Effects of different concentrations of sodium chloride on plant growth and glucosinolate content and composition in pakchoi. Afr. J. Biotechnol. 2010, 9, 4428–4433. [Google Scholar]
- Pramanik, M.H.R.; Imai, R. Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol. Biol. 2005, 58, 751–762. [Google Scholar] [CrossRef]
- Redillas, M.C.F.R.; Park, S.H.; Lee, J.W.; Kim, Y.S.; Jeong, J.S.; Jung, H.; Bang, S.W.; Hahn, T.R.; Kim, J.K. Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 2012, 6, 89–96. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Ul Rehman, R.; Hakeem, K.R.; Irfan Qureshi, M. Signaling in response to cold stress. Plant Signal. Underst. Mol. Crosstalk 2014, 9788132215424, 193–226. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément Christophe, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Fu, J.; Miao, Y.; Shao, L.; Hu, T.; Yang, P. De novo transcriptome sequencing and gene expression profiling of Elymus nutans under cold stress. BMC Genom. 2016, 17, 870. [Google Scholar] [CrossRef] [Green Version]
- Pedras, M.S.C.; Zheng, Q.A.; Gadagi, R.S.; Rimmer, S.R. Phytoalexins and polar metabolites from the oilseeds canola and rapeseed: Differential metabolic responses to the biotroph Albugo candida and to abiotic stress. Phytochemistry 2008, 69, 894–910. [Google Scholar] [CrossRef]
- Döll, S.; Kuhlmann, M.; Rutten, T.; Mette, M.F.; Scharfenberg, S.; Petridis, A.; Berreth, D.C.; Mock, H.P. Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J. 2018, 93, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Gzyl, J.; Chmielowska-Bak, J. Homocysteine over-accumulation as the effect of potato leaves exposure to biotic stress. Plant Physiol. Biochem. 2013, 63, 177–184. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.; Kronzucker, H.J.; Shi, W. Quantification and enzyme targets of fatty acid amides from duckweed root exudates involved in the stimulation of denitrification. J. Plant Physiol. 2016, 198, 81–88. [Google Scholar] [CrossRef]
- Murata, J.; Watanabe, T.; Sugahara, K.; Yamagaki, T.; Takahashi, T. High-resolution mass spectrometry for detecting Acetylcholine in Arabidopsis. Plant Signal. Behav. 2015, 10, e1074367. [Google Scholar] [CrossRef]

| m/z | Neutral Mass | RT 1 | Ionization | Molecular Formula | Theoretical Mass | Mass Deviation (ppm) | Putative Identification |
|---|---|---|---|---|---|---|---|
| 381.0803 | 342.1166 | 1.01 | [M+K]+ | C12H22O11 | 342.1162 | −0.98 | Glucose disaccharide |
| 136.0629 | 135.0551 | 1.45 | [M+H]+ | C5H5N5 | 135.0545 | −4.70 | Adenine |
| 166.0868 | 165.0790 | 3.48 | [M+H]+ | C9H11NO2 | 165.079 | −0.07 | Phenylalanine |
| 146.0818 | 145.0740 | 4.31 | [M+H]+ | C6H11NO3 | 145.0739 | −0.60 | L-Allysine |
| 477.0641 | 478.0719 | 8.92 | [M−H]− | C17H22N2O10S2 | 478.0716 | −0.69 | Methoxyglucobrassicin |
| 225.1566 | 224.1488 | 9.44 | [M+H]+ | ||||
| 251.0322 | 250.0244 | 14.85 | [M+H]+ | C11H10N2OS2 | 250.0235 | −3.73 | Spirobrassinin |
| 181.1229 | 180.1151 | 15.51 | [M+H]+ | C11H16O2 | 180.115 | −0.28 | Dihydroactinidiolide |
| 671.3268 | 648.3371 | 16.54 | [M+Na]+ | C31H52O14 | 648.3357 | −2.20 | Digalactosylmonoacylglycerol (DGMG; 16:3) |
| 200.2377 | 199.2299 | 16.72 | [M+H]+ | C13H29N | 199.23 | 0.45 | Tridecanamine |
| 299.2008 | 300.2086 | 16.75 | [M−H]− | C20H28O2 | 300.20893 | 1.10 | Dehydroabietic acid |
| 294.2431 | 293.2353 | 17.09 | [M+H]+ | C18H31NO2 | 293.2355 | 0.71 | Hexadecatrienoic acid ethanol amide |
| 301.2162 | 300.2084 | 17.43 | [M+H]+ | C20H28O2 | 300.2089 | 1.90 | Dehydroabietic acid |
| 235.0365 | 234.0287 | 17.65 | [M+H]+ | C11H10N2S2 | 234.0285 | −0.55 | Cyclobrassinin |
| 338.3045 | 338.3045 | 18.32 | [M]+ | C21H40NO2 | 338.3059 | 4.18 | 9,12-Hexadecadienoylcholine |
| 368.3526 | 367.3448 | 20.16 | [M+H]+ | ||||
| 375.2510 | 352.2613 | 20.26 | [M+Na]+ | C21H36O4 | 352.2614 | 0.11 | Glyceryl linolenate (enantiomer2) |
| 324.2898 | 323.2820 | 20.45 | [M+H]+ | C20H37NO2 | 323.2824 | 1.42 | Linoleoyl ethanolamide |
| 375.2516 | 352.2618 | 20.48 | [M+Na]+ | C21H36O4 | 352.2614 | −1.37 | Glyceryl linolenate (enantiomer1) |
| 377.2664 | 354.2766 | 21.47 | [M+Na]+ | C21H38O4 | 354.277 | 1.27 | Glyceryl linoleate |
| 601.4235 | 600.4157 | 21.64 | [M+H]+ | C31H52N8O4 | 600.4112 | −7.60 | N-[2-[2-(2-Aminoethoxy)ethoxy]ethyl]-4-[[[4-[(4-hydroxycyclohexyl)amino]-6-(octylamino)-1,3,5-triazin-2-yl]amino]methyl]benzamide (YTC_000721) |
| 282.2788 | 281.2710 | 22.3 | [M+H]+ | C18H35NO | 281.2719 | 3.07 | Oleamide |
| 296.2952 | 295.2874 | 23.14 | [M+H]+ | C19H37NO | 295.2875 | 0.28 | Palmitic amide propyl ester |
| 535.2704 | 534.2626 | 23.31 | [M+H]+ | C33H34N4O3 | 534.2631 | 0.99 | Pyropheophorbide A |
| 607.2917 | 606.2839 | 24.12 | [M+H]+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Urbano, M.; Velasco, P.; Cartea, M.E.; Rodríguez, V.M. Metabolism Reorganization in Kale (Brassica oleracea L. var acephala) Populations with Divergent Glucosinolate Content under Thermal Stresses. Agronomy 2022, 12, 2652. https://doi.org/10.3390/agronomy12112652
Díaz-Urbano M, Velasco P, Cartea ME, Rodríguez VM. Metabolism Reorganization in Kale (Brassica oleracea L. var acephala) Populations with Divergent Glucosinolate Content under Thermal Stresses. Agronomy. 2022; 12(11):2652. https://doi.org/10.3390/agronomy12112652
Chicago/Turabian StyleDíaz-Urbano, María, Pablo Velasco, María Elena Cartea, and Víctor M. Rodríguez. 2022. "Metabolism Reorganization in Kale (Brassica oleracea L. var acephala) Populations with Divergent Glucosinolate Content under Thermal Stresses" Agronomy 12, no. 11: 2652. https://doi.org/10.3390/agronomy12112652
APA StyleDíaz-Urbano, M., Velasco, P., Cartea, M. E., & Rodríguez, V. M. (2022). Metabolism Reorganization in Kale (Brassica oleracea L. var acephala) Populations with Divergent Glucosinolate Content under Thermal Stresses. Agronomy, 12(11), 2652. https://doi.org/10.3390/agronomy12112652







