Comparison of Yield and Important Seed Quality Traits of Selected Legume Species
Abstract
:1. Introduction
1.1. Importance of Legumes
1.2. Characteristics of the Selected Species
2. Materials and Methods
2.1. Field Data
- Soybean (Glycine max (L.) Merr.), cultivar Mavka,
- Faba bean (Vicia faba var. minor), cultivar Albus,
- Pea (Pisum sativum L.), cultivar Batuta,
- White lupin (Lupinus albus L.), cultivar Butan,
- Narrow-leafed lupin (Lupinus angustifolius L.), cultivar Regent,
- Yellow lupin (Lupinus luteus L.), cultivar Mister.
2.2. Agrotechnics
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results and Discussion
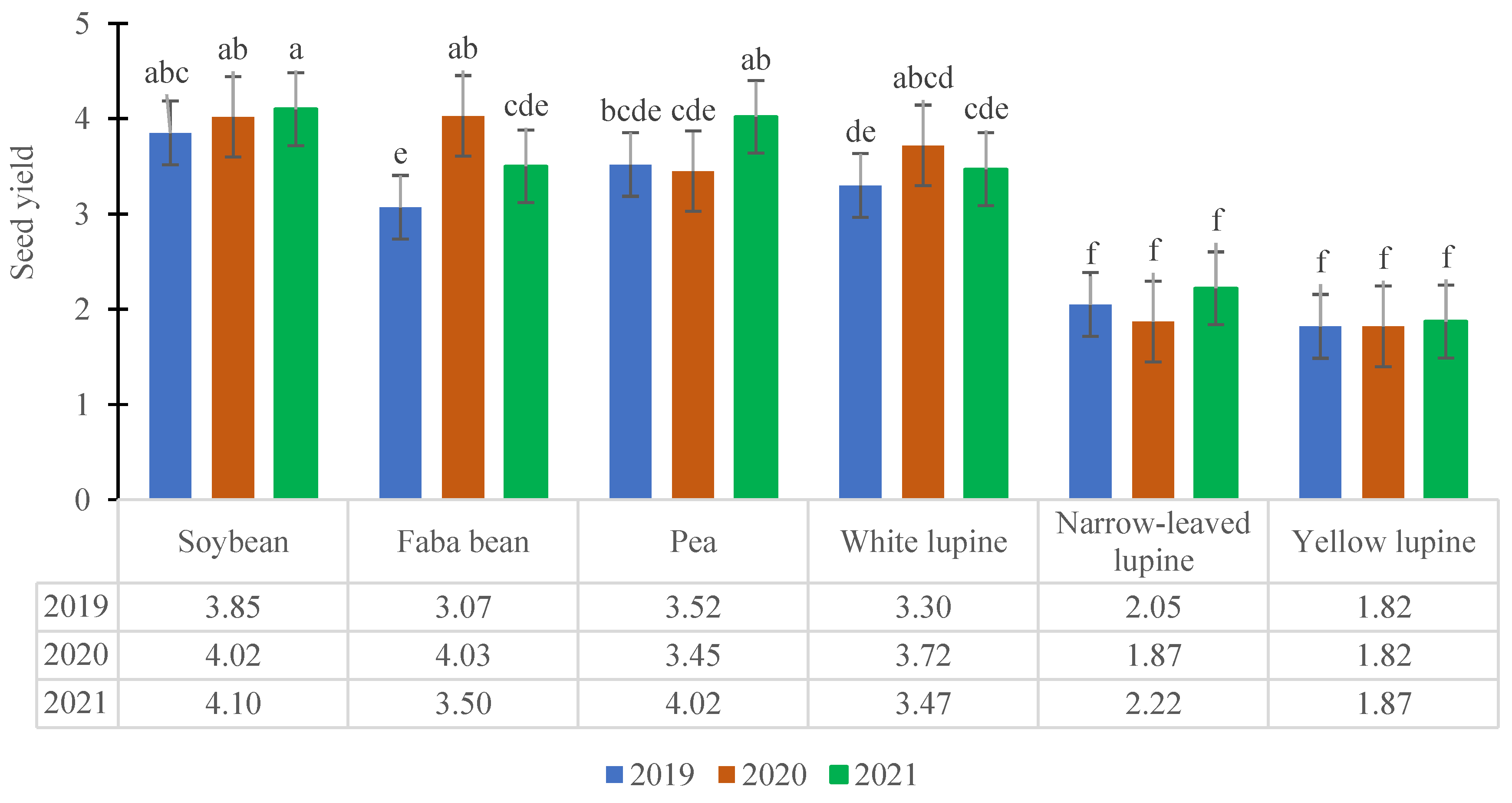
3.1. Seed Yield
3.2. Total Protein Yield
3.3. Chemical Composition of Seeds
3.4. The Content of Macronutrients
3.5. The Content of Micronutrients
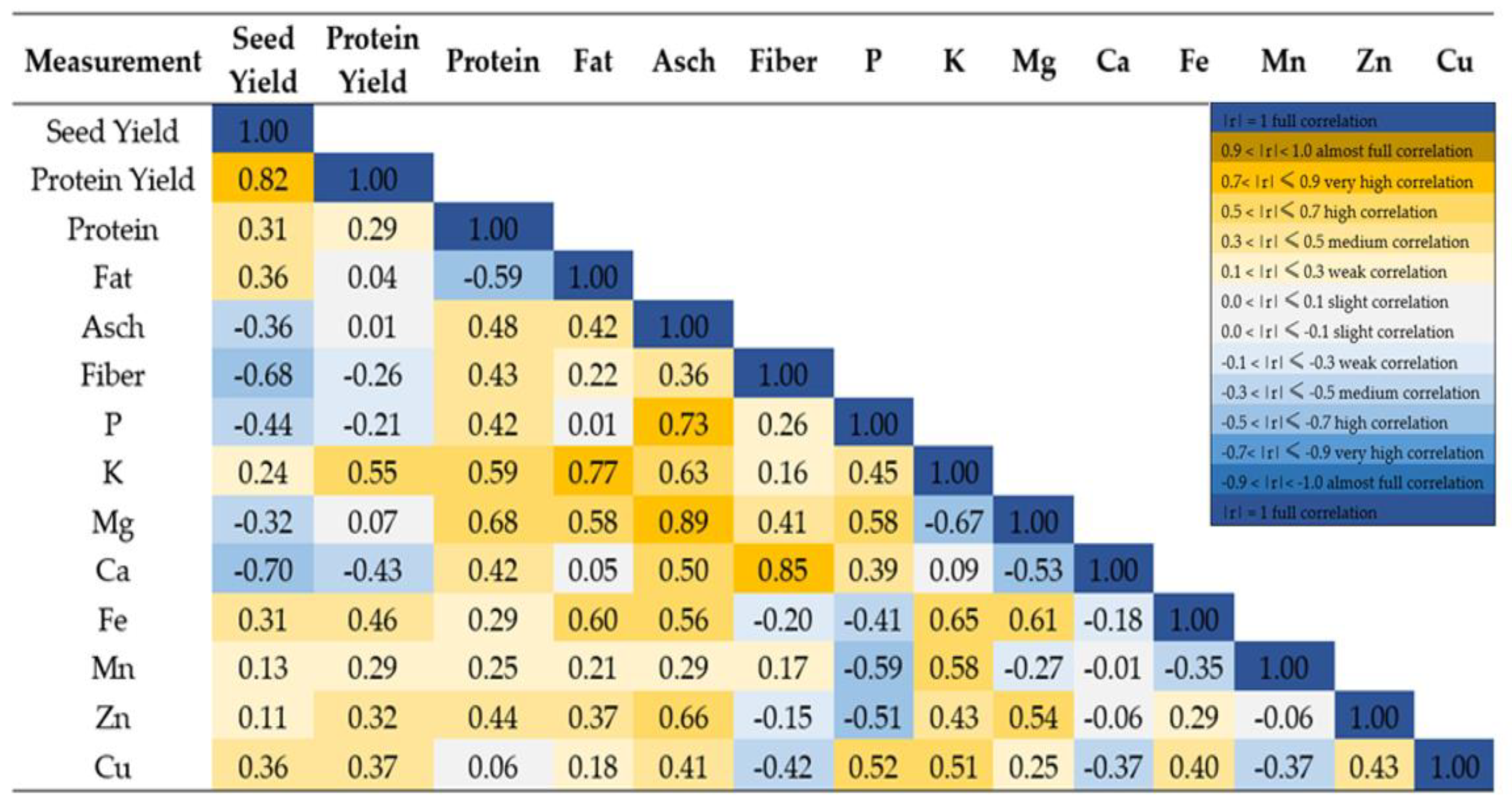
3.6. Statistical Dependencies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palmero, F.; Fernandez, J.A.; Garcia, F.O.; Ricardo, J.; Haro, R.J.; Prasad, P.V.; Salvagiotti, F.; Ignacio, A.; Ciampitti, I.A. A quantitative review into the contributions of biological nitrogen fixation to agricultural systems by grain legumes. Eur. J. Agron. 2022, 136, 126514. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [Green Version]
- Perera, T.; Russo, C.; Takata, Y.; Bobe, G. Legume Consumption Patterns in US Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014 and Beans, Lentils, Peas (BLP) 2017 Survey. Nutrients 2020, 12, 1237. [Google Scholar] [CrossRef]
- Sterna, V.; Zute, S.; Jansone, I.; Ence, E.; Strausa, E. Evaluation of various legume species and varieties grown in Latvia as a raw material of plant-based protein products. Agron. Res. 2020, 18, 2602–2612. [Google Scholar] [CrossRef]
- Diaz, D.; Morlacchini, M.; Masoero, F.; Moschini, M.; Fusconi, G.; Piva, G. Pea seeds (Pisum sativum), faba beans (Vicia faba var. minor) and lupin seeds (Lupinus albus var. multitalia) as protein sources in broiler diets: Effect of extrusion on growth performance. Ital. J. Anim. Sci. 2006, 5, 43–53. [Google Scholar] [CrossRef]
- Degola, L.; Sterna, V.; Jansons, I.; Zute, S. The nutrition value of soybeans grown in Latvia for pig feeding. Agron. Res. 2019, 17, 1874–1880. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Alghamdi, S.S. Chemical Composition of Faba Bean (Vicia faba L.) Genotypes under Various Water Regimes. Pak. J. Nutr. 2009, 8, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Popović, V.; Glamočlija, Đ.; Sikora, V.; Đekić, V.; Cervenski, J.; Simić, D.; Ilin, S. Genotypic specificity of soybean (Glicine max. (L.) Merr.) under conditions of foliar fertilization. Rom. Agric. Res. 2013, 30, 1–12. [Google Scholar]
- Jo, H.; Asekova, S.; Bayat, M.A.; Ali, L.; Song, J.T.; Ha, Y.-S.; Hong, D.-H.; Lee, J.-D. Comparison of Yield and Yield Components of Several Crops Grown under Agro-Photovoltaic System in Korea. Agriculture 2022, 12, 619. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, Z.; Cheng, Q.; Liu, R.; Li, M.; Yan, X.; Li, G.; Wang, D.; Fu, L.; Ma, Y.; et al. Estimation of plant height and yield based on UAV imagery in faba bean (Vicia faba L.). Plant Methods 2022, 18, 26. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Marinangeli, C.P.F.; Pigat, S.; Bompola, F.; Campbell, J.; Pan, Y.; Curran, J.M.; Cai, D.J.; Jaconis, S.Y.; Rumney, J. Pulse Intake Improves Nutrient Density among US Adult Consumers. Nutrients 2021, 13, 2668. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A. Legumes: Nutritional quality, processing and potential health benefits. In Legumes and Their Associated Health Benefits; Royal Society of Chemistry: London, UK, 2019; p. 353. [Google Scholar]
- Carbonaro, M.; Nucara, A. Legume Proteins and Peptides as Compounds in Nutraceuticals: A Structural Basis for Dietary Health Effects. Nutrients 2022, 14, 1188. [Google Scholar] [CrossRef]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer Understanding and Culinary Use of Legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef] [Green Version]
- Elamine, Y.; Alaiz, M.; Girón-Calle, J.; Guiné, R.P.F.; Vioque, J. Nutritional Characteristics of the Seed Protein in 23 Mediterranean Legumes. Agronomy 2022, 12, 400. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Pathania, S.; Brites, C.; Rosa, E.; Barros, A. Potential of Legumes: Nutritional Value, Bioactive Properties, Innovative Food Products, and Application of Eco-friendly Tools for Their Assessment. Food Rev. Int. 2021, 1, 1–25. [Google Scholar] [CrossRef]
- Sánchez-Chino, X.; Jiménez-Martinez, C.; Dávila-Ortiz, G.; Alvarez-Gonzalez, I.; Madrigal-Bujaidar, E. Nutrient and Nonnutrient Components of Legumes, and Its Chemopreventive Activity: A Review. Nutr. Cancer 2015, 67, 401–410. [Google Scholar] [CrossRef]
- Prusiński, J. White lupin (Lupinus albus L.)—Nutritional and health values in human nutrition—A review. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Tirdilova, I.; Vollmannova, A.; Siekel, P.; Zetochova, E.; Ceryova, S.; Trebichalsky, P. Selected legumes as a source of valuable substances in human nutrition. J. Food Nutr. Res. 2020, 59, 193–201. [Google Scholar]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein—Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Thompson, H. Motivating Pulse-Centric Eating Patterns to Benefit Human and Environmental Well-Being. Nutrients 2020, 12, 3500. [Google Scholar] [CrossRef]
- Teferra, T.F. Advanced and feasible pulses processing technologies for Ethiopia to achieve better economic and nutritional goals: A review. Heliyon 2021, 7, e07459. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Chakraborty, S. Optimization of extraction process for legume-based synbiotic beverages, followed by their characterization and impact on antinutrients. Int. J. Gastron. Food Sci. 2022, 28, 100506. [Google Scholar] [CrossRef]
- Palmer, S.M.; Winham, D.M.; Oberhauser, A.M.; Litchfield, R.E. Socio-Ecological Barriers to Dry Grain Pulse Consumption among Low-Income Women: A Mixed Methods Approach. Nutrients 2018, 10, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinangeli, C.; Curran, J.; Barr, S.; Slavin, J.; Puri, S.; Swaminathan, S.; Tapsell, L.; Patterson, C. Enhancing nutrition with pulses: Defining a recommended serving size for adults. Nutr. Rev. 2017, 75, 990–1006. [Google Scholar] [CrossRef] [Green Version]
- Muminova, S.S.; Tastanbekova, G.R.; Kashkarov, A.A.; Azhimetova, G.N.; Balgabaev, A.M. Effect of foliar mineral fertilizer and plant growth regulator application on seed yield and yield components of soybean (Glycine max) cultivars. Eurasian J. Soil Sci. 2022, 11, 322–328. [Google Scholar] [CrossRef]
- de Mello Cunha, G.O.; de Almeida, J.A.; Coelho, C.M.M. Chemical composition of soybean seeds subjected to fertilization with rock dusts. Acta Sci. Agron. 2021, 44, e53312. [Google Scholar] [CrossRef]
- Hong, H.; Yoosefzadeh-Najafabadi, M.; Rajcan, I. Correlations between soybean seed quality traits using a genome-wide association study panel grown in Canadian and Ukrainian mega-environments. Can. J. Plant Sci. 2022, 102, 1040–1052. [Google Scholar] [CrossRef]
- Gonyane, M.B.; Sebetha, E.T. The Effect of Plant Density, Zinc Added to Phosphorus Fertilizer Sources and Location on Selected Yield Parameters of Soybean. Legum. Res.-Int. J. 2022, 45, 196–202. [Google Scholar] [CrossRef]
- Plūduma-Pauniņa, I.; Gaile, Z.; Bankina, B.; Balodis, R. Field Bean (Vicia faba L.) Yield and quality depending on some agrotechnical aspects. Agron. Res. 2018, 16, 212–220. [Google Scholar] [CrossRef]
- Paul, S.K.; Mondal, M.; Sarker, U.K.; Sarkar, S.K. Response of yield and seed quality of faba bean (Vicia faba) to irrigation and nutrient management. Res. Crop 2021, 22, 256–264. [Google Scholar] [CrossRef]
- Ton, A.; Karaköy, T.; Anlarsal, A.E.; Türkeri, M. Genetic diversity for agro-morphological characters and nutritional compositions of some local faba bean (Vicia faba L.) genotypes. Turk. J. Agric. For. 2021, 45, 301–312. [Google Scholar] [CrossRef]
- Greveniotis, V.; Bouloumpasi, E.; Zotis, S.; Korkovelos, A.; Ipsilandis, C.G. Yield Components Stability Assessment of Peas in Conventional and Low-Input Cultivation Systems. Agriculture 2021, 11, 805. [Google Scholar] [CrossRef]
- Wozniak, A.; Soroka, M. and Stepniowska, A.; Makarski, B. Chemical composition of pea (Pisum sativum L.) seeds depending on tillage systems. J. Elem. 2014, 19, 1143–1152. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Fordoński, G.; Pszczółkowska, A.; Krzebietke, S.; Olszewski, J.; Okorski, A. Yield and mineral composition of seeds of leguminous plants and grain of spring wheat as well as their residual effect on the yield and chemical composition of winter oilseed rape seeds. J. Elem. 2015, 20, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Dymerska, A.; Grabowska, K. Predicting the yields of yellow lupin depending on the selected climate change scenarios. Acta Agroph. 2014, 2, 1–98. [Google Scholar]
- Fotyma, M.; Kęsik, K.; Lipiński, W.; Filipiak, K.; Purchała, L. Soil tests as the basis of fertilizer advisory. Stud. Rep. IUNG-PIB Puławy 2015, 42, 9–51. (In Polish) [Google Scholar]
- Jarecki, W.; Buczek, J.; Lachowski, T. The influence of seed inoculation and/or initial nitrogen dose on yield and chemical composition of faba bean. J. Elem. 2022, 27, 367–377. [Google Scholar] [CrossRef]
- Kozak, M.; Malarz, W.; Kotecki, A.; Černý, I.; Serafin-Andrzejewska, M. The effect of different sowing rate and Asahi SL biostimulator on chemical composition of soybean seeds and postharvest residues. Oilseed Crops 2008, 24, 217–230. (In Polish) [Google Scholar]
- Jarecki, W. Reaction of soybean (Glycine max (L.) Merr.) to seed inoculation with Bradyrhizobium japonicum bacteria. Plant, Soil Environ. 2020, 66, 242–247. [Google Scholar] [CrossRef]
- Jarecki, W.; Bobrecka-Jamro, D. Effect of sowing date on the yield and seed quality of soybean (Glycine max (L.) Merr.). J. Elem. 2021, 26, 7–18. [Google Scholar] [CrossRef]
- Csajbók, J.; Kutasy, E.T.; Melash, A.A.; Virág, I.C.; Ábrahám, É.B. Performance of Soybean [Glycine max (L.) Merrill] Cultivars under Irrigated and Rainfed Conditions. Legum. Res.-Int. J. 2022, 45, 594–600. [Google Scholar] [CrossRef]
- Csajbók, J.; Kutasy, E.T.; Melash, A.A.; Virág, I.C.; Ábrahám, É.B. Agro-biological traits of soybean cultivars, their yield quantity and quality under Central European conditions. Zemdirb. Agric. 2022, 109, 107–114. [Google Scholar] [CrossRef]
- Agapie, A.L.; Gorinoiu, G.; Horabaga, M.N. The mineral fertilization influence on soybean quality. Res. J Agric. Sci. 2019, 51, 3–8. [Google Scholar]
- Szpunar-Krok, E.; Wondołowska-Grabowska, A. Quality Evaluation Indices for Soybean Oil in Relation to Cultivar, Application of N Fertiliser and Seed Inoculation with Bradyrhizobium japonicum. Foods 2022, 11, 762. [Google Scholar] [CrossRef]
- Didinger, C.; Thompson, H.J. Defining Nutritional and Functional Niches of Legumes: A Call for Clarity to Distinguish a Future Role for Pulses in the Dietary Guidelines for Americans. Nutrients 2021, 13, 1100. [Google Scholar] [CrossRef]
- Cabrera, C.; Lloris, F.; Giménez, R.; Olalla, M.; López, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14. [Google Scholar] [CrossRef]
- Jankauskienė, J.; Brazaitytė, A.; Vaštakaitė-Kairienė, V. Potential of vegetable soybean cultivation in Lithuania. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12267. [Google Scholar] [CrossRef]



| Month | Sum of Precipitation [mm] | Temperature [°C] | ||||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | Multi-Years | 2019 | 2020 | 2021 | Multi-Years | |
| III | 23 | 20 | 18 | 37 | 5.9 | 5.1 | 3.2 | 2.8 |
| IV | 21 | 10 | 49 | 46 | 9.9 | 9.2 | 6.5 | 8.7 |
| V | 74 | 83 | 64 | 77 | 13.1 | 11.3 | 12.8 | 13.7 |
| VI | 31 | 163 | 47 | 80 | 21.5 | 18.1 | 18.8 | 17.1 |
| VII | 50 | 19 | 55 | 95 | 19.1 | 18.8 | 21.6 | 19.0 |
| VIII | 61 | 7 | 107 | 65 | 20.3 | 19.9 | 17.5 | 18.4 |
| IX | 32 | 44 | 86 | 62 | 14.7 | 15.0 | 13.1 | 13.6 |
| Sum/Mean | 292 | 346 | 426 | 462 | 14.9 | 13.9 | 13.4 | 13.3 |
| Legumes | Year | Total Protein | Crude Fat | Fiber | Ash |
|---|---|---|---|---|---|
| Soybean | 2019 | 354.5 ± 9.5 bcde | 211.4 ± 3.0 ab | 114.9 ± 2.4 cdf | 55.6 ± 0.8 bc |
| 2020 | 382.0 ± 10.4 abc | 219.5 ± 2.3 a | 108.3 ± 7.4 defg | 52.9 ± 0.9 cd | |
| 2021 | 399.2 ± 8.8 a | 208.3 ± 3.0 b | 108.0 ± 7.7 efg | 50.2 ± 1.3 d | |
| Faba bean | 2019 | 291.6 ± 3.9 h | 8.4 ± 0.7 e | 96.9 ± 2.4 fg | 36.9 ± 1.1 fgh |
| 2020 | 309.3 ± 2.9 fgh | 9.5 ± 0.5 e | 74.3 ± 3.6 hi | 36.4 ± 0.6 fgh | |
| 2021 | 292.1 ± 4.2 gh | 8.5 ± 0.6 e | 90.6 ± 8.0 gh | 41.8 ± 2.5 e | |
| Pea | 2019 | 234.8 ± 4.8 i | 14.0 ± 0.2 e | 63.3 ± 0.9 i | 34.0 ± 1.0 h |
| 2020 | 247.9 ± 3.2 i | 14.1 ± 1.0 e | 60.4 ± 1.4 i | 33.7 ± 2.2 h | |
| 2021 | 235.9 ± 4.5 i | 14.1 ± 0.3 e | 54.4 ± 14.8 i | 33.2 ± 0.8 h | |
| White lupine | 2019 | 347.9 ± 18.1 cde | 109.1 ± 8.3 c | 120.4 ± 9.8 cde | 36.5 ± 1.3 fgh |
| 2020 | 389.3 ± 11.7 ab | 104.8 ± 8.7 c | 112.6 ± 9.7 cdef | 34.8 ± 1.8 gh | |
| 2021 | 349.5 ± 19.2 cde | 109.5 ± 6.2 c | 120.6 ± 8.6 cde | 36.2 ± 1.9 fgh | |
| Narrow-leaved lupine | 2019 | 339.8 ± 8.7 def | 46.3 ± 4.3 d | 165.8 ± 11.8 a | 39.4 ± 0.6 ef |
| 2020 | 334.6 ± 11.6 ef | 46.6 ± 4.7 d | 159.3 ± 10.8 a | 38.4 ± 0.8 efg | |
| 2021 | 328.9 ± 9.0 efg | 44.1 ± 4.0 d | 149.0 ± 11.1 ab | 38.2 ± 1.5 efg | |
| Yellow lupine | 2019 | 406.4 ± 14.2 a | 50.7 ± 1.6 d | 129.2 ± 5.0 bcd | 60.8 ± 1.5 a |
| 2020 | 389.3 ± 15.9 ab | 49.4 ± 4.4 d | 131.4 ± 6.1 bc | 58.4 ± 1.8 ab | |
| 2021 | 375.4 ± 42.1 abcd | 53.4 ± 3.8 d | 128.3 ± 5.7 bcde | 59.6 ± 1.6 a | |
| Legumes × Year | *** | * | * | *** | |
| Legumes | Year | Phosphorus | Potassium | Magnesium | Calcium |
|---|---|---|---|---|---|
| Soybean | 2019 | 5.36 ± 0.24 de | 18.3 ± 0.37 a | 2.25 ± 0.06 a | 1.15 ± 0.12 de |
| 2020 | 5.84 ± 0.08 cd | 16.7 ± 0.41 ab | 2.12 ± 0.11 a | 0.85 ± 0.11 ef | |
| 2021 | 6.53 ± 0.26 ab | 16.1 ± 0.97 b | 2.14 ± 0.09 a | 0.63 ± 0.06 fg | |
| Faba bean | 2019 | 6.00 ± 0.17 bc | 11.1 ± 0.96 cdef | 1.15 ± 0.16 ef | 0.50 ± 0.12 fg |
| 2020 | 5.70 ± 0.27 cde | 10.2 ± 0.18 cdefg | 0.99 ± 0.08 f | 0.49 ± 0.09 fg | |
| 2021 | 6.54 ± 0.53 ab | 11.6 ± 0.38 c | 1.16 ± 0.13 def | 0.65 ± 0.09 fg | |
| Pea | 2019 | 4.19 ± 0.29 f | 9.2 ± 0.88 ghi | 1.20 ± 0.04 def | 0.31 ± 0.07 g |
| 2020 | 5.22 ± 0.11 e | 7.5 ± 0.53 i | 1.30 ± 0.04 cde | 0.35 ± 0.11 g | |
| 2021 | 3.43 ± 0.38 g | 7.4 ± 0.34 i | 1.18 ± 0.03 def | 0.28 ± 0.03 g | |
| White lupine | 2019 | 3.50 ± 0.13 g | 9.5 ± 0.68 fgh | 1.17 ± 0.10 def | 0.81 ± 0.12 ef |
| 2020 | 3.88 ± 0.10 fg | 10.2 ± 0.76 cdefg | 1.34 ± 0.08 cde | 1.16 ± 0.36 de | |
| 2021 | 3.81 ± 0.13 fg | 7.9 ± 0.75 hi | 1.32 ± 0.06 cde | 0.91 ± 0.14 ef | |
| Narrow-leaved lupine | 2019 | 5.47 ± 0.05 cde | 9.7 ± 1.08 defgh | 1.64 ± 0.16 b | 2.08 ± 0.09 a |
| 2020 | 5.36 ± 0.07 de | 9.6 ± 0.51 efgh | 1.54 ± 0.22 bc | 1.59 ± 0.06 bc | |
| 2021 | 5.24 ± 0.10 de | 9.8 ± 0.21 cdefg | 1.45 ± 0.20 bcd | 1.69 ± 0.02 abc | |
| Yellow lupine | 2019 | 7.10 ± 0.04 a | 11.2 ± 0.57 cdef | 2.22 ± 0.07 a | 1.97 ± 0.06 ab |
| 2020 | 7.07 ± 0.06 a | 11.5 ± 0.71 cd | 2.20 ± 0.04 a | 1.79 ± 0.14 abc | |
| 2021 | 6.90 ± 0.40 a | 11.4 ± 1.30 cde | 2.15 ± 0.12 a | 1.50 ± 0.45 cd | |
| Legumes × Year | *** | *** | * | *** | |
| Legumes | Year | Iron | Manganese | Zinc | Copper |
|---|---|---|---|---|---|
| Soybean | 2019 | 61.0 ± 8.4 c | 14.6 ± 0.5 c | 35.7 ± 1.3 de | 11.1 ± 1.1 cdef |
| 2020 | 75.8 ± 8.5 b | 18.2 ± 1.5 c | 47.6 ± 1.7 ab | 12.8 ± 0.6 abcd | |
| 2021 | 99.2 ± 3.6 a | 23.5 ± 0.4 c | 50.2 ± 2.3 a | 14.3 ± 0.3 ab | |
| Faba bean | 2019 | 35.4 ± 3.5 efg | 13.7 ± 1.6 c | 35.0 ± 1.4 de | 13.1 ± 0.9 abc |
| 2020 | 33.4 ± 2.9 fg | 12.7 ± 1.1 c | 33.5 ± 0.8 de | 11.7 ± 0.7 bcde | |
| 2021 | 46.8 ± 5.6 de | 14.7 ± 1.4 c | 38.4 ± 8.0 cd | 14.5 ± 2.0 a | |
| Pea | 2019 | 44.7 ± 2.8 def | 6.1 ± 0.5 c | 21.2 ± 3.1 g | 4.8 ± 0.7 hi |
| 2020 | 53.3 ± 3.1 cd | 9.2 ± 0.3 c | 39.8 ± 2.5 bcd | 9.9 ± 0.7 efg | |
| 2021 | 51.1 ± 1.6 cd | 8.3 ± 0.4 c | 35.4 ± 4.7 de | 10.3 ± 1.1 def | |
| White lupine | 2019 | 32.1 ± 1.2 g | 636.9 ± 8.8 a | 37.8 ± 1.9 de | 7.2 ± 1.2 gh |
| 2020 | 30.9 ± 2.3 g | 474.8 ± 96.2 b | 30.0 ± 2.0 ef | 5.8 ± 0.7 hi | |
| 2021 | 36.7 ± 4.9 efg | 608.5 ± 20.4 a | 34.1 ± 1.2 de | 4.5 ± 1.0 hi | |
| Narrow-leaved lupine | 2019 | 31.1 ± 1.3 g | 45.0 ± 7.5 c | 23.3 ± 2.9 fg | 3.8 ± 0.5 i |
| 2020 | 30.1 ± 2.5 g | 43.5 ± 9.3 c | 22.0 ± 2.9 fg | 3.6 ± 0.7 i | |
| 2021 | 30.7 ± 1.5 g | 42.9 ± 7.5 c | 20.8 ± 2.8 g | 3.5 ± 1.3 i | |
| Yellow lupine | 2019 | 55.9 ± 5.4 cd | 54.6 ± 8.8 c | 46.2 ± 2.3 abc | 12.0 ± 0.6 abcde |
| 2020 | 52.5 ± 6.2 cd | 41.1 ± 5.4 c | 48.1 ± 2.2 a | 8.7 ± 0.7 fg | |
| 2021 | 54.1 ± 6.9 cd | 51.7 ± 8.4 c | 47.2 ± 3.2 ab | 10.6 ± 2.2 cdef | |
| Legumes × Year | *** | *** | *** | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarecki, W.; Migut, D. Comparison of Yield and Important Seed Quality Traits of Selected Legume Species. Agronomy 2022, 12, 2667. https://doi.org/10.3390/agronomy12112667
Jarecki W, Migut D. Comparison of Yield and Important Seed Quality Traits of Selected Legume Species. Agronomy. 2022; 12(11):2667. https://doi.org/10.3390/agronomy12112667
Chicago/Turabian StyleJarecki, Wacław, and Dagmara Migut. 2022. "Comparison of Yield and Important Seed Quality Traits of Selected Legume Species" Agronomy 12, no. 11: 2667. https://doi.org/10.3390/agronomy12112667







