Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.2. Physiological Indexes
2.3. Quantification and Extraction of Carotenoids
2.4. RNA Extraction, Illumine Sequencing, and RNA-Seq Analysis
2.5. Experimental Validation of Candidate Genes
2.6. Statistical Analysis
3. Results
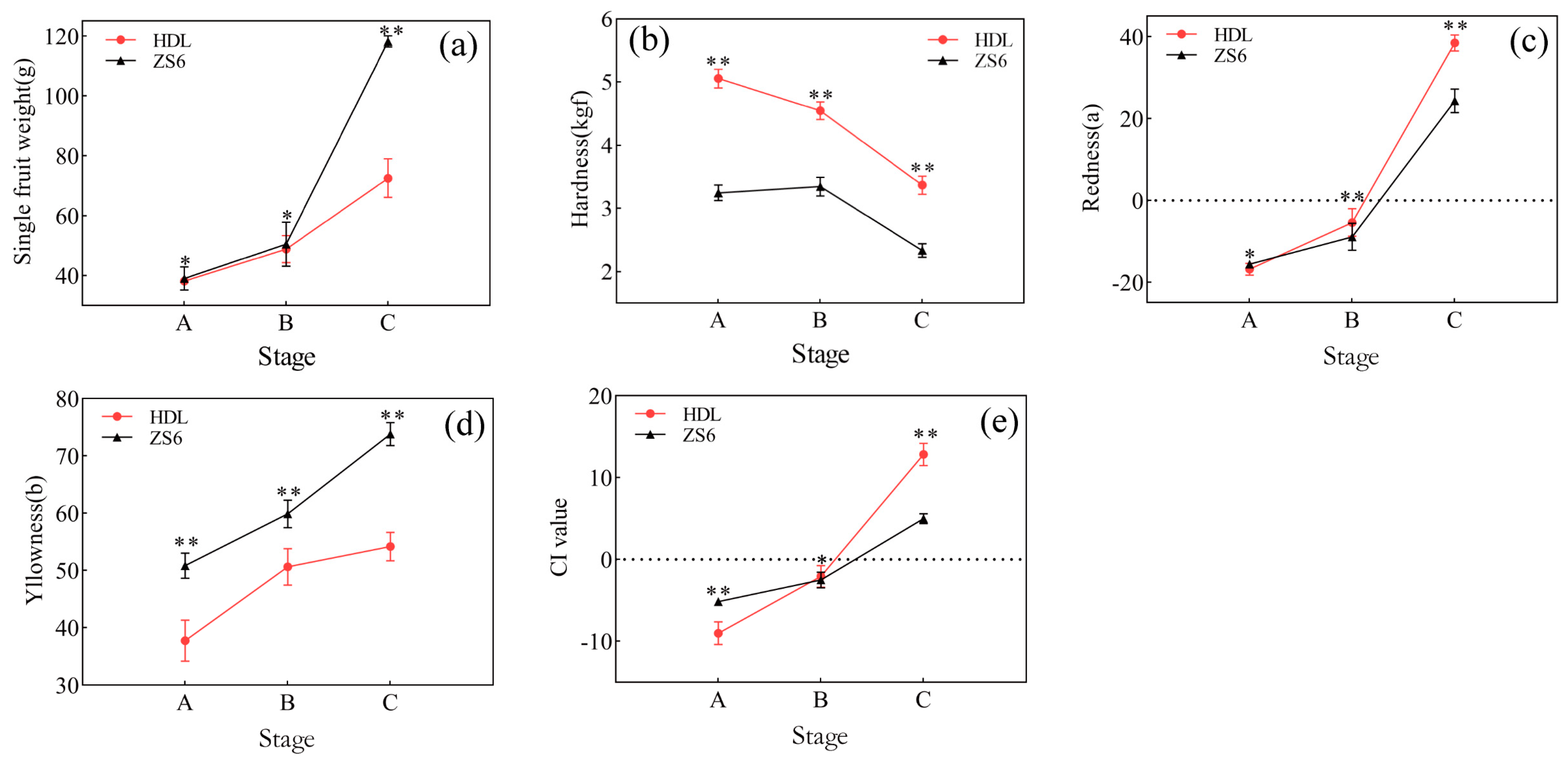
3.1. Physiological Changes Indicators during Development and Maturation
3.2. Carotenoids in Fruit Peel Change during Development and Ripening
3.3. RNA Sequencing and Sample Transcriptome Mapping
3.3.1. Sequencing Data Quality Assessment
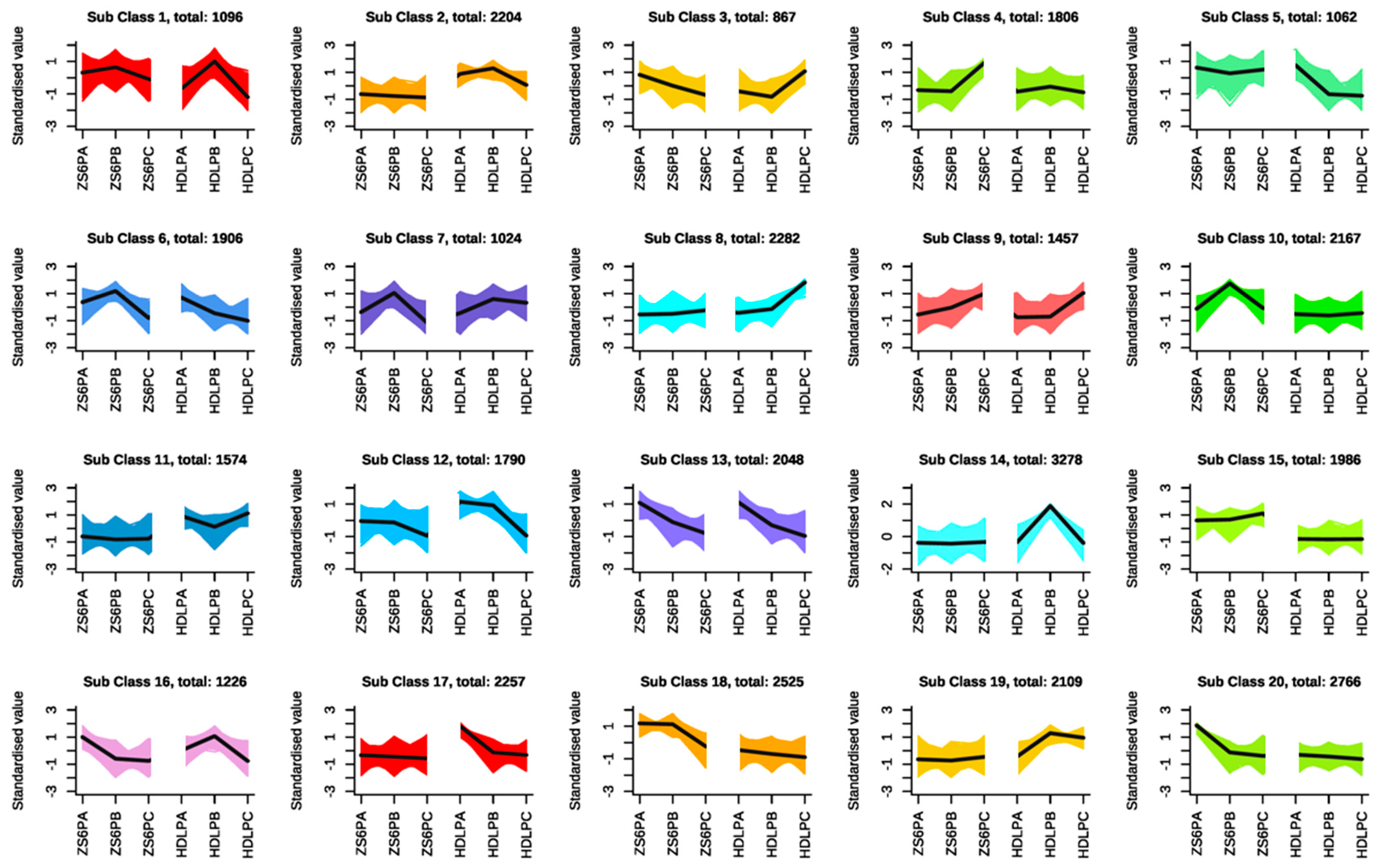
3.3.2. Analyzing the Differential Expression of Genes
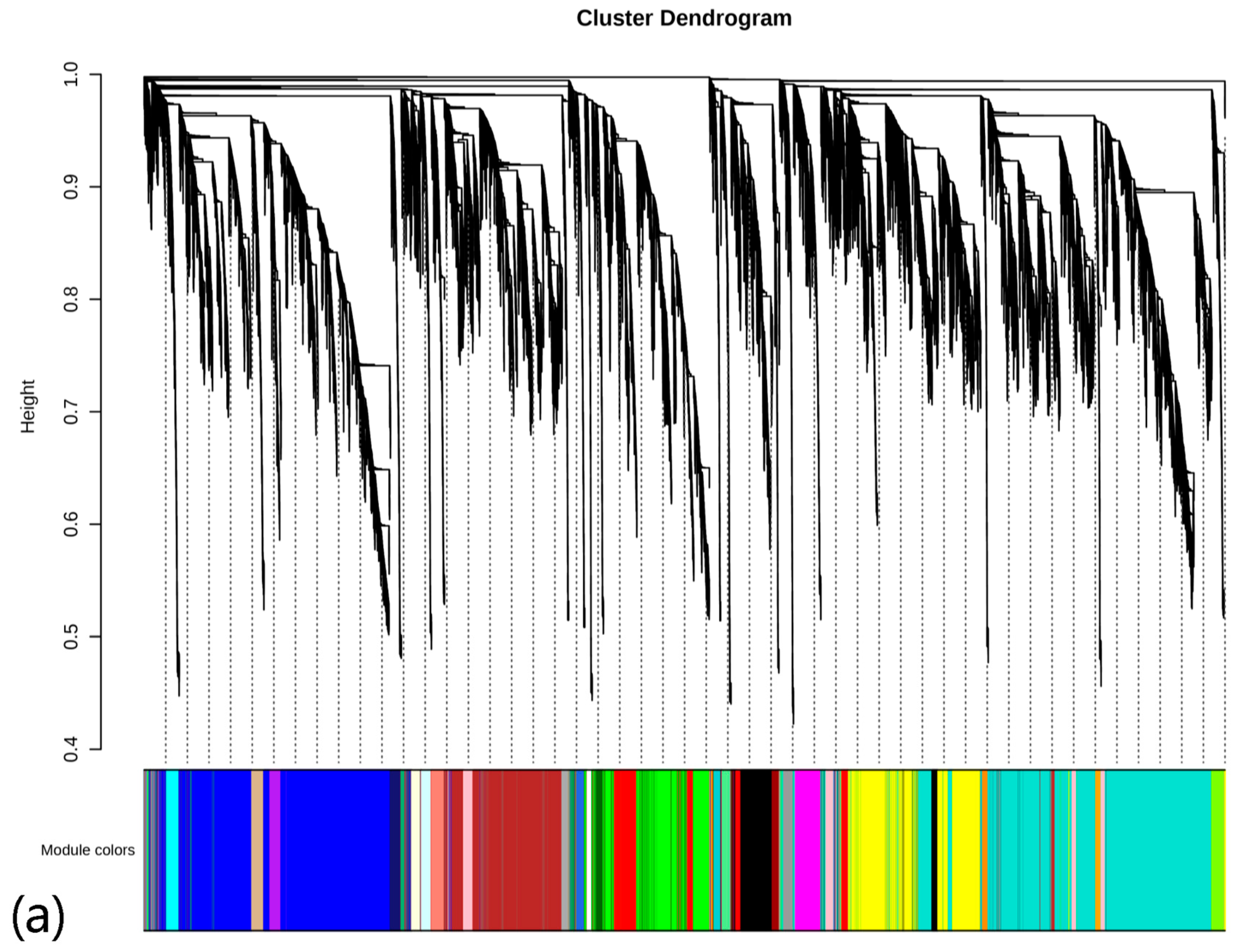
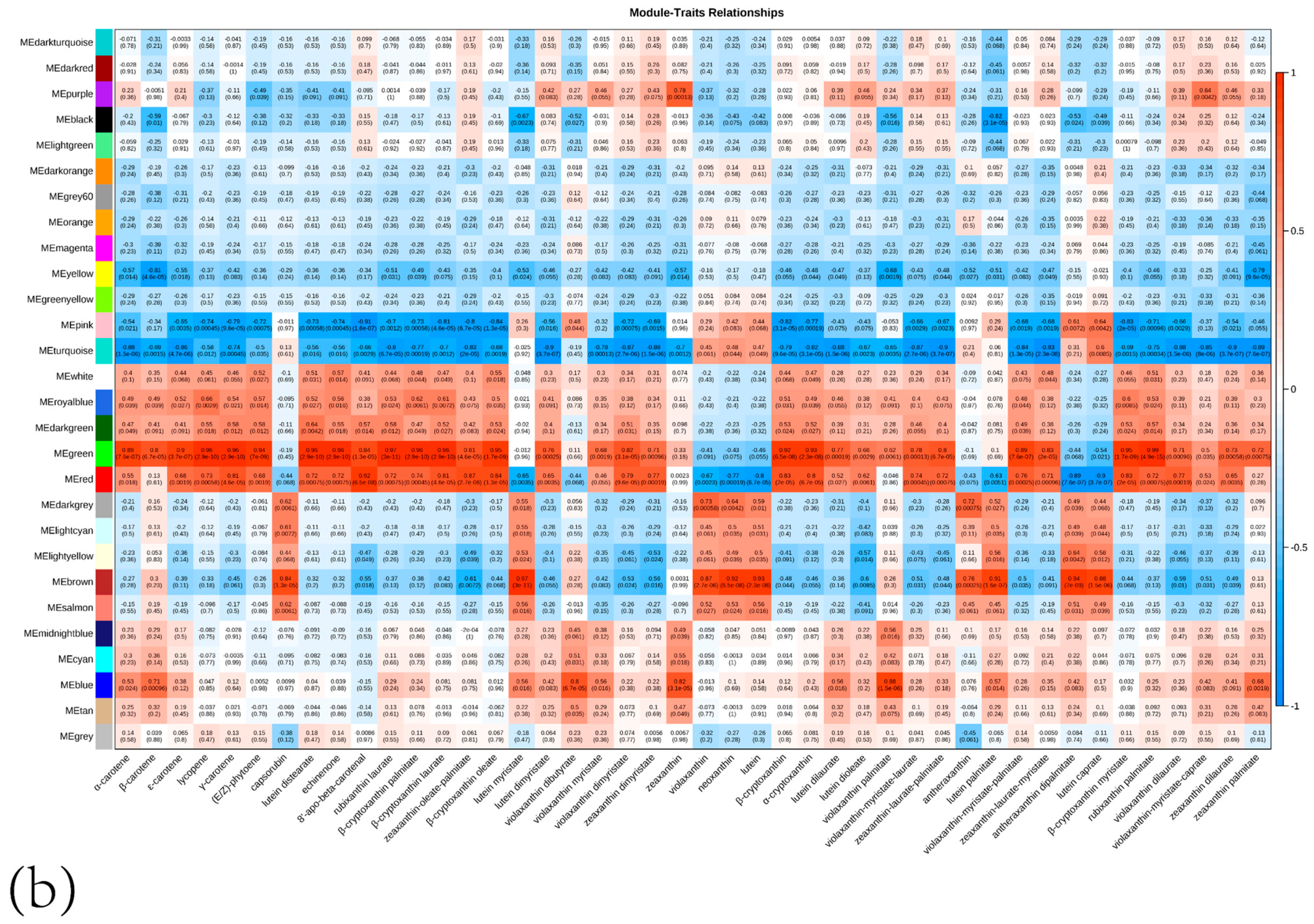
3.3.3. WGCNA Module Analysis
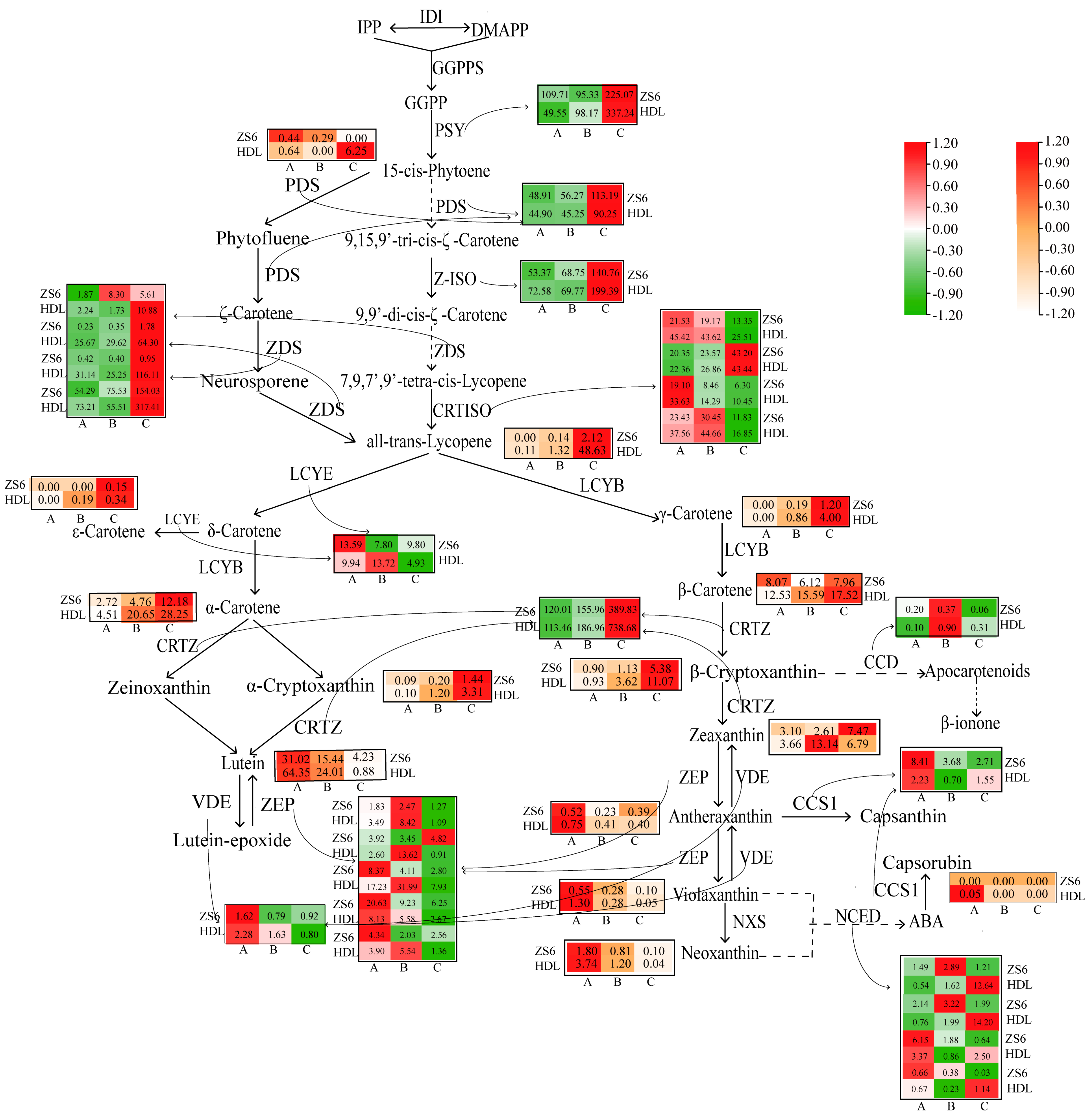
3.3.4. Metabolic Pathway Analysis of Carotenoids
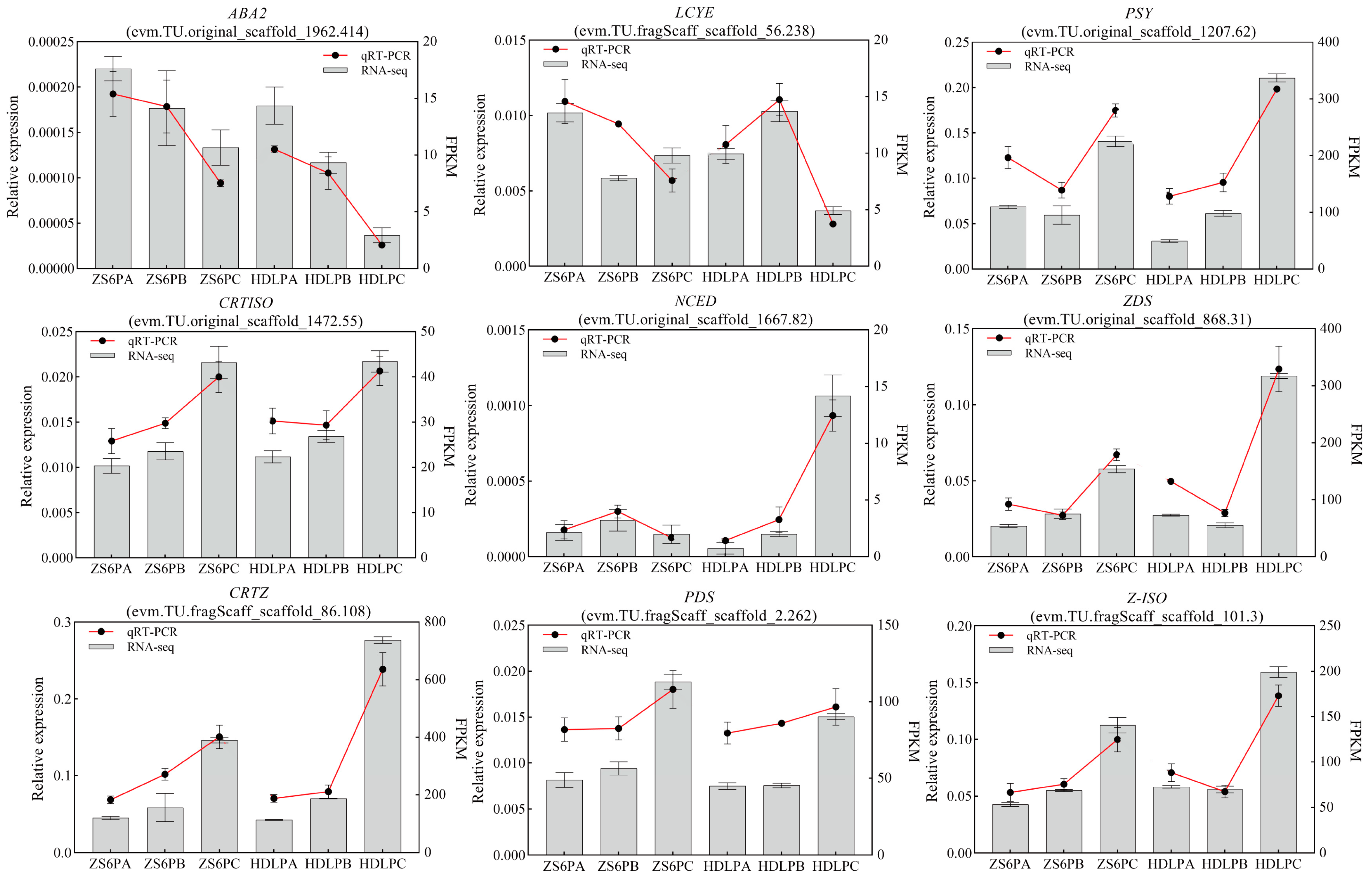
3.3.5. qRT-PCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miraei Ashtiani, S.; Salarikia, A.; Golzarian, M.R.; Emadi, B. Non-Destructive estimation of mechanical and chemical properties of persimmons by ultrasonic spectroscopy. Int. J. Food Prop. 2016, 19, 1522–1534. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, R. Persimmon in China: Domestication and traditional utilization of genetic resources. Adv. Hortic. Sci. 2008, 22, 1000–1005. [Google Scholar]
- Yu, X.; Wang, J.; Gao, R.; Gong, B.; Ai, C. Integrated Metabolomic-Transcriptomic analysis reveals diverse resource of functional ingredients from persimmon leaves of different varieties. Front. Plant Sci. 2022, 13, 904208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, M.; Li, Q.; Wang, T.; Zhang, B.; Zhao, H.; Fu, J. The beneficial effects of polysaccharide obtained from persimmon (Diospyros kaki L.) on the proliferation of Lactobacillus and gut microbiota. Int. J. Biol. Macromol. 2021, 182, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, J.; Xu, X.; Perl, A.; Chen, S.; Ma, H. Adoption of table grape cultivars: An attribute preference study on Chinese grape growers. Sci. Hortic. -Amst. 2017, 216, 66–75. [Google Scholar] [CrossRef]
- Wei, X.; Wu, L.; Ge, D.; Yao, M.; Bai, Y. Prediction of the maturity of greenhouse grapes based on imaging technology. Plant Phenomics 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Li, W.; Yang, C.; Lin, Y.; Wang, Y.; Chen, C.; Wan, C.; Chen, J.; Gan, Z. The Effects of Bagging on Color Change and Chemical Composition in ‘Jinyan’ Kiwifruit (Actinidia chinensis). Horticulturae 2022, 8, 478. [Google Scholar] [CrossRef]
- Ranganath, K.G. Pigments that colour our fruits: An overview. Erwerbs-Obstbau 2022. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, X.; Zhang, Q.; Wu, H.; Yan, D.; Liu, Y.; Zhu, X.; Ren, X.; Yang, Y. Carotenoid accumulation and gene expression in fruit skins of three differently colored persimmon cultivars during fruit growth and ripening. Sci. Hortic. -Amst. 2019, 248, 282–290. [Google Scholar] [CrossRef]
- Homnava, A.U.O.G.; Payne, J.; Koehler, P.; Eitenmiller, R. Characterization of changes during ripening of oriental persimmon. J. Food Qual. 1991, 14, 425–434. [Google Scholar] [CrossRef]
- Luan, Y.; Fu, X.; Lu, P.; Grierson, D.; Xu, C. Molecular mechanisms determining the differential accumulation of carotenoids in plant species and varieties. Crit. Rev. Plant Sci. 2020, 39, 125–139. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melendez-Martinez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel Carotenoid-Derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.W.; Lincoln, R.E. 1. Lysopersicon selections containing a high content of carotenes and colorless polyenes. 2. The mechanism of carotene biosynthesis. Arch. Biochem. 1950, 27, 390–403. [Google Scholar]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Ou, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, B.; Ma, Y.; Xu, W.; Wu, H.; Wang, S. Carotenoid accumulation and expression of carotenoid biosynthesis genes in mango flesh during fruit development and ripening. Sci. Hortic. -Amst. 2018, 237, 201–206. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, S.; Xu, M.; Niu, Y.; Nasier, M.; Fan, G.; Quan, S.; Zhang, S.; Wang, Y.; Liao, K. Identification of key genes controlling carotenoid metabolism during apricot fruit development by integrating metabolic phenotypes and gene expression profiles. J. Agric. Food Chem. 2021, 69, 9472–9483. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef]
- Blas, A.L.; Ming, R.; Liu, Z.; Veatch, O.J.; Paull, R.E.; Moore, P.H.; Yu, Q. Cloning of the papaya Chromoplast-Specific lycopene β-Cyclase, CpCYC-b, controlling fruit flesh color reveals conserved microsynteny and a recombination hot spot. Plant Physiol. 2010, 152, 2013–2022. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, K.; Sun, Q.; Zhang, W.; Wang, X.; Cao, H.; Tan, M.; Xie, Z.; Zeng, Y.; Ye, J.; et al. Natural variation in CCD4 promoter underpins Species-Specific evolution of red coloration in citrus peel. Mol. Plant 2019, 12, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Xu, K.; Luo, Y.; Chen, J.; Sheng, L.; Wang, J.; Han, J.; Zeng, Y.; Xu, J.; Chen, J.; et al. Distinct carotenoid and flavonoid accumulation in a spontaneous mutant of ponkan (Citrus reticulata blanco) results in yellowish fruit and enhanced postharvest resistance. J. Agric. Food Chem. 2015, 63, 8601–8614. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Xiao, Q.; Huang, X.; Li, C.; Gao, X.; Wang, Q.; Xiang, X.; Zhu, Y.; Wang, J.; et al. Carotenoids modulate kernel texture in maize by influencing amyloplast envelope integrity. Nat. Commun. 2020, 11, 5346. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Sun, P.; Suo, Y.; Han, W.; Diao, S.; Mai, Y.; Li, F.; Fu, J. Effects of Plant Growth Regulators, Soil Moisture Contents, and Carbon/Nitrogen Ratios on Sex Differentiation in Persimmon (Diospyros kaki Thunb.) Flowers. J. Plant Growth Regul. 2021, 40, 1121–1138. [Google Scholar] [CrossRef]
- Suo, Y.; Sun, P.; Cheng, H.; Han, W.; Diao, S.; Li, H.; Mai, Y.; Zhao, X.; Li, F.; Fu, J. A high-quality chromosomal genome assembly of Diospyros oleifera Cheng. GigaScience 2020, 9, giz164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Yin, Y.; Tan, G.; Wang, G.; Liu, H.; Zhuang, J.; Zhang, J.; Zhuang, F.; Xiong, A. Advances in engineering the production of the natural red pigment lycopene: A systematic review from a biotechnology perspective. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Shahid, M.Q.; Zhao, C.; Wang, M.; Bai, Y.; He, Y.; Lin, S.; Yang, X. Transcriptional analysis for the difference in carotenoids accumulation in flesh and peel of white-fleshed loquat fruit. PLoS ONE 2020, 15, e233631. [Google Scholar] [CrossRef]
- Heng, Z.; Sheng, O.; Huang, W.; Zhang, S.; Fernie, A.R.; Motorykin, I.; Kong, Q.; Yi, G.; Yan, S. Integrated proteomic and metabolomic analysis suggests high rates of glycolysis are likely required to support high carotenoid accumulation in the banana pulp. Food Chem. 2019, 297, 125016. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Karrer, P.; Morf, R.; Krauss, V.E.; Zubrys, A. Pflanzenfarbstoffe XXXIX. Vermischte beobachtungen über carotinoide (α-carotin, zeaxanthin, carotinoide aus kaki_x0002_früchten). Helv. Chim. Acta 1932, 15, 490–493. [Google Scholar] [CrossRef]
- Novillo, P.; Besada, C.; Tian, L.; Bermejo, A.; Salvador, A. Nutritional composition of ten persimmon cultivars in the “Ready-to-Eat crisp” stage. Effect of deastringency treatment. Food Nutr. Sci. 2015, 6, 1296–1306. [Google Scholar] [CrossRef]
- Brossard, J.; Mackinney, G. Fruit pigments, carotenoids of Diospyros kaki (Japanese persimmons). J. Agric. Food Chem. 1963, 11, 501–503. [Google Scholar] [CrossRef]
- Jeanagross, G.E. Carotenoid changes in the peel of ripening persimmon (Diospyros kaki) cv triumph. Phytochemistry 1984, 24, 29–32. [Google Scholar]
- Cheng, J.; Miller, B.; Balbuena, E.; Eroglu, A. Lycopene protects against Smoking-Induced lung cancer by inducing base excision repair. Antioxidants 2020, 9, 643. [Google Scholar] [CrossRef]
- Baranska, M.; Schutze, W.; Schulz, H. Determination of lycopene and beta-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Polinati, R.M.; Teodoro, A.J.; Correa, M.G.; Casanova, F.A.; Passos, C.; Silva, J.L.; Fialho, E. Effects of lycopene from guava (Psidium guajava L.) derived products on breast cancer cells. Nat. Prod. Res. 2022, 36, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, Q.; Jin, X.; Han, F.; Huang, X.; Cai, S.; Yang, L. A spontaneous bud mutant that causes lycopene and β-carotene accumulation in the juice sacs of the parental Guanxi pummelo fruits (Citrus grandis (L.) Osbeck). Sci. Hortic.-Amst. 2016, 198, 379–384. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, Y.; Zhou, P.; Fatima, M.; Lin, J.; Yue, J.; Zhang, X.; Chen, L.; Ming, R. Papaya CpbHLH1/2 regulate carotenoid biosynthesis-related genes during papaya fruit ripening. Hortic. Res. 2019, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, J.; Li, J.; Yang, C.; Wang, T.; Ouyang, B.; Li, H.; Giovannoni, J.; Ye, Z. A STAY-GREEN protein SlSGR1 regulates lycopene and β-carotene accumulation by interacting directly withSlPSY 1 during ripening processes in tomato. New Phytol. 2013, 198, 442–452. [Google Scholar] [CrossRef]
- Alquézar, B.; Zacarías, L.; Rodrigo, M.J. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Zhang, R.; Khadr, A.; Tian, Y.; Xu, Z.; Xiong, A. Transcript profiling of genes involved in carotenoid biosynthesis among three carrot cultivars with various taproot colors. Protoplasma 2020, 257, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, H.; Guo, S.; Ren, Y.; Li, M.; Wang, J.; Zhang, H.; Gong, G.; Xu, Y. Decreased protein abundance of lycopeneβ-Cyclase contributes to red flesh in domesticated watermelon. Plant Physiol. 2020, 183, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, P.K.; Beyer, P.; Wünn, J.; Klöti, A.; Armstrong, G.A.; Schledz, M.; Lintig, J.; Potrykus, I. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin a biosynthesis. Plant J. Cell Mol. Biol. 1997, 11, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G. Plant carotenoids: Genomics meets multi-gene engineering. Curr. Opin. Plant Biol. 2017, 19, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, Y.; Hu, H.; Wang, C.; Zhang, W.; Li, H.; Cheng, Z.; Yang, L. Functional validation of phytoene synthase and lycopene epsilon-Cyclase genes for high lycopene content in autumn olive fruit (Elaeagnus umbellata). J. Agric. Food Chem. 2020, 68, 11503–11511. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Fraser, M.R.T.C. Carotenoid Biosynthesis during Tomato Fruit Development. Plant Physiol. 1994, 105, 405–413. [Google Scholar]
- Pecker, I.; Gabbay, R.; Cunningham, F.J.; Hirschberg, J. Cloning and characterization of the cDNA for lycopene beta-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Mol. Biol. 1996, 30, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Chen, J.; Shen, H.; Yang, W. Genetics of flesh color and nucleotide sequence analysis of phytoene synthase gene 1 in a yellow-fruited tomato accession PI114490. Sci. Hortic. -Amst. 2008, 118, 20–24. [Google Scholar] [CrossRef]
- Ma, C.; Ma, B.; He, J.; Hao, Q.; Lu, X.; Wang, L. Regulation of carotenoid content in tomato by silencing of lycopene β/ε-Cyclase genes. Plant Mol. Biol. Rep. 2011, 29, 117–124. [Google Scholar] [CrossRef]
- Li, M.; Gan, Z.; Cui, Y.; Shi, C.; Shi, X. Cloning and characterization of the -Carotene desaturase gene from Chlorella protothecoides CS-41. J. Biomed. Biotechnol. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Yan, P.; Gao, X.Z.; Shen, W.T.; Zhou, P. Cloning and expression analysis of phytoene desaturase and zeta-carotene desaturase genes in Carica papaya. Mol. Biol. Rep. 2011, 38, 785–791. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Mai, Y.; Wang, Y.; Yuan, J.; Suo, Y.; Li, H.; Han, W.; Sun, P.; Diao, S.; Fu, J. Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits. Agronomy 2022, 12, 2688. https://doi.org/10.3390/agronomy12112688
Ye L, Mai Y, Wang Y, Yuan J, Suo Y, Li H, Han W, Sun P, Diao S, Fu J. Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits. Agronomy. 2022; 12(11):2688. https://doi.org/10.3390/agronomy12112688
Chicago/Turabian StyleYe, Lingshuai, Yini Mai, Yiru Wang, Jiaying Yuan, Yujing Suo, Huawei Li, Weijuan Han, Peng Sun, Songfeng Diao, and Jianmin Fu. 2022. "Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits" Agronomy 12, no. 11: 2688. https://doi.org/10.3390/agronomy12112688
APA StyleYe, L., Mai, Y., Wang, Y., Yuan, J., Suo, Y., Li, H., Han, W., Sun, P., Diao, S., & Fu, J. (2022). Metabolome and Transcriptome Analysis Reveal the Accumulation Mechanism of Carotenoids and the Causes of Color Differences in Persimmon (Diospyros kaki Thunb.) Fruits. Agronomy, 12(11), 2688. https://doi.org/10.3390/agronomy12112688






