Abstract
Apples are one of the most popular crops in the world, grown for fresh consumption, processing, and ornamental purposes. In the present study, the seeds of thirty crab apple (Malus spp.) genotypes were analyzed to evaluate the tocopherol composition and find a crop-specific profile. The mean proportion (%) of tocopherol (T) homologues (α, β, γ, and δ) was as follows: α-T (45.8%), β-T (21.8%), γ-T (24.3%), δ-T (8.1%) with a mean content of 22.41, 10.89, 12.35, and 4.08 mg/100 g dry weight, respectively. The coefficient of variation was higher in γ-T (0.748), δ-T (0.648) and β-T (0.540), and about two times lower for α-T (0.320). The total content of tocopherols varied much less in studied genotypes (coefficient of variation 0.164). α-T was the predominant tocopherol homologue in twenty-four genotypes (33.4–79.0%), while γ-T (36.4–64.9%) was the predominant in the remaining six studied genotypes. Principal component analysis identified six groups based on the tocopherol profile. Variety, purpose (ornamental vs. edible), and species appear to be associated with tocopherol profile. Most Malus sp., M. × prunifolia, and edible genotypes were located in two groups characterized by twice the content of α-T over β-T, and similar content of both (α-T and β-T), respectively. In both cases the sum of α-T and β-T constituted about 80% of total tocopherols. Significant correlations among tocopherol homologues were obtained: positive between α-T vs. β-T and γ-T vs. δ-T, and negative between α-T vs. γ-T, α-T vs. δ-T, and γ-T vs. β-T. These can be explained by the biosynthetic pathway of those lipophilic bioactive compounds.
1. Introduction
Apples are one of the most popular crops in the world, being the third most produced fruit, with the total worldwide production amounting to over 86 million tons in 2020 [1]. According to a publication from 2013, more than 30,000 apple varieties exist in the world [2] and new cultivars, generally distinguished into dessert apples, cider apples, and crab apples, are registered every year. By-products of apple trees and apples can become an important source of bioactive constituents, like phloridzin and flavonoids. Their content is affected by many factors, such as genotype, locality of cultivation, time of harvesting, fruit part, and treatment [3,4]. Further, crab apples can be divided into two types: ornamental and edible. Edible crab apples are mainly processed for preserves, juice, and cider, while ornamental apples may have fruits that are too small, too astringent or not as juicy. Malus domestica Borkh includes dessert and cider apples, while crab apples belong to several species and are often interspecific crosses [5]. A large number of different cultivars determines the broad variability of the quality attributes of apples [6,7]. Since the breeding process takes a long time and involves many steps, it is important to involve different process streamlining tools [7].
The term “tocochromanols” is often used for a group of lipophilic antioxidants composed of a chromanol ring with different structures and shorter or longer fully saturated or partly saturated side-chains. Tocochromanols include such bioactive compounds as tocopherols, tocotrienols, plastochromanol-8, and other rare prenyllipids [8]. The profile of tocochromanols in seeds and grains is affected by several factors, such as genotype, time of harvest, climatic conditions, and cultivation [9,10,11]. Tocopherols have a role in many relevant physiological processes in seeds and plants; for instance, germination, growth, leaf senescence, response to abiotic stresses, antioxidant function, and export of photoassimilates. Several physiological processes, such as plant responses to biotic stresses and flowering, are still not well understood. Nevertheless, under certain conditions tocopherols can be particularly important in plants by activating alternative defense mechanisms [12]. Due to tocochromanol functions in plants, some studies have demonstrated that those lipophilic molecules possess chemotaxonomic value in some families and can play a major role in taxonomic studies [13,14]. Other studies show that tocopherols are suitable as a plant functional trait biomarker and are useful for monitoring the physiological response of plants to, for instance, stress [15]. On the other hand, positive and negative correlations have been observed between specific tocopherol homologues in different oak species [16], which can be explained by the biosynthetic pathways of those bioactive compounds. Despite significant knowledge about tocochromanol functions, several issues associated with those lipophilic molecules are not completely clear [12,15].
Despite the large number of developed apple genotypes/varieties/cultivars, few studies, most of them only partly, have discussed the topic of tocochromanols in apple seeds. Moreover, only three, six, twelve, one, and one apple variety, respectively, were analyzed [17,18,19,20,21]; mainly M. domestica was investigated. In a study of seven crab apple genotypes [17], four tocopherol homologues (α, β, γ, and δ) were found in apple seeds and their proportions were dependent on apple cultivar [17]. The effect of different rootstocks on phenolic content and antioxidant activity in apples was investigated [22]. The impact of rootstock on the tocochromanol profile in apple seeds was not investigated. In the present study, we aimed to investigate the distribution of tocopherol homologues in seeds of crab apples (Malus spp.) to examine the possible relation between rootstock, species, their purpose (ornamental and edible), and tocopherol profile. We evaluated the tocopherol homologue composition in seeds of 30 genotypes used for different purposes (ornamental and edible) and of several species and hybrids. The results of this study represent the first large-scale screening of tocopherol homologues (α, β, γ and δ) in crab apple seeds.
2. Materials and Methods
2.1. Plant Material
The seeds of 30 crab apple genotypes were obtained from fruits collected in September-November 2013 at the Institute of Horticulture in Dobele, Latvia (GPS location: N: 56°36′39″ E: 23°17′50″). The climate of the location is among the warmest in Latvia, but rather unstable with temperatures below −30 °C in winter every 5–10 years and frequent thaws in other years. The vegetation season with temperatures above 5 °C is 198 days. The sum of temperatures over 10 °C is between 2000–2100 °C. The summer temperatures may exceed 30 °C. Average annual precipitation is relatively low at 581 mm. In 2013 (January-December), the average air temperature was 7.3 °C, the sum of precipitation (rain) 606.5 mm, and the average humidity 81.2%. Detailed meteorological conditions during each month of the 2013 year, including average air temperature (°C), precipitation (rain) (mm), and average humidity (%), are provided in the Supplementary Materials (Table S1). Apple flowering takes place from beginning to end of May depending on year. The apple harvest takes place from end of July to 1st or 2nd day of October (first autumn frosts). The soil of the orchard site is sandy loam, sodium carbonate gleyic, with organic matter 2.3%. Soil pHKCl is 6.7, content of phosphorus (P2O5) 207 mg kg−1, potassium (K2O) 255 mg kg−1, magnesium (Mg) 230 mg kg−1.
Each genotype was represented by three biological replicates obtained from 1–3 apple trees grafted on different rootstocks (Table 1). In the case of one apple tree, crab apples were shared in three groups/replications. Fully ripe apples were harvested. The seeds were separated from apple flesh and cores, frozen, and freeze-dried (FreeZone, Labconco, Kansas City, MO, USA) for 24 h. Dry seeds (1–5 g) were milled with an A 11 basic analytical mill (IKA, Staufen, Germany) to obtain a powder with mesh size ≤ 0.5 mm. Powdered samples were extracted immediately after milling. Dry weight basis (dw) in studied samples was measured gravimetrically.

Table 1.
Detailed information about studied crab apples (Malus spp.).
2.2. Solvents, Reagents and Standards
All solvents (HPLC grade) and reagents (analytical grade) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Standards of four tocopherol homologues with purity ≥ 95% were provided by Merck (Darmstadt, Germany).
2.3. Sample Preparation for Tocopherols Analysis
Crab apple seed samples were prepared according to the previous micro-saponification and extraction protocols [17].
2.4. Determination of Tocopherols via Reverse Phase High Performance Liquid Chromatography with Fluorescence Detector (RP-Hplc/FLD)
The chromatographic separation was carried out using PFP column (3 μm, 150 × 4.6 mm) protected with the guard column (3 μm, 4 × 3 mm) (Phenomenex, Torrance, CA, USA) on a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan). All chromatographic conditions were in accordance with a previously validated and reported method [23].
2.5. Statistical Analysis
The results of all measurements were obtained at the turn of 2013/2014. The results for each genotype were presented as means (n = 3) of the three independent measurements from each biological replication of seeds. The p-value < 0.05 was used to denote significant differences between mean values determined using one-way analysis of variance (ANOVA). The Bonferroni post-hoc test was used to denote statistically significant values at p < 0.05. A multivariate statistical analysis of the mean values obtained for the Malus spp. (n = 30) and four variables (n = 4)—four tocopherol homologues (α, β, γ, and δ)—was performed using principal component analysis (PCA). The ANOVA and unequal n Tukey test were used to find statistically significant differences among groups at p < 0.05 assigned by the PCA of content proportions of tocopherol homologues (%). All statistical analyses were performed with the assistance of Statistica 10.0 (StatSoft, Tulsa, OK, USA) software.
3. Results and Discussion
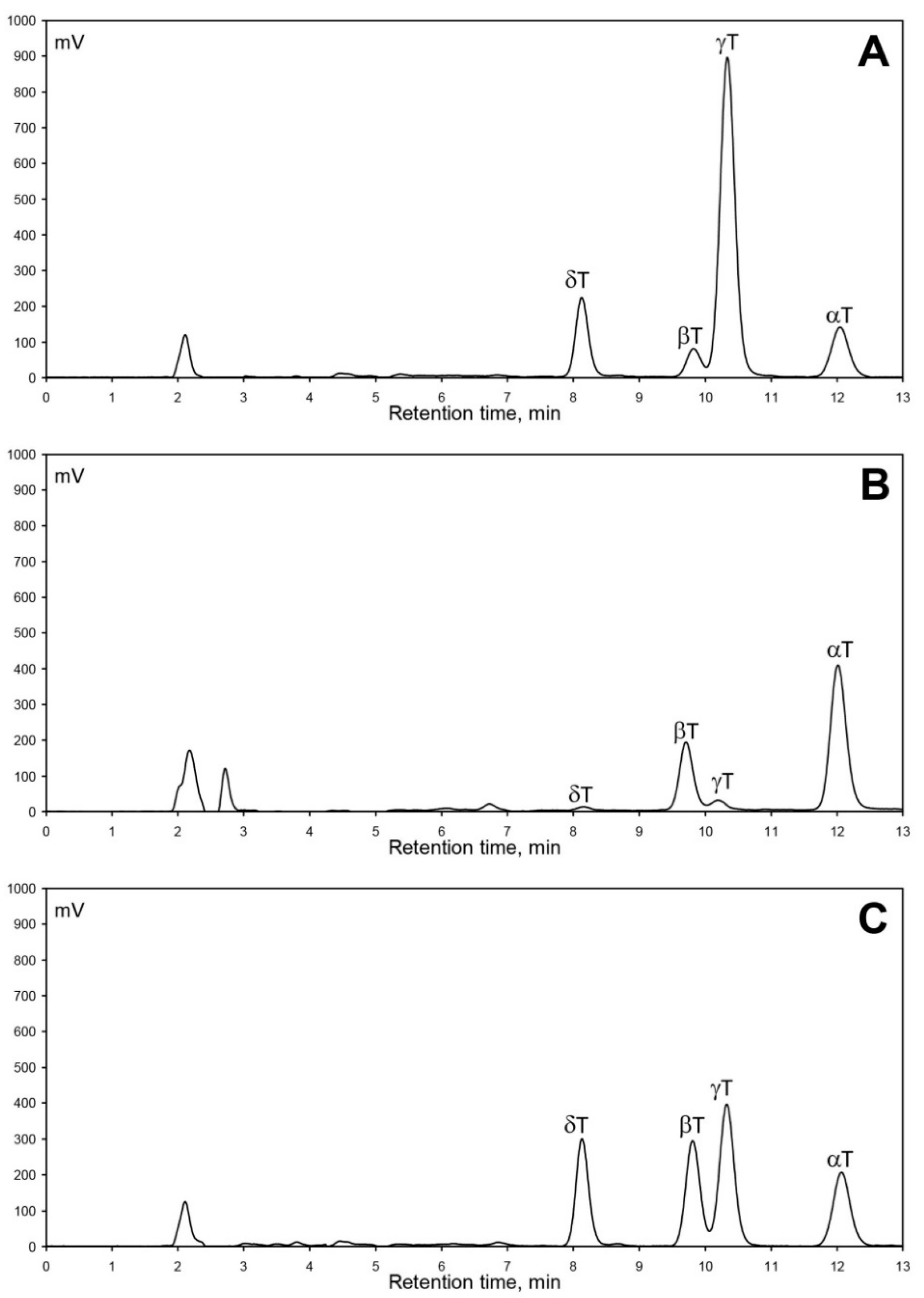
Generally, the predominance of a specific tocopherol homologue, usually α-T or γ-T, is characteristic for a specific plant in Rosaceae fruit crops. Cultivar/variety/genotype [24,25,26,27], development stage [10], harvest year (abiotic factors) [11], or even various species for the specific fruit crop [24] do not affect the predominant tocopherol characteristic for this specific plant. The situation is completely different in apples, as can be seen in Figure 1A–C, which presents obtained RP-HPLC/FLD chromatograms of tocochromanol separation in seeds of three selected crab apple varieties characterized by a different tocopherol homologue proportion—‘Sarkanlapu Krebs’ (A), H-17-05-1 (B), and ‘Pundurkrebs’ (C). Analyzed crab apple genotypes have diverse geographical origins and include advanced modern cultivars, selected hybrids, and landraces; with different pedigrees and biological and growing properties (Table 1). Cultivars from six countries of origin have been included in this study (Estonia, Latvia, Russia, Sweden, Japan, and United States), along with two widely planted cultivars of uncertain origin, ensuring high genetic diversity. Several of them have high scab resistance, while not showing the presence of the wide-spread Rvi6 gene when investigated with molecular markers (data not shown). Genotypes with different tree structure (standard and columnar), different fruit use (ornamental and edible), and leaf color were analyzed to evaluate the content of tocopherol homologues and to find a crop-specific profile. In the thirty crab apple genotypes, all four tocopherol homologues were detected and quantified.
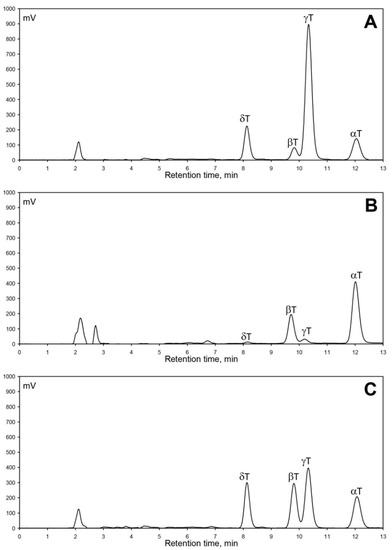
Figure 1.
Chromatograms of four tocopherols (α-T, β-T, γ-T, and δ-T) separated by RP-HPLC/FLD in seeds of three crab apple varieties: ‘Sarkanlapu Krebs’ (A); H-17-05-1 (B); ‘Pundurkrebs’ (C).
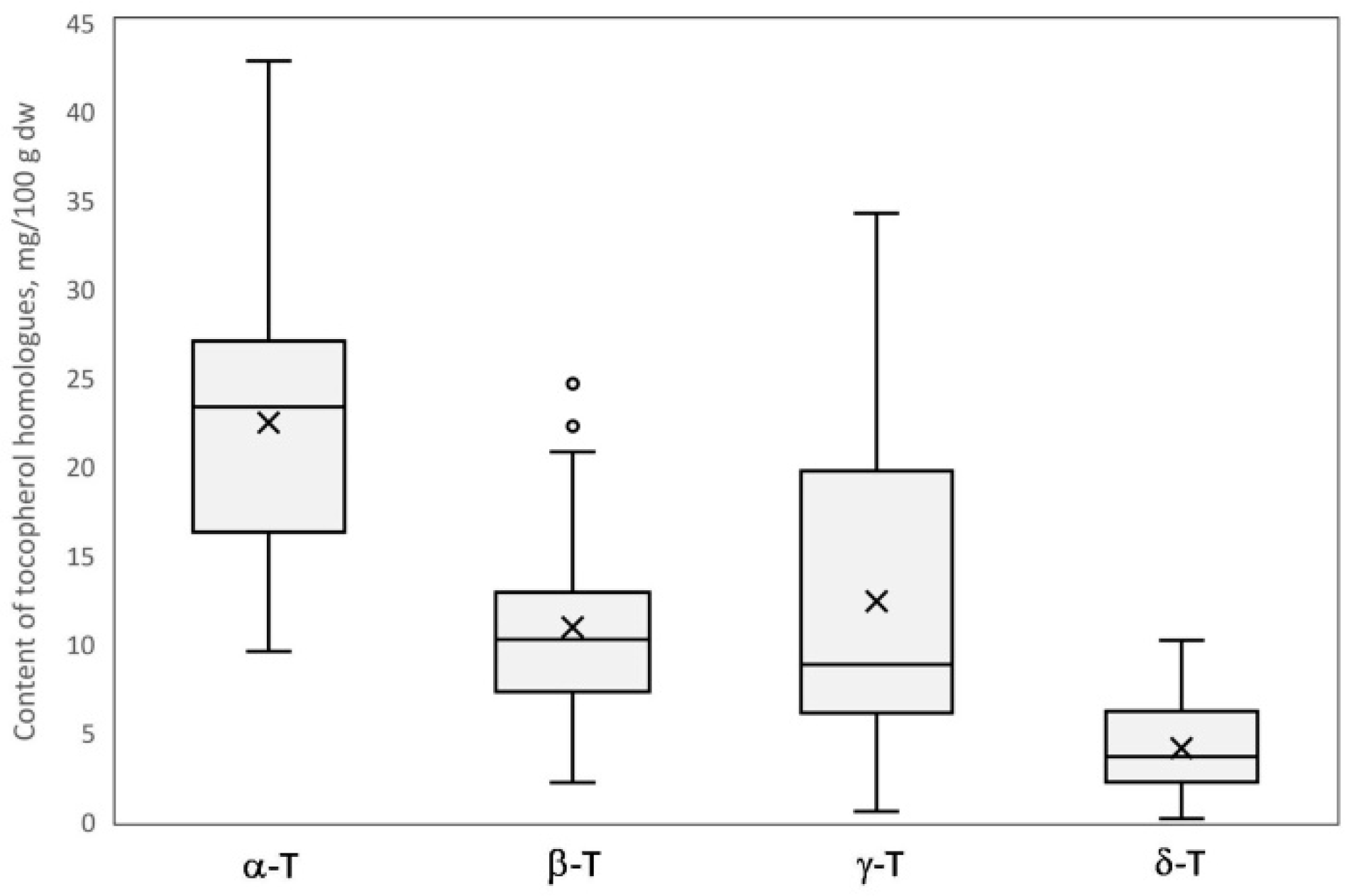
The content of individual tocopherol homologues (α, β, γ, and δ) as well as the total value was statistically and significantly different (p < 0.05) between investigated genotypes (Table 2); it showed a wide range of values and high variability among tested samples, best illustrated in a box-plot (Figure 2). Obtained values for the coefficient of variation showed high diversity in the content of γ-T (0.748), δ-T (0.648), and β-T (0.540), and was about two times lower for α-T (0.320). The total tocopherol content was much less varied and more stable in the studied genotypes (coefficient of variation 0.164). The total content of tocopherols ranged from 36.62 to 68.12 mg/100 g dw for genotype H-17-05-5 and ‘Purpura Vēlais Krebs’, respectively. A similar range of concentration of the lowest bound and about 25% lower of the upper bound was reported previously in seven crab apple genotypes [17]. The mean proportion (%) of tocopherol homologues was as follows: α-T (45.8%), β-T (21.8%), γ-T (24.3%), and δ-T (8.1%) represented by mean content 22.41, 10.89, 12.35, and 4.08 mg/100 g dry weight, respectively. Generally, α-T was a predominating homologue in twenty-four genotypes (80% of investigated samples) and constituted from 33.4 to 79.0% of total tocopherols, while in the remaining six studied genotypes (20% of investigated samples) γ-T was the predominant tocopherol, constituting 36.4 to 64.9%. In previous studies α-T was found to be a predominant tocopherol in all seven crab apple genotypes [17], while the predomination of γ-T in some genotypes is reported in the present study for the first time. Previously, a predominance of α-T or β-T has been reported, depending on the variety, in dessert and cider apples [17,18]; previous reports have analyzed a relatively low number of apple varieties—four, six, twelve, one, and one [17,18,19,20,21]. In contrast to apples, the impact of variety on the characteristically dominant tocopherol homologue for the individual crop, mainly α-T or γ-T, has not been observed in other pome fruits, such as quince, Japanese quince, and pears [11,18,26] or the Prunus genus [25,27,28,29]. The lowest content of α-T and β-T was found for cv. ‘Purpura Ābele’ (9.53 and 2.14 mg/100 g dw, respectively), which at the same time was characterized by the highest content of γ-T (34.22 mg/100 g dw). The highest content of α-T was found for cv. ‘Purpura Vēlais Krebs’ (42.78 mg/100 g dw) while β-T was highest in ‘Dzeltenais Saldais Krebs’ and VK-P-1 (24.61 and 24.74 mg/100 g dw, respectively). Those two genotypes, as well as cv. ‘Riku’ with β-T content 22.21 mg/100 g dw, were marked as outliers in the box-plot (Figure 2). The lowest level of γ-T and δ-T was found for cv. ‘Sarkanais Vēlais Krebs’ (0.52 and 0.11 mg/100 g dw, respectively). The highest level of δ-T was found for cv. ‘Tumšsarkanais Krebs’ (10.16 mg/100 g dw), which was over two, three, and four times lower in comparison to the highest levels noted for β-T, γ-T, and α-T, respectively.

Table 2.
Tocopherol homologue profile in different crab apple seeds (Malus spp.).
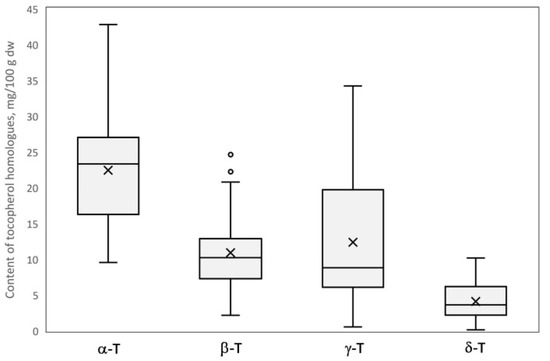
Figure 2.
The box-plot of the content of tocopherol homologues (α, β, γ, and δ) in thirty crab apple genotypes.
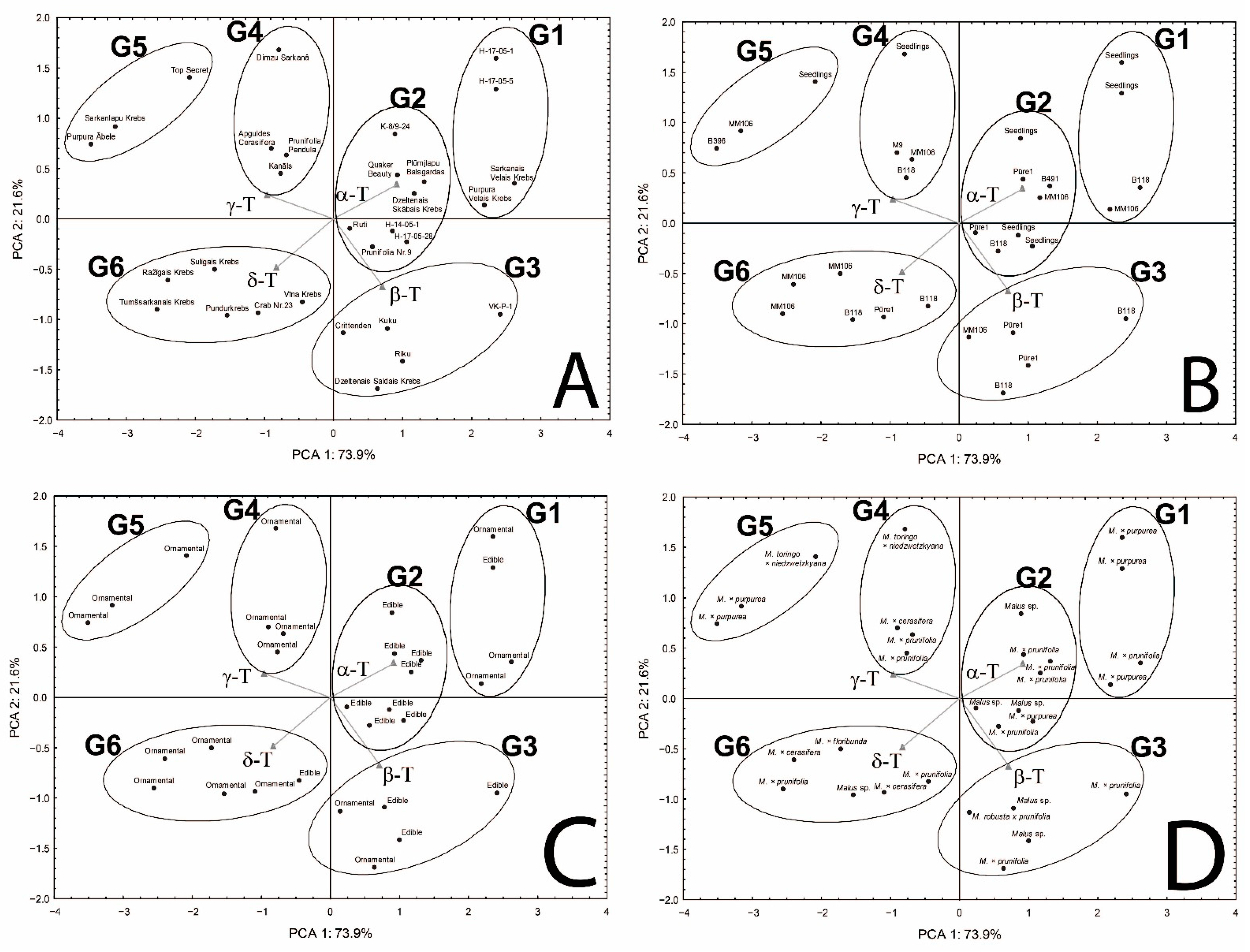
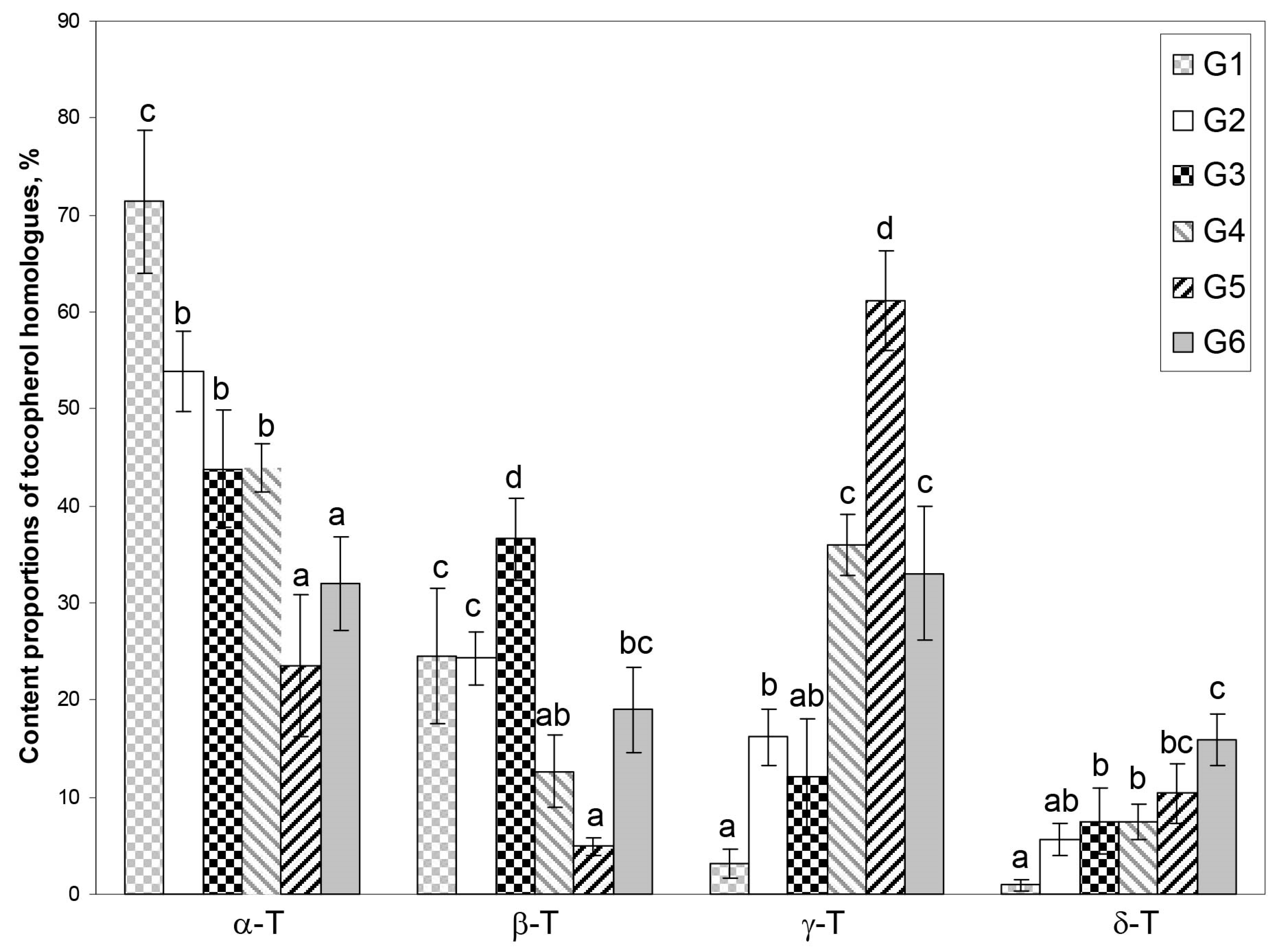
Principal component analysis (PCA) was applied to reveal potential groups depending on the tocopherol homologue content (mg/100 g dw) as well as homologue proportion (%) and the possibility of their association with several factors (rootstock, species, and purpose). The results of PCA showed that the first two principal components (PC1 × PC2) explain 88.2% and 95.5% of the information in the original data for tocopherol homologue content and proportion (%), respectively (Figure S1—Supplementary Material and Figure 3, respectively). It was chosen for further analysis since the tocopherol homologue proportion (%) has higher data loading and the tocopherol concentration in seeds of the same species is affected by maturity, genotype, and abiotic factors, while the proportion of tocopherol homologues does not change significantly; for instance, in Chaenomeles japonica [10,11]. PC1 has high loads for four tocopherol homologues (α-T and β-T—positive correlation, and γ-T and δ-T—negative correlation), whereas PC2 is moderately correlated with β-T and δ-T (negative correlation for both). According to PCA, crab apple genotypes could be classified into six groups (G1–G6) characterized by different proportions of four tocopherol homologues in sample (Figure 3A–D and Figure S2—Supplementary Material). Group 1 (G1) includes four genotypes characterized by the predominance of α-T, nearly three times lower content of β-T compared to α-T and very low content of γ-T and δ-T, which gives average proportions 71.37, 24.56, 3.14, and 0.92%, and coefficient of variation 0.104, 0.283, 0.472, and 0.672, for α, β, γ, and δ, respectively. Group 2 (G2) includes twice as many genotypes as in G1, also characterized by the predominance of α-T, but with about 20% lower content and similar content of β-T and about five times higher γ-T and δ-T content in comparison to G2, which gives average proportions 53.87, 24.28, 16.20, and 5.65%, and coefficient of variation 0.076, 0.114, 0.179, and 0.299, for α, β, γ, δ, respectively. Group 3 (G3) includes five genotypes characterized also by the predominance of α-T; however, its content is lower than in G2 and more similar to β-T, while γ-T and δ-T contents were slightly lower and higher, respectively, in comparison to G2, and gives average proportions 43.83, 36.55, 12.09, and 7.54%, and coefficient of variation 0.140, 0.114, 0.492, and 0.443, for α, β, γ, and δ, respectively. In Group 4 (G4), four genotypes were characterized by nearly the same content of α-T (dominant homologue), and δ-T as genotypes in G3, while β-T and γ-T have mirrored content compared to genotypes in G3, which gives average proportions 43.89, 12.65, 35.98, and 7.49%, and coefficient of variation 0.056, 0.297, 0.086, and 0.237, for α, β, γ, δ, respectively. Group 5 (G5) includes three genotypes and was characterized by the predominance of γ-T. The contents of α-T and β-T were about two times lower, while γ-T and δ-T were higher than in G4, and gives average proportions 23.53, 4.90, 61.20, and 10.37%, and a coefficient of variation 0.307, 0.188, 0.084, and 0.293, for α, β, γ, δ, respectively. Group 6 (G6) includes six genotypes with the most evenly distributed composition of all tocopherol homologues with slight dominance of α-T over γ-T and vice versa (three genotypes of each). The contents of β-T and δ-T were lower by 1/3 and 1/2 in comparison to α-T and γ-T characterized by similar contents, respectively, which gives average proportions 32.06, 19.02, 33.06, and 15.86%, and coefficient of variation 0.150, 0.231, 0.208, and 0.165, for α, β, γ, and δ, respectively. To find a potential association between the tocopherol profile and plant specification, the names of genotypes in each group were replaced by the name of rootstock (3B), purpose of crab apples (ornamental or edible) (3C), and species (3D). As can be seen in Figure 3B, there was no association between the tocopherol profile with the rootstock (3B). In each group, various rootstocks are present. When crab apples were classified by purpose, three groups formed: G4 and G5 were represented only by ornamental genotypes, while G2 was represented by edible ones. G1 and G6 were characterized by the domination of ornamental genotypes with only one exception of edible genotype in each of those groups. G3 contains three edible and two ornamental genotypes. Classification by species of crab apples among the groups was not as successful as classification by crab apple purpose. However, groups G2 and G3, which contained mainly edible genotypes, were represented mainly by the group Malus sp. (complex hybrids) and M. prunifolia, with only one exception of M. purpurea in G2 (Figure 3D). It seems that the purposes of crab apples (ornamental or edible) and species are partly associated with the tocopherol profile; a relation with edible types of crab apples and the group Malus sp. and M. prunifolia can be seen (Table 1, Figure 3C,D and Figure S2—Supplementary Material). ANOVA and unequal n Tukey tests for genotype groups identified in PCA were performed to evaluate their reliability. Statistically significant differences (p < 0.05) can be seen in part of the groups (Figure 4).
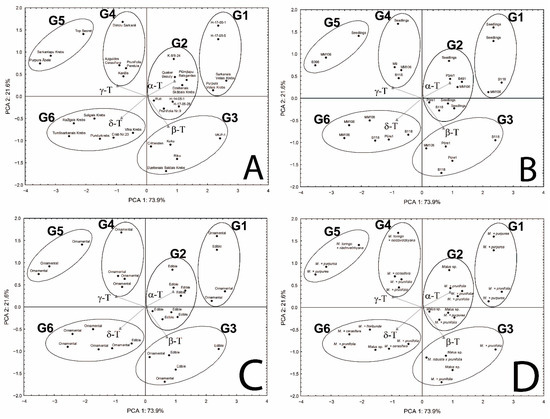
Figure 3.
Distribution of study samples based on the principal component analysis (PCA) according to content proportions of tocopherol homologues (α, β, γ, and δ) (%) with different indicators: genotype (A), rootstock (B), purposes of crab apples (ornamental and edible) (C), and species (D).
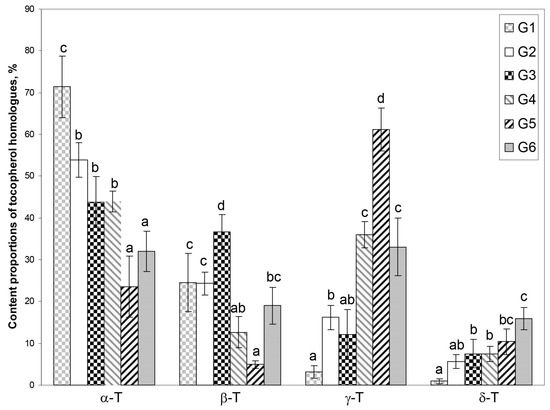
Figure 4.
The content proportions of tocopherol homologues (α, β, γ, and δ) (%) in groups (G1–G6) assigned by the PCA. Statistically significant differences among groups were assigned by ANOVA and unequal n Tukey test (p < 0.05).
Results of tocopherol analysis discovered a close correlation between the content (mg/100 g dw) and proportion (%) of particular homologues in analyzed samples (Figure S3—Supplementary Material). In both cases, there were positive correlations between α-T vs. β-T, and γ-T vs. δ-T and negative correlations between α-T vs. δ-T, α-T vs. γ-T, γ-T vs. β-T, and β-T vs. δ-T. The correlation between β-T and δ-T was statistically insignificant (p > 0.05). Similar correlations between tocopherol homologues were found in Quercus rubra and Quercus robur acorns [16]. Based on the present and previous study [16], positive correlations between γ-T vs. δ-T and α-T vs. β-T, and negative correlations between α-T vs. δ-T, α-T vs. γ-T, β-T vs. γ-T, and β-T vs. δ-T can be explained by the biosynthesis pathways of tocopherols. Tocopherol cyclase (TC) cyclizes 2-methyl-6-phytyl-1,4-benzoquinol (MPBQ) and 2,3-dimethyl-6-phytyl-1,4-benzoquinol (DMPBQ—product of methylation MPBQ by a methyltransferase) to produce δ-T and γ-T, respectively, while δ-T and γ-T are precursors of β-T and α-T, respectively, catalyzed by the γ-tocopherol methyltransferase (γ-TMT) [30,31]. Since δ-T and γ-T are primary products and are at the same time substrates for further biosynthesis of β-T and α-T (final products) catalyzed by the same enzyme, positive (primary-primary or secondary-secondary product) and negative (primary-secondary products) correlations between specific tocopherol homologues can be explained by their biosynthesis pathways.
4. Conclusions
The content and proportions of individual tocopherol homologues had higher variability than the total content of tocopherols in thirty studied crab apple genotypes. The vast majority (80%) of studied genotypes were predominated by α homologue while the remaining 20% were predominated by γ. PCA revealed six groups characterized by specific tocopherol profiles, where the tocopherol profile appears to be at least partly associated with the purpose of the crab apples (ornamental or edible) and their species. Significant correlations between tocopherol homologues were observed—positive α-T vs. β-T and γ-T vs. δ-T, and negative α-T vs. γ-T, α-T vs. δ-T, and γ-T vs. β-T. These relationships can be explained by the underlying pathways of compound biosynthesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy12112736/s1: Table S1: The meteo conditions; average air temperature (°C), precipitation (rain) (mm), and humidity (%), during the 2013 year (January–December).; Figure S1: Distribution of study samples based in principal component analysis (PCA) according content of tocopherol homologues (α, β, γ, and δ) (mg/100 g dw); Figure S2: Proportion (%) of four tocopherol homologues in crab apple seeds (Malus spp.) of thirty samples characterized by high genetic diversity; Figure S3: Correlation between the individual homologues of tocopherol expressed as tocopherol content (mg/100 g dw) (A–F) and proportion (%) (G–L) in crab apple seeds.
Author Contributions
P.G.: Investigation, Resources, Formal analysis, Validation, Data Curation, Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing, Software, Visualization, Supervision, Funding acquisition; I.M.: Formal analysis; L.I.: Writing—Original Draft, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, project “Dicotyledonous plant families and green tools as a promising alternative approach to increase the accessibility of tocotrienols from unconventional sources”, project No. lzp-2020/1-0422.
Data Availability Statement
The data used to support the findings of this study are available in Supplementary Material and from the corresponding author upon request.
Conflicts of Interest
All other authors declare no conflict of interest.
References
- FAOSTAT FAO Statistical Database. Available online: http://www.fao.org (accessed on 12 April 2022).
- Strohm, K. Of the 30,000 Apple Varieties Found All Over the World only 30 Are Used and Traded Commercially. Available online: http://www.agribenchmark.org (accessed on 12 April 2022).
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Chen, P.; Li, Z.; Zhang, D.; Shen, W.; Xie, Y.; Zhang, J.; Jiang, L.; Li, X.; Shen, X.; Geng, D. Insights into the effect of human civilization on Malus evolution and domestication. Plant Biotechnol. J. 2021, 19, 2206–2220. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; Tillman, J.; Luby, J.; Evans, K.; Musacchi, S. Characterization of a novel S-RNase allele and genotyping of new apple cultivars. Sci. Hortic. 2020, 273, 109630. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Novel and rare prenyllipids—Occurrence and biological activity. Plant Physiol. Biochem. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Orsák, M.; Popov, M.; Martinek, P. Tocotrienols and tocopherols in colored-grain wheat, tritordeum and barley. Food Chem. 2018, 240, 725–735. [Google Scholar] [CrossRef]
- Mišina, I.; Sipeniece, E.; Rudzińska, M.; Grygier, A.; Radzimirska-Graczyk, M.; Kaufmane, E.; Segliņa, D.; Lācis, G.; Górnaś, P. Associations between oil yield and profile of fatty acids, sterols, squalene, carotenoids, and tocopherols in seed oil of selected Japanese quince genotypes during fruit development. Eur. J. Lipid Sci. Technol. 2020, 122, 1900386. [Google Scholar] [CrossRef]
- Sipeniece, E.; Mišina, I.; Grygier, A.; Qian, Y.; Rudzińska, M.; Kaufmane, E.; Segliņa, D.; Siger, A.; Górnaś, P. Impact of the harvest year of three cultivars of Japanese quince (Chaenomeles japonica) on the oil content and its composition. Sci. Hortic. 2021, 275, 109683. [Google Scholar] [CrossRef]
- Falk, J.; Munne-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Goffman, F.D.; Thies, W.; Velasco, L. Chemotaxonomic value of tocopherols in Brassicaceae. Phytochemistry 1999, 50, 793–798. [Google Scholar] [CrossRef]
- De Agostini, A.; Cogoni, A.; Cortis, P.; Vacca, A.; Becerril, J.M.; Hernández, A.; Esteban, R. Heavy metal tolerance strategies in metallicolous and non-metallicolous populations of mosses: Insights of γ+β-tocopherol regulatory role. Environ. Exp. Bot. 2022, 194, 104738. [Google Scholar] [CrossRef]
- Górnaś, P. Oak Quercus rubra L. and Quercus robur L. acorns as an unconventional source of gamma- and beta-tocopherol. Eur. Food Res. Technol. 2019, 245, 257–261. [Google Scholar] [CrossRef]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Fromm, M.; Bayha, S.; Kammerer, D.R.; Carle, R. Identification and quantitation of carotenoids and tocopherols in seed oils recovered from different rosaceae species. J. Agric. Food Chem. 2012, 60, 10733–10742. [Google Scholar] [CrossRef]
- Arain, S.; Sherazi, S.T.H.; Bhanger, M.I.; Memon, N.; Mahesar, S.A.; Rajput, M.T. Prospects of fatty acid profile and bioactive composition from lipid seeds for the discrimination of apple varieties with the application of chemometrics. Grasas Aceites 2012, 63, 175–183. [Google Scholar]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 2015, 659541. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; Suárez Valles, B. Characterization of apple seeds and their oils from the cider-making industry. Eur. Food Res. Technol. 2018, 244, 1821–1827. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Abukauskas, V.; Janulis, V.; Kviklys, D. Phenolic content and antioxidant activity in apples of the ‘Galaval’ cultivar grown on 17 different rootstocks. Antioxidants 2022, 11, 266. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Lācis, G.; Radenkovs, V.; Olšteine, A.; Segliņa, D.; Kaufmane, E.; Rubauskis, E. Composition of tocochromanols in the kernels recovered from plum pits: The impact of the varieties and species on the potential utility value for industrial application. Eur. Food Res. Technol. 2015, 241, 513–520. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Soliven, A.; Kaufmane, E.; Segliņa, D. Tocochromanols composition in kernels recovered from different apricot varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Nat. Prod. Res. 2015, 29, 1222–1227. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lāce, B.; Lācis, G.; Segliņa, D. Tocochromanols composition in seeds recovered from different pear cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. LWT-Food Sci. Technol. 2015, 62, 104–107. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Ruisa, S.; Rubauskis, E.; Lācis, G.; Segliņa, D. Composition of tocochromanols in kernels recovered from different sweet cherry (Prunus avium L.) cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur. Food Res. Technol. 2015, 240, 663–667. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzinska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Lācis, G.; Seglina, D. Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J. Agric. Food Chem. 2016, 64, 898–905. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Soliven, A.; Segliņa, D. Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind. Crops Prod. 2016, 82, 44–50. [Google Scholar] [CrossRef]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).