Abstract
Pineapple cultivation in nitrogen deficient and acidic peat soils leads to poor growth, yield, and fruit quality of pineapples. A study was conducted to determine whether clinoptilolite zeolite (CZ) could improve soil nitrogen availability, growth, yield, and fruit quality of pineapples grown in drained peat soils. Laboratory leaching experiments were conducted to determine the effectiveness of CZ in controlling nitrogen loss from peat soils, whereas an ion-exchange resin method was used to determine nitrogen availability in pineapple cultivation. Treatments evaluated were: (i) different amounts of CZ (25, 50, 70, and 100%) + NPK fertilizer, (ii) NPK fertilizer, and (iii) peat soil only. The peat soils with CZ reduced ammonium and nitrate losses because of the sorption of ammonium within the lattices of the CZ via ion exchange. Co-application of CZ (25%) and NPK fertilizers was more effective in increasing soil ammonium availability, whereas the use of CZ (25% to 100%) improved nitrogen uptake and use efficiency, growth, yield, and fruit quality of pineapple because CZ could regulate the availability of nitrogen ions for pineapple uptake. The buffering capacity of CZ increased soil pH and facilitated organic nitrogen mineralization. The co-application of CZ and NPK fertilizers can be used to improve nitrogen availability and pineapple productivity in tropical peat soils.
Keywords:
ammonium; natural zeolite; nitrate; nutrient leaching; organic soil; pineapple; soil amendments 1. Introduction
Tropical peat soils are one of the major groups of problem soils in the world, including in Malaysia. Tropical peat soils in Malaysia cover approximately 2.6 million hectares and constitute 8% of the total land area in Malaysia [1,2]. The peat soils are regarded as one of the marginal soils in Class 4 with severe limitations [3]. The limitations dictate the types of crops for cultivation. Several agronomic challenges are encountered in developing and managing tropical peatlands. Some of the challenges are high acidity, low nutrient content, irreversible drying, waterlogging conditions, poor root anchorage, low bearing capacity, and mechanization difficulties [4,5].
Tropical peat soil is defined as an organic soil containing a minimum of 65% fibrous organic matter by weight, but has less than a 35% mineral content in which the thickness of the accumulated organic soil matter is between 40 cm to 50 cm in the upper 100 cm of the soil profile [6,7,8,9]. Although agronomic and crop production technologies (for example, fertilizer needs, water management, and crop performance) have made it possible to develop tropical peatlands for agriculture, low nutrient (macro- and micronutrients) levels in available forms and the poor nutrient retention capacity of peat soils are some of the persistent problems in peatland management [10,11,12,13]. Moreover, crop productivity decreases with the increasing time of using peat soils for agriculture because the oligotrophic tropical peat soils are low in nutrients [14]. Thus, frequent and higher fertilization is required to maintain crop yield, but nutrient concentrations are affected because of the high leaching of nutrients in peat soils [15,16]. Currently, approximately 1.3 million hectares of the peatland in Malaysia have been cultivated with oil palm, sago, coconut, rubber, pineapple, and mixed horticulture [17,18].
Pineapple (Ananas comosus L. Merr) is an important cash crop which is cultivated at a commercial scale in peat soils in Malaysia because it is economically rewarding [19]. Pineapple cultivation in peat soils was estimated at 12,665 ha with a total fresh fruit production of 325,038 metric tons in 2021 [20]. Although Malaysia was once a major pineapple producer in the world (from 1960 to 1970), the country’s pineapple production has been declining since 1972. The reduction in pineapple production could be attributed to land conversion to other premium crops, namely, palm oil and rubber [21,22]. However, the nematode Paratylenchus species infestation is the main cause for the reduction in pineapple crop yield in peat soils in Malaysia [23] besides the diminishing quality of peat soil, which is attributed to the prolonged use for pineapple cultivation [24]. Nevertheless, pineapple remains an economic fruit for export and continues to contribute significantly to Malaysia’s economic growth and development. The export value for Malaysia’s pineapple production is approximately USD 6.08 million annually [25], suggesting that the contribution of the pineapple industry to the economy of Malaysia, especially for the livelihood of pineapple growers, cannot be ignored. Thus, there is a need to improve the productivity of peat soils in which this cash crop is grown.
Pineapples require a regular nutrient supply because nutrition plays a significant role in the growth and development of pineapple plants, yield, and fruit quality [26,27]. Nitrogen (N) in the pineapple plants’ nutrition is important to ensure a high growth rate of pineapple plants and the production of good fruit yields. Additionally, N plays an important role in plant photosynthesis and overall plant health [27]. Nitrogen is required in low amounts during the early vegetative phase of pineapple plants. However, the N supply is increased at four months after planting until the flower induction stage to ensure optimum growth and the production of good pineapple fruit yields. The recommended fertilizer rates for pineapples grown in peat soils are the Bordeaux mixture (foliar fertilization: 50 to 100 mL/plant) and compound 30:1:32 (N:P2O5:K2O: 20 g/plant) fertilizers [28]. The Bordeaux mixture is a foliar fertilizer containing 42 g of copper sulfate, 21 g of iron sulfate, 42 g of zinc sulfate, and 640 g of lime. This foliar fertilizer is applied to pineapple plants at 1.5 and 4.5 months after planting to reduce micronutrient deficiency that is a common problem in peat soils, whereas compound NPK fertilizer is applied thrice at three, six, and nine months after pineapple planting. Nitrogen deficiency in pineapple is commonly characterized by slow growth and colorful fruit with a small crown [27]. The nutritional status of pineapple plants is influenced by the peat soil nutrient content, soil–water movement, and root system formation [27]. Hence, to ensure N availability in tropical peat soils, particularly where nutrient loss through leaching is high, it is essential to ensure optimum pineapple growth and productivity through the balanced use of fertilizers.
Total N in tropical peat soils is high and it ranges from 1% to 2.1% [29]. However, most of the N is unavailable and remains in the organic form, such as a lignoprotein. Moreover, only a small amount of N is mineralized because of the high C:N ratio and acidity of peat soils. This causes low inorganic N uptake unless a large amount of N fertilizer is applied [2,13]. Furthermore, a substantial amount of mineralized N is lost through leaching [16]. Additionally, the availability of N for pineapple plants is affected by temperature, moisture, aeration, and acidity [4]. Unlike potassium ions, ammonium ions do not leach rapidly because they are attracted to the negatively charged soil colloids of peat soils [30], but ammonium ions are rapidly converted to nitrate because they are relatively unstable in oxygen-rich peat soils [31,32]. Nitrates are soluble but they are not easily adsorbed in peat soils because nitrate ions are negatively charged, and thus, they are easily leached from the root zone of plants [31,33,34]. However, the concentration of nitrate ions are significantly high in drained cultivated peat soils because the oxidation of peat increases the nitrification of ammonium ions [35]. Although pineapple requires a regular N supply [36], it is not advisable to apply high levels of N fertilizers to build up ammonium and nitrate ions in drained peat soils because this nutrient leaches, especially under high rainfall. Because of the specific functions of N in pineapple productivity, N should not be only supplied in sufficient quantity, but it should also be applied in synchrony with plant nutrient uptake to obtain maximum yield and fruit quality [4,37]. Thus, to improve the N availability of peat soils, the ion-exchange properties of natural zeolite, particularly CZ, could be utilized in the fertilization scheme of pineapple cultivation in drained tropical peat soils.
Besides vermiculite, alkaline fly ash, and lime, natural zeolites, such as CZ, are one of the inorganic amendments that have an extensive application in agriculture [38,39,40]. Natural zeolites are inexpensive because of their abundant availability and their annual production is approximately 1 to 1.3 million tons [41,42]. Several studies have successfully demonstrated the effectiveness of CZ in improving soil physicochemical properties such as water-holding capacity, acidity, nutrient retention, uptake, and use efficiency, particularly of N, potassium (K), and phosphorus (P) in acid, sandy, and clayey soils [43,44,45,46], and significantly increased crop yields for barley, maize, potatoes, rice, strawberries, sunflowers, wheat, and vegetables [40,47,48,49,50]. The effectiveness of CZ in improving soil and crop productivity relates to its unique, open, three-dimensional structure made up of hydrated aluminosilicate with interconnected pores and voids linked by shared oxygen atoms that provides a large, internal, microporous area for cation exchange, ion adsorption, and catalytic activity [51,52,53]. These important characteristics of CZ enable it to be a medium for releasing nutrients in addition to serving as a nutrient carrier which selectively controls the types of ions to be retained or passed through its surface [43,46,54]. In Malaysia, the selective nutrient sorption (adsorption and desorption) and ion-exchange properties of natural zeolites were capitalized on by amending mineral acid soils with CZ to improve soil pH, N, K, and P availability, and improve yields for lowland rice (cv. MR219) and maize (Zea mays L.) cultivation [55,56,57,58,59,60]. Although the use of CZ as a soil amendment in acid, sandy, and poor clayey soils is well established, information on the use of natural zeolites in drained tropical peat soils, particularly for pineapple cultivation, is still lacking. Moreover, there are limited data on the optimization of the use of CZ, the time and frequency of application, and the exact technique of applying CZ to improve the structure and the fertility of drained peat soils where pineapples are grown.
Based on the aforementioned rationale, the first objective of the study was to determine the effects of CZ on retaining and reducing the leaching of ammonium and nitrate in a tropical peat soil which is cultivated with Moris pineapple. The second objective of the study was to determine the effects of CZ on the N uptake and use efficiency, growth performance, fresh fruit yield, and quality of pineapple fruits. Moris is one of the popular varieties which is cultivated in Malaysia. Moris is widely cultivated for domestic consumption because it is not susceptible to pests, diseases, and stress compared with other pineapple varieties, but it is also an important cultivar for export [61]. In this present study, we hypothesized that CZ would reduce the leaching of ammonium and nitrate and that this process would increase the amount of exchangeable ammonium and available nitrate in drained peat soils to ensure their optimum uptake and use efficiency. This hypothesis is based on the assumption that the selective nutrient retention and high cation-exchange properties of CZ will enable timely sorption (adsorption and desorption) of ammonium and nitrate via ion-exchange and physical-sorption mechanisms. Additionally, CZ is expected to increase pineapple growth, fresh yield, and fruit quality through improved N uptake. This assumption is based on the buffering capacity of CZ reputed for reducing peat soil acidity in addition to improving N availability. To test the afore-stated hypothesis, laboratory leaching experiments were conducted to determine losses of ammonium and nitrate at different soil depths throughout the wet and dry seasons. Additionally, a field study using the ion-exchange resin method was carried out to determine if the co-application of compound NPK fertilizers and CZ could improve soil exchangeable ammonium and the available nitrate, growth, fruit yield, and fruit quality of Moris pineapple cultivation in a sapric peat soil. Information obtained from this study could provide insights on the use of CZ as a potential cost-effective soil amendment for improving N use efficiency, pineapple productivity, and fertilizer management in drained tropical peat soils.
2. Materials and Methods
2.1. Experimental Site Description
The field study was conducted from December 2016 to March 2018 at the Malaysian Agricultural Research and Development Institute (MARDI) Peat Research Station at Saratok, Sarawak, Malaysia (latitude 1°55′30.9″ N and longitude 111°14′15.1″ E). The peatland at the research station was heavily logged for high-value timber species (Hopea and Shorea) from 1970 to 1990. The area has a flat topography from 5 m to 6 m above mean sea level. The soil at the experimental site is classified as highly decomposed sapric peat (von Post Scale: H7 to H9), consisting of amorphous material with a scarcely indistinguishable plant structure. The sapric peat is dark brown with a thickness ranging from 0.5 m to 3 m. The peat soil sporadically overlies a clayey substratum, which is sulfidic in nature. The research area receives an annual mean rainfall of 3923 mm, whereas the monthly rainfall distribution exhibits an extreme wet period between November and January (450 mm to 514 mm) and a dry period in July (172 mm). The mean relative humidity and mean temperature at the experimental site ranges from 55.2% to 60.1% and 22.8 °C to 32.5 °C, respectively, throughout the year.
Before setting up the field experiment in 2016, the study area (one hectare) was planted with Bentong ginger (Zingiber officinale Roscoe) and Moris pineapple from 2012 to 2015, but thereafter, it was left to lie fallow for approximately one and a half years. In December 2016, land clearing was carried out using the felling and burying method. This technique involves the felling of trees and shrubs using a hydraulic excavator, after which plant debris are cut into appropriate sizes using a chain saw and stacked in dug-out pits, and finally, are buried at a minimum depth of two meters. The water table depth ranges from 29 cm to 38 cm at the experimental area.
2.2. Characterization of Physical and Chemical Properties of Peat Soil
The physical and chemical properties of the peat soil, such as bulk density, water-holding capacity, moisture, pH, electrical conductivity, cation-exchange capacity (CEC), total organic carbon, total N, exchangeable ammonium, available nitrate, available P, and exchangeable K, were determined. Peat soil sampling was performed systematically at depths from 0 cm to 20 cm, 20 cm to 40 cm, and 40 cm to 60 cm, at 16 points over a 20 m × 20 m grid. The collected soil samples were air-dried at room temperature for seven days, after which they were ground and sieved to pass a 2 mm sieve. Soil bulk density was determined using the core method [62], whereas soil water-holding capacity was determined using the method of Dugan et al. [63]. Soil moisture was determined using the gravimetric method [62], whereas soil pH and electrical conductivity were measured based on a 1:5 soil to water suspension [64]. Soil CEC and total organic carbon were determined using the Harada and Inoko [65] and Walkley and Black [66] methods, respectively. Soil total N was measured using the Kjeldahl method [67], whereas the steam distillation method was utilized to determine exchangeable ammonium and available nitrate [68]. Soil available P was determined using the method of Olsen and Sommers [69], whereas exchangeable K was measured using the method of Knudsen et al. [70].
2.3. Characterization of Clinoptilolite Zeolite
The CZ used in this present study was greyish white and in powder form. It was imported from Indonesia, where it is produced at a commercial scale. The CZ was not subjected to any chemical pretreatment prior to the laboratory and field studies. The pH of the CZ was measured based on a 1:2 zeolite to water suspension [71], whereas the cesium chloride method was used to determine CZ’s CEC [72]. A Fourier Transform Infrared Spectrometer (FTIR) (Nicolet 6700, Thermo Electron Corporation, Madison, WI, USA) was used to identify functional groups in CZ, whereas an Ultra-High Resolution Scanning Electron Microscope (FESEM) with an Energy-Dispersive X-Ray (EDX) (Nova NanoSEM 230, Fei Company, Hillsboro, OR, USA) was used to determine CZ’s elemental and morphological characteristics. The surface area of the CZ was determined using a surface area analyzer (ASAP 2460, Micromeritics Instrument Corp, Norcross, GA, USA) through a N desorption isotherm. The Brunauer–Emmet–Teller (BET) model was used to determine their surface area.
2.4. Laboratory Leaching Experiment
Soil column experiments were used to determine exchangeable ammonium and available nitrate leaching [72]. The laboratory soil leaching experiments were designed to simulate the dry and wet seasons of the research station (MARDI Saratok). Each season consisted of six treatments with three replicates and the completely randomized design (CRD) was used as the experimental design in this leaching study. The details of the treatments evaluated are summarized in Table 1. The treatments comprised different amounts of CZ (25%, 50%, 70%, and 100% of the existing recommended rate of CZ). Amounts of the CZ (5 g, 10 g, 14 g, and 20 g) utilized were computed based on the compound NPK fertilizer requirement of Moris pineapple at the vegetative and fruiting stages cultivated in tropical peat soils [28]. The ratio of the compound N:P2O5:K2O fertilizer used in the leaching study was 30:1:32. The compound fertilizer (100 kg) is a mixture containing ammonium sulfate (72 kg), Christmas Island rock phosphate (CIRP) (1 kg), and muriate of potash (MP) (27 kg). For each treatment (T1 to T5), 20 g of compound NPK 30:1:32 fertilizer was used.

Table 1.
Clinoptilolite zeolite and compound nitrogen, phosphorus, and potassium fertilizer application rates used in the laboratory leaching experiments.
Cylindrical soil columns were fabricated using an acrylic material measuring 90 cm in height and 5 cm in diameter. The base of each column was attached with stainless steel mesh to prevent soil loss. The leaching experiment was performed using peat soils at a 65% moisture content to reduce peat oxidation. Soil columns were assembled by filling the columns with soil according to the following soil depths: 10 cm to 30 cm, 30 cm to 60 cm, and 60 cm to 90 cm. At the depth of 0 cm to 10 cm, the CZ and compound NPK fertilizer were meticulously homogenized with the peat soil based on the information listed in Table 1 (treatments T1 to T5). For each soil column, about 975 g of peat soil was used, after which it was rinsed with a one-tenth pore volume of deionized water before commencing the leaching experiment.
Approximately 586.7 mL and 1175.5 mL of deionized water were used for the dry (9 rainy days in 24 days) and wet (19 rainy days in 30 days) seasons, respectively. The total volume of deionized water used for the leaching experiments was calculated based on nineteen years of rainfall data (1996 to 2015) acquired from an existing weather station (WatchDog 2900ET, Spectrum Technologies Inc, Plainfield, IL, USA) installed at the experimental site. Thereafter, leachates were collected and analyzed for pH, ammonium, and nitrate. The pH of the leachates was determined using a digital pH meter (Cyberscan pH1500, Eutech Instruments Pte Ltd., Singapore), whereas ammonium and nitrate were measured using the method of Keeney and Nelson [73]. Soil samples at 24 and 30 days of the leaching experiment were analyzed for pH, exchangeable ammonium, and available nitrate at depths from 0 cm to 10 cm, 10 cm to 30 cm, 30 cm to 60 cm, and 60 cm to 90 cm using the standard procedures as outlined previously [64,68]. Amounts of ammonium and nitrate leached and retained in the treatment with peat soils only (T6) were subtracted from those of fertilized treatments (T1 to T5) to account for the effects of the CZ and compound NPK fertilizers.
2.5. Field Experimental Design and Nutrient Measurements in a Peat Soil Grown with Pineapples
The laboratory soil leaching treatments, as outlined in Table 1, were also evaluated in the field experiment. However, sampling time and soil depth were included in the field study. The field trial was a 6 × 3 × 3 factorial experiment in a randomized complete block design (RCBD) with three blocks involving: (i) six rates of fertilizer treatments (a mixture of CZ and compound NPK 30:1:32 fertilizer); (ii) a sampling time of 7 days, 15 days, and 30 days after fertilization; and (iii) a soil depth from 0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm. In December 2016, a total of eighteen raised beds were constructed measuring 1 m (width) × 3.5 m (length) × 0.4 m (height), and the distance between each raised bed was 0.5 m. Shallow ditches measuring 0.3 m in width and 0.5 m in depth were constructed between the blocks to avoid interplot contamination and to function as water discharge outlets. Moris suckers were planted in two rows on each raised bed at a planting distance of 30 cm × 60 cm. There was a total of eighteen Moris pineapple plants for each raised bed. The Moris pineapples were planted on raised beds to avoid flooding during the wet monsoon season. Crop care and maintenance of the pineapple plants were carried out using standard agronomic practices for pineapple cultivated in drained tropical peat soils [28]. The water table depth varies between 36 cm and 87 cm at the experimental site throughout the pineapple growth period.
The ion-exchange resin method [74,75] was used to determine the leaching of ammonium and nitrate in the raised beds. Leached nutrients were measured at the aerobic (0 cm to 30 cm) and saturated (0 cm to 60 cm and 0 cm to 90 cm) soil layers, and this was based on the mean water table for pineapples (40 cm to 60 cm). The leached ammonium and nitrate were collected using polyethylene fabric bags containing ion-exchange resins attached to plastic cores that were installed in the raised beds at soil depths from 0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm. The ion-exchange resins used in the field experiment were a mixture of hydrogen and hydroxide-exchange resins (Dowex Marathon MR3) in a ratio of 1:4 ion-exchange resins to acid-washed sand. The cation-anion-exchange resins were not pretreated chemically before use to ensure excellent nutrient recovery. The resin cores were fabricated using high-density polyethylene wire mesh measuring 8 cm in diameter and at heights of 30 cm, 60 cm, and 90 cm to mimic nutrient leaching depths. The diameter of the resin core used in the field study was based on variability of nutrient leaching influenced by the preferential flow of the peat soil water. The resin cores were installed vertically, after which peat soil columns were placed above the resin cores. A total of 162 resin cores were installed in the raised beds prior to each fertilization month.
Fertilization was carried out at three months, six months, and nine months of pineapple growth in March, June, and September 2017, respectively. For this, CZ and compound NPK 30:1:32 fertilizers (20 g/plant) were meticulously mixed according to the treatments (T1 to T5) listed in Table 1. The mixture containing CZ and compound NPK fertilizer (T1 to T5) was applied circularly onto the peat soil, approximately 5 cm from the collar of the pineapple plants. Resin cores were collected every 7 days, 15 days, and 30 days after fertilization. Thereafter, ammonium and nitrate in the mixture (hydrogen-hydroxide resin and sand) were extracted using a 1 M potassium chloride solution [74]. The extracted resins were analyzed for ammonium and nitrate using the method of Keeney and Nelson [73], whereas soil exchangeable ammonium and available nitrate in the resin cores were determined using the steam distillation method [68].
2.6. Growth Performance, Nutrient Uptake and Use Efficiency, Pineapple Fresh Yield, and Fruit Quality
Pineapple vegetative growth parameters, particularly height and leaf area, were recorded at three, six, and nine months after planting. Pineapple height was measured (m) from the base of the stem at ground level to the tip of the tallest leaf of the plant using a measuring tape, whereas leaf area was determined using the grid method [76]. For this, the D-leaves of the pineapple plants were collected because these leaves represent the nutrient movement within the plant and are sensitive to nutrient changes in the soil solution [77]. The D-leaves are the tallest and longest leaves that grow approximately 45° toward the soil surface. Pineapple fresh fruit was harvested at 14 months after planting in February 2018. Harvested pineapple plants were separated into stem, leaf, and root, after which the plant tissues were oven dried (60 °C), ground, and determined for total N using the micro Kjeldahl method [78]. The concentrations of N in leaf, stem, and root were multiplied by the respective dry weight of the plant parts to determine the amount of N taken up by the pineapple plants. Nitrogen use efficiency in pineapple was determined using the following equation [79]:
where A = total N uptake in plants with fertilizer at harvest (kg ha−1). B = total N uptake in plants without fertilizer at harvest (kg ha−1). C = total amount of N fertilizer applied (kg ha−1).
Nitrogen use efficiency (%) = [(A − B)/C] × 100%
Fruit quality characteristics, namely, fresh fruit weight, total soluble solids (TTSs), titratable acidity (TA), and juice pH of the fresh pineapple fruits were also determined. Pineapple fruit yield was calculated based on the mean fruit weight and plant density [80], whereas pineapple fresh fruit weight was determined using a digital weighing balance (EK-15KL, A&D Company, Toshima-ku, Tokyo, Japan) [80]. Pineapple fruits with similar attributes (maturity index 5: 50% ripe) were selected, hand-peeled, cored, sliced, and blended using a food processor [61,80]. Total soluble solids were determined using a handheld refractometer (Atago PAL-1, Spectrum Technologies Inc, Aurora, IL, USA) [61]. Titratable acidity contents were measured using the method of Hajar et al. [81], whereas pineapple fresh fruit juice pH was measured using a pH meter (Laqua PH1200, Horiba Scientific, Kyoto, Tokyo, Japan).
2.7. Statistical Analysis
An analysis of variance (ANOVA) was used to evaluate treatment effects, whereas significant differences between treatment means were compared using Tukey’s new multiple range test with p ≤ 0.05. Statistical Analysis System (SAS) Version 9.1 (SAS Institute Incorporated, Cary, NC, USA) was used for the statistical analysis.
3. Results
3.1. Peat Soil Physical and Chemical Characteristics
Physical and chemical properties of the peat soils at the study site (MARDI Saratok, Sarawak, Malaysia) are presented in Table 2. Results of the peat soil physicochemical characteristics were compared to those reported in the literature for tropical peatlands in Southeast Asia. The peat soil bulk density and water-holding capacity were similar irrespective of soil depth, whereas moisture content increased with increasing depth. Soil pH, electrical conductivity, CEC, total carbon, and available P demonstrated no significant differences with soil depth. Total N, exchangeable ammonium, and available nitrate contents showed significant differences with increasing soil depths, whereas exchangeable K generally decreased with increasing depth. The soil test results for bulk density, moisture content, pH, electrical conductivity, CEC, total carbon, total N, available P, and exchangeable K are within the reported range, whereas exchangeable ammonium and available nitrate contents are higher than those reported in the literature.

Table 2.
Selected physical and chemical properties of a drained peat soil at different soil depths at the Malaysian Agricultural Research and Development Institute (MARDI) Peat Research Station, Saratok, Malaysia.
3.2. Characteristics of Clinoptilolite Zeolite
Selected physicochemical properties of the CZ are presented in Table 3, whereas the functional groups and surface morphology of the natural zeolite are presented in Figure 1 and Figure 2, respectively. Results of the chemical characteristics of the CZ were compared to those reported in the literature [42,52,60,89,90,91,92]. pH and CEC of the CZ were high. Based on the EDX analysis, the chemical composition of the CZ comprised silicone oxide, aluminum oxide, iron oxide, potassium oxide, calcium oxide, magnesium oxide, and sodium oxide. The chemical compositions of magnesium oxide and the silicone oxide to aluminum oxide ratio were within the reported range [42,52,60], whereas silicone oxide, iron oxide, and potassium oxide were higher than the reported range [90]. However, aluminum oxide, calcium oxide, and sodium oxide values were lower than the reported range [90]. The FTIR analysis demonstrates or suggests six functional groups detected for CZ in the wavenumber region of 3400.33 cm−1, 1634.66 cm−1, 922.85 cm−1, 776.99 cm−1, 688.64 cm−1, and 518.17 cm−1 (Figure 1). The surface area of the CZ was low and it is comparable to those reported in the literature for natural zeolites from Indonesia [42,91,92]. From the FESEM micrographs, it was seen that the clinoptilolite zeolite is crystalline with irregular rod-shaped particles and has sharp edges (Figure 2).

Table 3.
Selected chemical and physical properties of clinoptilolite zeolite.
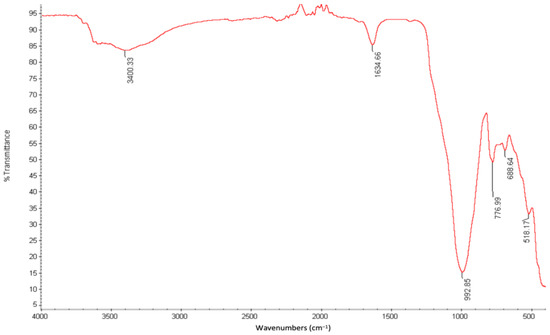
Figure 1.
Fourier Transform Infrared (FTIR) spectrum of clinoptilolite zeolite.
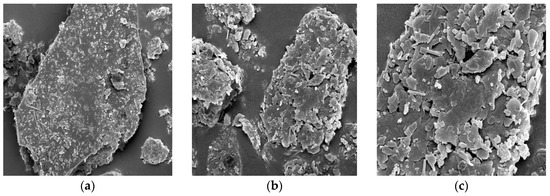
Figure 2.
Ultra-High Resolution Scanning Electron Microscope (FESEM) micrographs of clinoptilolite zeolite at different optical magnifications: (a) 10 μm; (b) 4 μm; and (c) 2 μm.
3.3. Laboratory Soil Leaching Experiment
Ammonium and nitrate losses in the wet and dry seasons are presented in Figure 3. The CZ significantly affected ammonium and nitrate leaching, but the losses of these nutrients differed based on the amount of CZ applied and seasons. In the wet season, CZ-treated peat soil (T4) significantly reduced ammonium loss compared with the NPK fertilization (T5) (Figure 3a). Conversely, all the treatments with CZ were effective in controlling the loss of nitrate compared with the control (T5), except T2 (Figure 3b). In the dry season, T1, T2, and T3 were able to minimize ammonium and nitrate losses compared with NPK fertilization (T5) (Figure 3a,b).
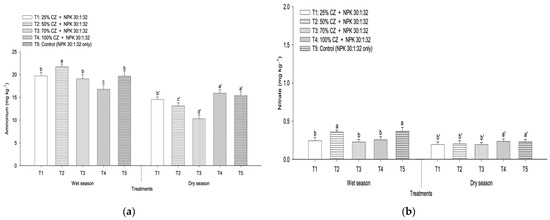
Figure 3.
Total amount of (a) ammonium and (b) nitrate losses in the wet and dry seasons. Means with different letters indicate significant differences between treatments according to Tukey’s test at p ≤ 0.05. Error bars represent standard error of the mean. CZ: clinoptilolite zeolite. Letters without primes represent the wet season and single primes represent the dry season.
At the end of the leaching experiment, the soil exchangeable ammonium ions of T1, T2, T3, and T4 were higher compared with the control (T5) regardless of season (Figure 4a). Likewise, in the wet season, all the treatments with CZ (T1 to T4) improved available nitrate retention compared with NPK fertilization (T5) (Figure 4b), whereas T1, T2, and T3 demonstrated higher nitrate content in the dry season (Figure 4b). Irrespective of season, T4 was more effective in retaining exchangeable ammonium relative to other treatments, including NPK fertilization, whereas T2 demonstrated higher nitrate content (Figure 4a,b).
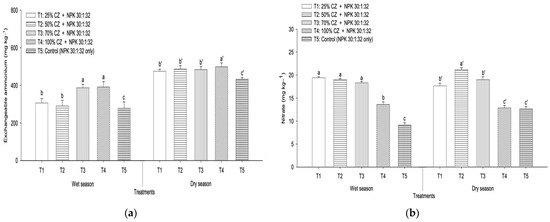
Figure 4.
Effects of clinoptilolite zeolite and nitrogen, phosphorus, and potassium fertilization on mean retention of (a) soil exchangeable ammonium and (b) available nitrate in the wet and dry seasons. Means with different letters indicate significant differences between treatments according to Tukey’s test at p ≤ 0.05. Error bars represent standard error of the mean. CZ: clinoptilolite zeolite. Letters without primes represent the wet season and single primes represent the dry season.
In the wet and dry seasons, exchangeable ammonium and available nitrate distribution in the peat soil varied with treatments and soil depth. Among the treatments (wet season), CZ-treated peat soil (T4) consistently improved the exchangeable ammonium retention, particularly at depths from 0 cm to 10 cm and 10 cm to 30 cm, whereas T3 significantly demonstrated higher exchangeable ammonium at the lower soil depths (30 cm to 60 cm and 60 cm to 90 cm) (Figure 5a). Similarly, in the dry season, T4 consistently improved exchangeable ammonium at depths from 0 cm to 10 cm, 10 cm to 30 cm, and 60 to 90 cm, whereas T1 and T2 demonstrated higher ammonium retention at a depth from 30 cm to 60 cm (Figure 5a).
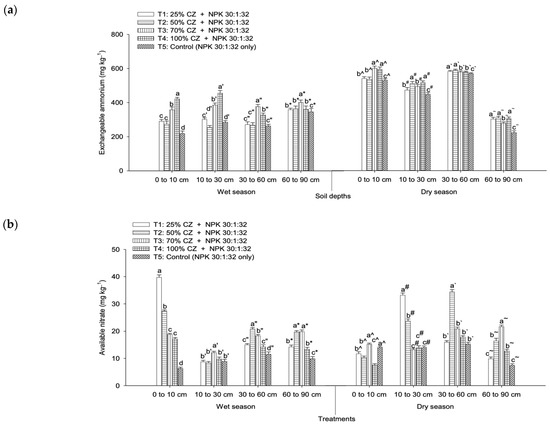
Figure 5.
Effects of clinoptilolite zeolite and nitrogen, phosphorus, and potassium fertilization on the retention of (a) soil exchangeable ammonium and (b) available nitrate with soil depths in the wet and dry seasons. Means with different letters indicate significant differences between treatments according to Tukey’s test at p ≤ 0.05. Error bars represent standard error of the mean. CZ: clinoptilolite zeolite. Letters without primes, single primes, double primes, and asterisks represent the wet season, whereas letters with carets, hashes, backticks, and tildes represent the dry season.
In the wet season, the peat soil with CZ (T2) consistently improved the available nitrate retention at the deeper soil layer (30 cm to 60 cm and 60 cm to 90 cm), whereas T1 and T3 demonstrated a higher nitrate retention at depths from 0 cm to 10 cm and 10 cm to 30 cm, respectively (Figure 5b). On the contrary, in the dry season, all the treatments with CZ (T1 to T4) were not effective in retaining available nitrate at a depth from 0 cm to 10 cm, whereas T1, T2, and T3 demonstrated higher nitrate retention at depths from 10 cm to 30 cm, 30 cm to 60 cm, and 60 cm to 90 cm, respectively (Figure 5b).
Clinoptilolite zeolite-treated peat soils (T1 to T4) significantly improved soil pH relative to NPK fertilization (T5) irrespective of season and soil depth (with exceptions of depths from 10 cm to 30 cm, 30 cm to 60 cm, and 60 cm to 90 cm) (Table 4).

Table 4.
Mean pH of peat soil in the wet and dry seasons.
3.4. Availability of Ammonium and Nitrate in a Tropical Peat Soil Cultivated with Pineapple
Clinoptilolite zeolite application significantly affected ammonium and nitrate leaching, but the losses of these nutrients differed based on the amount of zeolite applied and the vegetative phase of the pineapple plants. Ammonium leaching at three, six, and nine months after planting the pineapple suckers is demonstrated in Figure 6. During the early development of the pineapple plants (three months old), the treatments with CZ were not effective in minimizing ammonium leaching (Figure 6a). However, at six months after planting, the treatment with CZ (T1) demonstrated lower ammonium loss compared with other treatments including NPK fertilization (T5) and non-treated peat soil (T6) (Figure 6a). In the flower induction phase of the pineapple plants (nine months old), the CZ-treated peat soils (T1 and T4) significantly reduced ammonium loss compared with the controls (T5 and T6) (Figure 6a). Throughout the pineapple growth and development, the peat soils with CZ (T1 to T4) were not effective in controlling the loss of ammonium relative to the NPK fertilization (T5) and non-treated peat soil (T6) (Figure 7a).
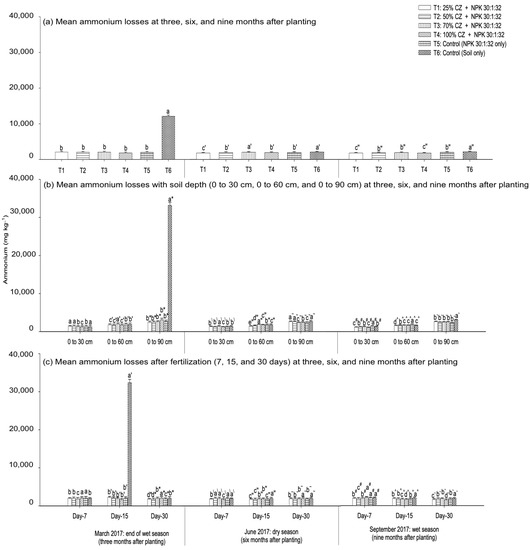
Figure 6.
Amount of ammonium leached from pineapple-cultivated peat soils treated with clinoptilolite zeolite at different vegetative stages (three, six, and nine months after planting): (a) mean amount of ammonium losses, (b) ammonium losses with soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm), and (c) ammonium losses after fertilization (seven, fifteen, and thirty days) throughout the wet (March and September 2017) and dry (June 2017) seasons. Error bars indicate the standard error of the mean (n = 162). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and double primes represent mean ammonium losses respectively at three (March 2017), six (June 2017), and nine (September 2017) months after planting. Letters without primes, single primes, and asterisks represent ammonium losses at the end of the wet season (March 2017), letters with backslashes, double primes, and tildes represent the dry season (June 2017), and letters with hashes, carets, and backticks represent the wet season (September 2017).
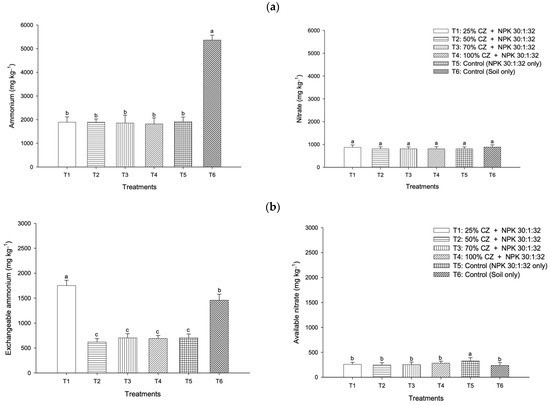
Figure 7.
Mean amount of ammonium and nitrate leached (a) and retained (b) from peat soils treated with clinoptilolite zeolite throughout the pineapple growing season. Error bars indicate the standard error of the mean (n = 486). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite.
During the growth period of the pineapple plants, the amount of ammonium leached from the peat soil varied with treatments, fertilization periods (seven, fifteen, and thirty days after fertilization), and soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm). With the exception of T3, at three and six months after planting the pineapple suckers, compared with NPK fertilization (T5) and non-treated peat soil (T6), the treatments with CZ were effective in controlling ammonium loss at depths from 0 cm to 30 cm (T4) and 0 cm to 60 cm (T1, T2, and T4) (Figure 6b). Similarly, in the flower induction phase of the pineapple plants, all the treatments with CZ (T1 to T4) were able to minimize ammonium leaching at depths from 0 cm to 30 cm and 0 cm to 60 cm compared with the control (T5) (Figure 6b). Regardless of the pineapple growth period, the CZ-treated peat soils (T1 to T4) were not effective in reducing ammonium loss at the saturated depth (0 cm to 90 cm) (Figure 6b). Throughout the pineapple growth phases, the mean ammonium loss (overall treatments: T1 to T6) was higher at depths from 0 cm to 90 cm, but lower from 0 cm to 30 cm and 0 cm to 60 cm (Figure 8a).
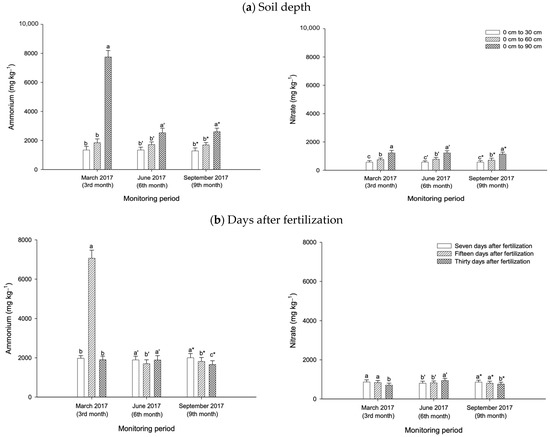
Figure 8.
Mean losses of ammonium and nitrate from peat soils cultivated with pineapple (a) according to soil depth and (b) after fertilization overall treatments (T1 to T6). Error bars indicate the standard error of the mean (n = 486). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and asterisks represent ammonium losses at the end of the wet season.
Compared with the fertilization period, at three months after planting, the CZ-amended peat soils in T1 and T2 consistently demonstrated lower ammonium loss at seven and thirty days after fertilization compared with other treatments including the control (T5) (Figure 6c), whereas all the treatments with CZ were not able to minimize ammonium leaching at day fifteen after fertilization. Similarly, at six months after planting, the CZ treatments (T1 to T4) were not effective in controlling ammonium loss at days fifteen and thirty after fertilization, whereas T1 and T4 demonstrated lower ammonium loss at day seven after fertilization (Figure 6c). In the flower induction phase, T2 demonstrated lower ammonium loss at day seven after fertilization, whereas T4 consistently minimized ammonium loss at days fifteen and thirty after fertilization relative to other treatments including the controls (T5 and T6) (Figure 6c). Additionally, the mean ammonium loss (overall treatments: T1 to T6) was significantly higher at day fifteen after fertilization during the early pineapple growth period, whereas ammonium loss was higher at days seven and thirty after fertilization at six months after planting (Figure 8b). During the flower induction phase, the mean ammonium loss was lower at days fifteen and thirty after fertilization (Figure 8b).
Nitrate leaching at three, six, and nine months after fertilization is presented in Figure 9. During the early growth of the pineapple plants (three months old), the soils with CZ (T2 and T4) significantly reduced nitrate loss compared with the NPK fertilization (T5) and non-treated soil (T6) (Figure 9a). Conversely, at six months after planting the pineapple suckers and during flower induction (nine months old), the peat soils with CZ (T1 to T4) were not effective in controlling the loss of nitrate compared with NPK fertilization (T5) (Figure 9a). Throughout pineapple growth and development, all the treatments with CZ (T1 to T4) were not able to minimize nitrate loss compared with other treatments, including NPK fertilization (T5) and non-treated peat soil (T6) (Figure 7a).
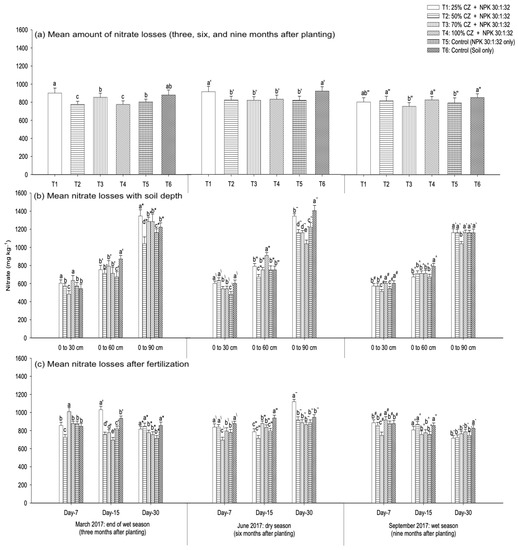
Figure 9.
Amount of nitrate leached from pineapple-cultivated peat soils treated with clinoptilolite zeolite at different vegetative stages (three, six, and nine months after planting): (a) mean amount of nitrate losses, (b) nitrate losses with soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm), and (c) nitrate losses after fertilization (seven, fifteen, and thirty days) throughout the wet (March and September 2017) and dry (June 2017) seasons. Error bars indicate the standard error of the mean (n = 162). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and double primes represent mean nitrate losses respectively at three (March 2017), six (June 2017), and nine (September 2017) months after planting. Letters without primes, single primes, and asterisks represent nitrate losses at the end of the wet season (March 2017), letters with backslashes, double primes, and tildes represent the dry season (June 2017), and letters with hashes, carets, and backticks represent the wet season (September 2017).
Similar to ammonium, the amount of nitrate leached from the peat soil varied with treatments, the fertilization period, and the peat soil depth (throughout the growth phases of the pineapple plants). At three months after planting (March 2017), the treatments with CZ (T3 and T2) minimized nitrate loss compared with the controls (T5 and T6). This observation is true for the aerobic zone (0 cm to 30 cm) (T3) and lower soil depth (0 cm to 90 cm) (T2) (Figure 9b). At six months after planting, T2 and T4 demonstrated lower nitrate leaching compared with NPK fertilization (T5) and non-treated peat soils (T6). This occurred at depths from 0 cm to 60 cm (T2) and 0 cm to 90 cm (T4) (Figure 9b). During the flower induction phase, compared with the controls (T5 and T6), all the treatments with CZ were not effective in controlling nitrate loss regardless of soil depth (Figure 9b). An exception to this finding is the effectiveness of T3 in reducing nitrate loss at the saturated depth (0 cm to 90 cm) (Figure 9b). Irrespective of the pineapple plant growth period, the mean nitrate loss (overall treatments: T1 to T6) was higher at the saturated depth (0 cm to 90 cm), but lower at the aerobic zone (0 cm to 30 cm) (Figure 8a).
Compared with the fertilization period, at three months after planting the suckers, the treatment with CZ (T2) consistently minimized nitrate loss at days seven and fifteen after fertilization (Figure 9c) compared with other treatments, including the controls (T5 and T6), whereas T3 consistently demonstrated lower nitrate loss at day seven after fertilization, six and nine months after planting (Figure 9c). The mean nitrate loss (overall treatments: T1 to T6) was higher at days seven and fifteen after fertilization, but lower at day thirty during the wet season (three and nine months after planting) (Figure 8b). Conversely, at six months after planting, the nitrate loss was higher at day thirty after fertilization but lower at days seven and fifteen (Figure 8b).
3.5. Retention of Soil Exchangeable Ammonium and Available Nitrate in a Tropical Peat Soil Cultivated with Pineapple
Soil exchangeable ammonium availability at three, six, and nine months after planting the pineapple suckers is presented in Figure 10. During the early development of the pineapple plants, that is, at three months after planting (March 2017), the peat soil with CZ (T1) significantly improved the exchangeable ammonium retention compared with NPK fertilization (T5) and non-treated peat soil (T6) (Figure 10a). At six months after planting (June 2017), the treatments with CZ (T1 to T4) demonstrated higher exchangeable ammonium compared to that of NPK-fertilized peat soil (T5) (Figure 10a). However, during the flower induction phase (nine months old, September 2017), the treatments with CZ were not effective in retaining exchangeable ammonium compared with the NPK fertilization (T5) and non-treated peat soil (T6) (Figure 10a). Throughout pineapple growth and development, T1 was more effective in retaining exchangeable ammonium relative to other treatments, including the controls (T5 and T6) (Figure 7b).
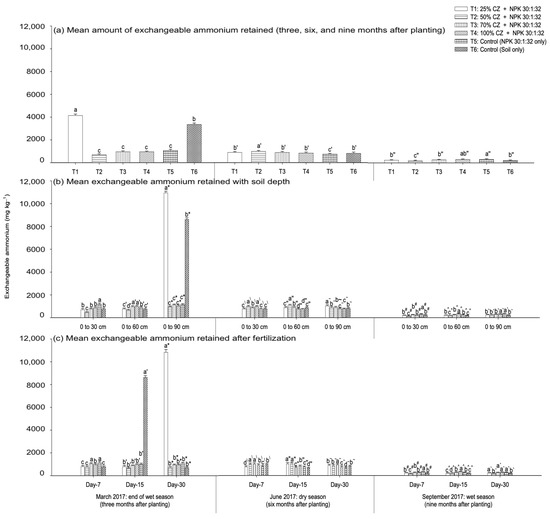
Figure 10.
Effects of clinoptilolite zeolite on soil exchangeable ammonium retention at different vegetative stages (three, six, and nine months after planting): (a) mean amount of ammonium retained, (b) ammonium retention with soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm), and (c) ammonium retention after fertilization (seven, fifteen, and thirty days) throughout the wet (March and September 2017) and dry (June 2017) seasons. Error bars indicate the standard error of the mean (n=162). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and double primes represent mean ammonium retention respectively at three (March 2017), six (June 2017), and nine (September 2017) months after planting. Letters without primes, single primes, and asterisks represent ammonium retention at the end of the wet season (March 2017), letters with backslashes, double primes, and tildes represent the dry season (June 2017), and letters with hashes, carets, and backticks represent the wet season (September 2017).
During the growth period of the pineapple plants, exchangeable ammonium distribution in the soil varied with treatments (T1 to T6), fertilization period (seven, fifteen, and thirty days after fertilization), and soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm). At three months after planting the pineapple suckers, the CZ-treated soils (T3 and T4) demonstrated a higher exchangeable ammonium retention at a depth from 0 cm to 60 cm compared with the controls (T5 and T6), whereas T1 had higher exchangeable ammonium at the lower soil depth (0 cm to 90 cm) (Figure 10b). However, all the treatments with CZ were not effective in improving ammonium retention at the aerobic zone (0 cm to 30 cm) (Figure 10b). Among the treatments (at six months after planting), the peat soils with CZ (T2 and T3) consistently improved the exchangeable ammonium retention, regardless of depth, compared with NPK fertilization (Figure 10b). During the flower induction phase, the CZ-treated soils in T4 demonstrated a higher exchangeable ammonium retention at a depth from 0 cm to 60 cm compared with the controls (T5 and T6), whereas all the treatments with CZ were not effective in improving ammonium retention at the aerobic (0 cm to 30 cm) and saturated (0 cm to 90 cm) depths (Figure 10b). The mean retention of exchangeable ammonium (overall treatments) was similar irrespective of soil depth and pineapple growth period (except at three months) (Figure 11a).
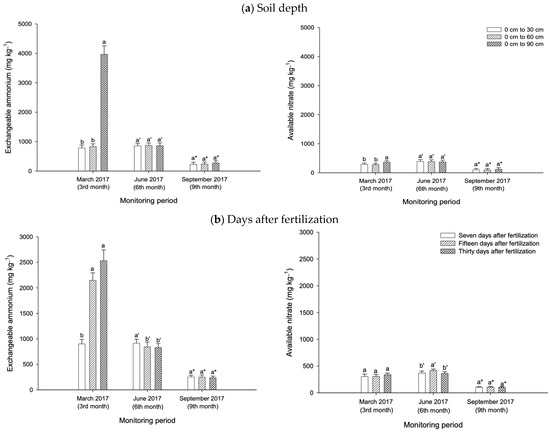
Figure 11.
Mean retention of exchangeable ammonium and available nitrate from peat soils cultivated with pineapple (a) according to soil depth and (b) after fertilization with overall treatments (T1 to T6). Error bars indicate the standard error of the mean (n = 486). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and asterisks represent nitrate retention at the end of the wet season.
Compared with the fertilization period (Figure 10c), the peat soils with CZ (T1) demonstrated a higher exchangeable ammonium retention at day thirty after fertilization during the early vegetative phase of the pineapple plants. At six months after planting the pineapple suckers, T2 consistently improved exchangeable ammonium retention, regardless of the fertilization period, compared with NPK fertilization (T5) (Figure 10c). Irrespective of the fertilization period, all the treatments with CZ were not effective in improving exchangeable ammonium relative to other treatments, including the controls (T5 and T6), during the flower induction phase (Figure 10c). An exception to this finding was the effectiveness of T4, which demonstrated a higher exchangeable ammonium retention at day fifteen after fertilization. The mean retention of soil exchangeable ammonium (overall treatments) was higher at days fifteen and thirty after fertilization during the early growth phases of the pineapple plants (Figure 11b). Conversely, mean ammonium retention was lower at days fifteen and thirty after fertilization at six months after planting the pineapple suckers (Figure 11b). However, the mean soil exchangeable ammonium was similar regardless of fertilization period during the flower induction phase (Figure 11b).
The peat soil available nitrate availability at three, six, and nine months after planting the pineapple suckers is presented in Figure 12.
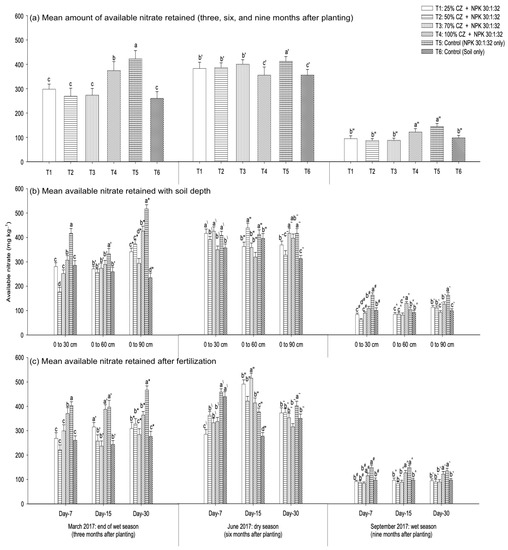
Figure 12.
Effects of clinoptilolite zeolite on soil available nitrate retention at different vegetative stages (three, six, and nine months after planting): (a) mean amount of nitrate retained, (b) nitrate retention with soil depth (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm), and (c) nitrate retention after fertilization (seven, fifteen, and thirty days) throughout the wet (March and September 2017) and dry (June 2017) seasons. Error bars indicate the standard error of the mean (n = 162). Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite. Letters without primes, single primes, and double primes represent mean nitrate retention respectively at three (March 2017), six (June 2017), and nine (September 2017) months after planting. Letters without primes, single primes, and asterisks represent nitrate retention at the end of the wet season (March 2017), letters with backslashes, double primes, and tildes represent the dry season (June 2017), and letters with hashes, carets, and backticks represent the wet season (September 2017).
Compared with the NPK fertilization (T5) and non-treated peat soil (T6), all the treatments with CZ (T1 to T4) were not effective in improving available nitrate retention regardless of the pineapple growth phases (Figure 12a), soil depth (Figure 12b), and fertilization period (Figure 12c). An exception to this finding is the effectiveness of T3 and T4, which demonstrated higher available nitrate at day fifteen after fertilization (six months after planting) (Figure 12c) and at a depth from 0 cm to 60 cm (nine months after planting), respectively (Figure 12 b). Throughout the pineapple plants’ growth and development, the peat soil mean available nitrate retention (overall treatments: T1 to T6) was similar irrespective of soil depth and fertilization period (Figure 11a,b). An exception to this finding is the higher nitrate retention at the saturated depth (0 cm to 90 cm) during the early development of the pineapple plants and the lower nitrate content at days seven and thirty after fertilization at six months after planting (Figure 11a,b).
Irrespective of the pineapple growth period, the pH of the soils with CZ (T1 to T4) significantly improved compared with NPK fertilization (T5) and non-treated soil (T6) (Table 5).

Table 5.
Mean pH of peat soils cultivated with pineapples at different vegetative stages.
3.6. Growth, Nutrient Uptake and Use Efficiency, Yield, and Fruit Quality of Ananas Comosus L. Merr var. Moris
Regardless of the pineapple plant growth period, the heights of the pineapple plants with CZ (T1) significantly increased compared to those with other treatments, including NPK fertilization (T5) and non-treated soil (T6) (Table 6). Compared with the controls (T5 and T6), all the treatments with CZ (T1 to T4) significantly increased the leaf area of the pineapple plants irrespective of the pineapple growth phases (Table 6). Compared with NPK fertilization (T5) and non-treated soil (T6), the peat soils with CZ in T2, T3, and T4 significantly improved the root N content (Table 6), whereas T3 and T4 improved the leaf N content. Conversely, all the treatments with CZ (T1 to T4) significantly increased stem N concentration compared with the controls (T5 and T6) (Table 6). The effects of CZ on the total N uptake and use efficiency of Ananas comosus L. Merr var. Moris are presented in Figure 13. Compared with the controls (T5 and T6), the peat soils with CZ (T1 to T4) significantly increased the total N uptake and use efficiency of pineapples (Figure 13).

Table 6.
Vegetative growth and nitrogen concentration in leaves, stems, and roots of Ananas comosus L. Merr var. Moris at harvest cultivated in a tropical peat soil treated with clinoptilolite zeolite and compound NPK fertilizer.
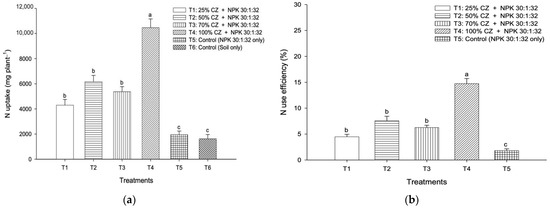
Figure 13.
Effects of clinoptilolite zeolite and NPK fertilizer on (a) total nitrogen uptake (leaves, stems, and roots) and (b) use efficiency of Ananas comosus L. Merr var. Moris at harvest. Error bars indicate the standard error of the mean. Means with different letters are significantly different using Tukey’s test with p ≤ 0.05. CZ: clinoptilolite zeolite.
Fruit yield and fruit quality characteristics of pineapple var. Moris with CZ and NPK fertilizers are presented in Table 7. All the treatments with CZ (T1 to T4) significantly increased fruit yield per plot and fruit weight, and improved fruit sweetness (TTS) and the pH of the pineapple fruit juice (Table 7). However, TTA of the pineapple fruit is generally similar regardless of treatments.

Table 7.
Fruit yield and fruit quality characteristics of Ananas comosus L. Merr var Moris in a tropical peat soil treated with different amounts of clinoptilolite zeolite and compound NPK fertilizer.
4. Discussion
4.1. Peat Soil Physical and Chemical Properties
The soil sampling (prior to the establishment of the field study) was carried out during the wet season in December 2016. The water table at the experimental site was high and fluctuated between 29 cm and 38 cm throughout the soil sampling. The bulk density of the peat soil is low, and this could be attributed to the high water table at the experimental site which may have increased the peat pore volume. However, the bulk density of the peat soil depends on the degree of organic material (sapric) decomposition once the peatland is drained and cultivated. Additionally, the high water table at the study site relatively explains the higher soil moisture content with increasing soil depth. The water-holding capacity of the peat soil in this present study was below the reported range because its measurement was based on the oven-dry weight method. Nevertheless, tropical peat soils have a high water-holding capacity due to the loose and hollow fibers of organic particles in peat soils, which can hold a large amount of water [5,8].
The low pH of the soil indicates that the peat soil is acidic. Generally, the composition of peat soils (organic acids such as carboxylic, hydroxylic, and phenolic acids) controls the acidity of peat soils [2,86]. The low electrical conductivity value of the peat soil suggests that the soil is not saline because the intrusion of salt water into the research station is prevented by the construction of a tidal gate located at the primary outlet drain of the station. The CEC of the peat soil is high because of the presence of exchangeable hydrogen from organic acids (carboxylic, phenolic, and amino acids) in peat soils where ion-exchange and adsorption occurs, both on the surface and inside the loose particles of these organic colloids [2,4,86]. Moreover, the CEC in peat soils depends on the pH, nature of organic materials, and degree of decomposition [4,8,13]. The peat soil has a high organic carbon content, which is typical of sapric peat. The high organic carbon of the peat soil is related to the high amount of ligneous material from woody tree trunks, particularly in deep peat soils [29,86]. The total N the peat soil is high, but it is mostly in organic forms. The high C:N ratio of the peat soils suggests that minimal N is mineralized, resulting in low inorganic nitrogen availability for plant uptake unless a substantial amount of N fertilizers is applied. The low oxidative decomposition of organic matter with increasing water content down the soil profile relatively explains the decreasing total N, exchangeable ammonium, and available nitrate contents with increasing peat soil depth. However, the soil exchangeable ammonium and available nitrate were higher compared with the reported range because this present study was carried out on a drained cultivated sapric peatland, whereas the reported inorganic N values [84] were based on a partially drained mixed swamp forest. Additionally, the agronomic practices and fertilization activities for pineapple and ginger cultivation at the experimental site from 2012 to 2015 before the commencement of the study might have contributed to the increase in N mineralization.
The low available P and exchangeable K of the peat soils are typical of drained peat soils. Generally, peat soils have a low P fixation because organic acid colloids have a low affinity for phosphate ions [93]. The low available P relates to the absence of low iron and aluminum ions, forming insoluble phosphate compounds [4,31]. The peat soil exchangeable K is low because humic compounds of these organic soils do not form coordination bonds with K ions, as the hydrogen ions are tightly bonded to the functional groups in humic compounds [4,31]. However, the relatively higher concentration of exchangeable K at a soil depth from 0 cm to 20 cm relative to a depth from 20 cm to 40 cm and 40 cm to 60 cm relates to the past, previous fertilization of ginger and pineapple at the experimental site prior to the study. Drainage and cultivation of the peat soils can decrease the void ratio and porosity of the peat soils because of the collapse of large pores of organic particles [8,94,95]. Thus, the reduced porosity might have restricted the leaching of K ions, leading to a higher retention below the peat ground surface.
4.2. Characteristics of Clinoptilolite Zeolite
The high pH of the CZ suggests this natural zeolite is alkaline, which reflects its ability to buffer soil pH and reduce soil acidity. The high CEC of the CZ indicates its nutrient-holding capacity through ion exchange, depending on the exchange kinetics, ion-exchange capacity, and cation selectivity of the zeolite [96]. The exchange kinetics of the CZ indicates the time needed for the counter-ion to reach the zeolite’s exchange site and replace the cation within its structure. Conversely, the ion-exchange capacity refers to the total amount of cations (in milliequivalents per gram) the CZ can retain on its exchange sites, whereas cation selectivity denotes the preferred cation selectivity order of clinoptilolite zeolite, which is influenced by cationic hydration energies and cation radii.
Variation in the mineralogical composition of the CZ compared to those reported in the literature could be attributed to the mineral deposits at different locations in Indonesia, which scarcely exist in a pure phase of the zeolite structure [42]. This further demonstrates that a type of natural zeolite could occur at the same deposit site [97]. Moreover, the chemical composition of the CZ is determined by its Si to Al ratio and structured framework [96,98]. The low surface area of the CZ is typical of natural zeolite. Generally, natural zeolites have a low surface area and crystallinity, in addition to having a small pore diameter ranging for 0.3 nm to 4 nm (for CZ) with high impurities compared with synthetic zeolite [42,96,98].
The main functional group of CZ is the hydroxyl stretching vibration (symmetric and asymmetric) and it appears at the broad wavenumber of 3400.33 cm−1. The presence of the hydroxyl group is further supported by the bending vibration (H-O-H) of absorbed water at 1634.66 cm−1. However, the FTIR spectra wavenumber regions from 518.17 cm−1 to 992.85 cm−1 are typical of CZ. The absorption at 922.85 cm−1 and 776.99 cm−1 is because of the symmetric and asymmetric stretching vibration of Si–O and Al–O, respectively [99]. The weak absorption band at 688.64 cm−1 is because of the rotational movement of water molecules, whereas the wavenumber at 518.17 cm−1 indicates the presence of tetrahedral oxygen units arranged in five-membered ring blocks [96]. The presence of water molecules and hydroxyl functional groups in the CZ compares to the general properties of natural zeolites, because 10 to 25% of the weight of natural zeolite is composed of water [96].
4.3. Laboratory Soil Leaching Experiment
Physicochemical properties of the CZ played a role in the sorption and retention of ammonium and nitrate in the peat soils. Throughout the wet and dry seasons, the significant reduction in ammonium loss for the treatments with CZ was because of the ability of CZ to adsorb ammonium ions from the peat solution within the lattices of CZ via ion exchange. This was possible because the CEC, which is caused by the high negative charges of CZ, enabled sorption of the ammonium ions [51,100,101]. Moreover, CZ has a high affinity for ammonium ions [45,51,100]. Additionally, a higher amount of CZ decreases the ammonium loss because of the increasing number of negatively charged surfaces for ammonium sorption [102]. This explains the effectiveness of T4 (100% CZ) in minimizing ammonium loss in the wet season. Conversely, the ineffectiveness of T4 in controlling the loss of ammonium in the dry season was unexpected. The reason for this observation is unclear, but this anomaly could be attributed to the competitive adsorption between ammonium and other cations, particularly K+ ions, onto the charged surface and within the lattices of the CZ. This was possible because the CZ has a higher selectivity for K+ ions compared with ammonium ions, thus resulting in low ammonium absorption. Generally, the selectivity for cation absorption by CZ is cesium, followed by rubidium, K, ammonium, barium, strontium, sodium, calcium, iron, aluminum, magnesium, and lithium [103,104].
At the end of the leaching experiment, the significant retention of soil exchangeable ammonium for all the treatments with CZ regardless of season is consistent with the increase in their soil pH (range from 4.63 to 5.33) because mineralization of organic N to ammonium increases with increasing pH [71]. The pH of NPK-fertilized peat soils (T5: control) remained acidic, and this might have impeded the mineralization of organic N to ammonium ions. Moreover, the negatively charged sites of the CZ contributed to the retention of ammonium ions. Among the treatments, the effectiveness of T4 in retaining exchangeable ammonium irrespective of season is because of the higher amount (100%) of CZ in T4. The higher amount of CZ increased the number of negatively charged surfaces for ammonium ion adsorption via ion exchange [102]. Likewise, the significant retention of exchangeable ammonium by the soils treated with CZ (T4) at depths from 0 cm to 10 cm and 10 cm to 30 cm in the wet and dry seasons is also associated with the higher amount of zeolite used in this treatment. The amount of zeolite in T4 provided a sufficient number of actively charged surfaces for ammonium adsorption at the aerobic zone [102]. Furthermore, the significant ammonium retention at a depth from 0 cm to 10 cm might have been influenced by the oxidation of peat soil at the aerobic zone. It is generally believed that the biochemical processes driven by many microorganism pathways occur mainly at the upper part of peat soils [105]. This improves mineralization of organic N, which indirectly increases the availability of ammonium ions to be sorbed on the charged surface of the zeolite. Moreover, this observation is consistent with the increase in soil pH in T4 at a depth from 0 cm to 10 cm throughout the wet and dry seasons as the mineralization of organic N to ammonium is high at a higher pH [45]. Similarly, the higher retention of ammonium in T3 at the lower soil depths (30 cm to 60 cm and 60 cm to 90 cm) in the wet season, and T1 and T2 (30 cm to 60 cm) in the dry season, is associated with the amounts of zeolite in these treatments. The amounts of zeolite (25% to 70%) corresponded to the optimum and equilibrium sorption of ammonium ions onto the negatively charged surface of the CZ via ion exchange [102,106].
The effectiveness of the CZ in controlling the loss of nitrate throughout the wet and dry seasons demonstrates the ability of zeolite to adsorb negatively charged anions within its lattices [71], particularly nitrate. The lower nitrate ions in the leachates of the treatments with CZ indicate that nitrate ions might have been sorbed from the peat soil solution through diffusion onto the negatively charged sites of the zeolite. Moreover, the effectiveness of CZ in minimizing nitrate loss during the wet and dry seasons is related to the adsorption of ammonium ions at the negative sites of zeolite. This reaction physically protected the ammonium ions from rapid nitrification to produce nitrate ions which are prone to leaching [35]. On the contrary, the ineffectiveness of T2 and T4 in minimizing nitrate loss during the wet and dry seasons, respectively, relates to the competitive adsorption between nitrate and other ions such as ammonium, phosphate, and K+ on the charged surfaces of the CZ. The competitive interactions between cations and anions can decrease adsorption of nitrate because the available channels are not sufficient to adsorb nitrate, ammonium, phosphate, and K+ ions simultaneously. At the end of the leaching experiment, the significant retention of available nitrate by the peat soils with CZ (T1 to T4) could be attributed to the buffering capacity of the CZ, and this partly increased the soil pH of these treatments because nitrification increases at a higher pH [71]. Although the treatments with CZ improved nitrate retention, the relatively higher amounts of zeolite in T4 (100%) were less effective in retaining available nitrate in the dry season, which was unexpected. The reason for this observation is unclear, but this anomaly relates to the zeolite’s higher affinity for cations, particularly ammonium and K+ ions, compared with that of anions such as nitrate and phosphate [103,104]. Additionally, the higher amount of CZ in T4 increased the number of negatively charged surfaces for ammonium ion adsorption via ion exchange [102]. Low rainfall increases peat oxidation at the upper part of the peat soils, and this facilitates organic N mineralization to indirectly increase availability of ammonium ions to be sorbed by CZ [105]. This reaction physically protects ammonium ions from rapid nitrification, resulting in low nitrate retention during the dry season [45,71]. Among the treatments, the effectiveness of T2 (50% CZ) in retaining available nitrate throughout the wet and dry seasons is consistent with the optimal and equilibrium sorption of nitrate ions within the lattices of the CZ. Furthermore, the effectiveness of T2 in retaining nitrate could be influenced by the competitive adsorption between ammonium, K+, and phosphate on the active sites of the CZ.
The different contents of available nitrate at the different soil depths (0 cm to 10 cm, 10 cm to 30 cm, 30 cm to 60 cm, and 60 cm to 90 cm) relate to the preferential flow of the peat soil water, which is influenced by high and low rainfall conditions [94,107]. In addition, the diffusion of CZ through peat macro- and micropores during contact with water throughout the wet and dry seasons might have influenced the sorption of available nitrate ions at different soil layers [106,108,109]. However, CZ (T1 to T4) was less effective in retaining available nitrate at 0 cm to 10 cm during the dry season, which was unexpected. The cause for this finding is uncertain, but this discrepancy relates to the oxidation of the peat soil at the aerobic zone during dry spells. This process indirectly increases the availability of ammonium ions to be sorbed onto the active sites of the CZ. This reaction might have temporarily protected ammonium ions from rapid nitrification, and this partly explains the low retention of available nitrate at the upper part of the peat soils. However, peat oxidation at the aerobic zone also facilitates the nitrification of ammonium ions, particularly during dry spells [105]. Moreover, because nitrate ions are negatively charged and soluble, ions are easily leached into deeper soil layers during contact with water, thus limiting the availability of nitrate ions for sorption by CZ at the aerobic zone.
The buffering capacity of the CZ reduces peat soil acidity, and this explains the higher soil pH for T1 to T4, particularly at depths from 0 cm to 10 cm and 10 cm to 30 cm throughout the wet and dry seasons because of the accumulation of CZ at the upper soil layer. This directly causes increases in peat soil pH. However, it is possible that the diffusive adhesion forces of zeolite minimized the leaching of CZ into peat soil solutions at lower soil depths, and thus, limit the buffering capacity of zeolite to improve soil pH. This explains the low soil pH from CZ-treated soils at depths from 30 cm to 60 cm and 60 cm to 90 cm.
From the foregoing rationale, findings from the laboratory leaching experiments demonstrated the ability of CZ in retaining and reducing the leaching of ammonium and nitrate in peat soils. The heterogeneous surface and polar functional groups in CZ provided negatively charged surfaces for ammonium and nitrate sorption and retention in the peat soils. These findings were consistent with the hypothesis of this present study.
4.4. Improving Soil Nitrogen Availability and Pineapple Productivity in a Drained Tropical Peat Soil Using Clinoptilolite Zeolite
In the field trial, during the growth phases of the pineapple plants (six months old and the flower induction phase), the significant reduction in the ammonium loss from the soil with CZ (T1 and T4) substantiates the ability of CZ to adsorb ammonium within its lattices via ion exchange [51,100,101] because of CZ’s high CEC and affinity for ammonium ions [103]. The significant retention of the soil exchangeable ammonium of the treatments with CZ in T1 at three months after planting is consistent with the optimal and equilibrium sorption of ammonium ions onto the charged surfaces and lattices of CZ via ion exchange [51,101]. However, at six months after planting, the retention of exchangeable ammonium by the soils with CZ (T1 to T4) might have been influenced by the oxidation of the peat soil at the aerobic zone, which is influenced by the low water table during the dry season. The low water table increases peat oxidation [105], and this process facilitates organic N mineralization to indirectly increase the availability of ammonium ions to be sorbed on the charged surface of the CZ. This observation is consistent with the low rainfall (285 mm) during the dry season in June 2017. Moreover, the significant retention of exchangeable ammonium by the peat soils with CZ (T1 to T4) is related to the buffering capacity of the CZ that increased the soil pH of these treatments because N mineralization increases at a higher pH [71]. Although CZ has a high affinity for cations such as ammonium and K [52,103], its effectiveness in retaining exchangeable ammonium and controlling ammonium loss throughout the growth phases of the pineapple plants were not consistent. The ineffectiveness of CZ (T1 to T4) in controlling ammonium loss and retaining exchangeable ammonium following NPK fertilization throughout the pineapple growth period might have been influenced by the competing interactions between ammonium, nitrate, phosphate, K+, organic acids, and dissolved organic carbon (DOC) onto the charged surfaces and within the lattices of the CZ. This process decreases ammonium sorption because the limited available channels of the zeolites are not sufficient to adsorb ammonium, cations, anions, organic acids, and DOC simultaneously [110], as water movement in the soil is affected by the high preferential flow of the peat soil water and high rainfall conditions in March and September 2017. This explains the ineffectiveness of the CZ (T1 to T4) in minimizing ammonium loss and retaining exchangeable ammonium during the early vegetative growth period (three months old, March 2017) and flower induction phase (nine months old, September 2017), respectively.
The effectiveness of the CZ (T2 and T4) in controlling the loss of nitrate during the early growth of the pineapple plants demonstrates the ability of zeolite to adsorb negatively charged anions, particularly nitrate and phosphate. The lower nitrate contents in the leachates of these treatments (T2 and T4) suggest that nitrate ions might have been sorbed from the peat soil solution onto the actively charged sites of the CZ. However, the ineffectiveness of the treatments with CZ (T1 to T4) in controlling the loss of nitrate at six and nine months after planting could be attributed to the competitive adsorption between nitrate, ammonium, phosphate, K+, organic acids, and DOC on the charged surfaces of the CZ, which might have decreased the sorption of nitrate ions from the peat soil solution. This also explains the ineffectiveness of the CZ-treated soils (T1 to T4) in minimizing nitrate loss throughout the growth phases of the pineapple plants. Unlike exchangeable ammonium, the peat soils with CZ (T1 to T4) were less effective in retaining available nitrate throughout the growth period of the pineapple plants regardless of soil depth and fertilization period, which relates to the zeolite’s higher selectivity for cations compared with that of anions [52,103]. The adsorption of ammonium ions onto the charged sites and cavities of CZ via ion exchange protects ammonium ions from rapid nitrification [45,71], thus resulting in low nitrate availability in these treatments (T1 to T4). Additionally, this observation is consistent with the higher retention of soil exchangeable ammonium in CZ-treated peats (T1) compared with other treatments, including NPK fertilization (T5), throughout the pineapple growth phases. This might have caused the low available nitrate retention in the zeolite-treated peat soils. Furthermore, the soils with CZ were less effective, which could also be associated with the lower surface area of the CZ. The lower surface area of CZ might have limited the number of porous surfaces for the sorption of negatively charged nitrate ions [108].
The losses of ammonium and nitrate at depths from 0 cm to 30 cm, 0 to 60 cm, and 0 cm to 90 cm relates to the preferential flow of the peat soil water, which is influenced by high (March 2017: end of wet season, and September 2017: beginning of the wet season) and low (June 2017: dry season) rainfall conditions, and water table fluctuation [2,94,107]. This is because infiltrating rainwater and the lateral movement of peat water indirectly influenced the mobilization of nutrients along the preferential pathway, thus resulting in changes to the availability of ammonium and nitrate ions to be sorbed onto the charged surfaces of the clinoptilolite zeolite. Throughout the pineapple plants’ growth and development, the effectiveness of the CZ (T1 to T4) in controlling the loss of ammonium at depths from 0 cm to 30 cm and 0 cm to 60 cm gives credence to the ability of this mineral to adsorb ammonium ions onto its charged surface and within its lattices via ion exchange. On the contrary, the ineffectiveness of all the treatments with CZ (T1 to T4) in minimizing ammonium loss at the lower soil depth (0 cm to 90 cm) regardless of the pineapple growth period could be attributed to the high preferential flow of the peat soil water, and high rainfall at the experimental site (in March and September 2017). The high soil water enabled the rapid transport of water and mobilization of nutrients, thus limiting the amount of ammonium ions to be sorbed on the active sites of the CZ. Conversely, the higher nitrate loss at the saturated depth (0 cm to 90 cm) is due to the peat soil oxidation at the aerobic zone. The oxidation improves nitrification of ammonium ions to nitrate ions [105]. This reaction indirectly increases the availability of nitrate ions in the peat soil solution. The preferential flow of the peat soil enables the nitrate ions to diffuse easily into deeper soil layers during initial contact with water because nitrate ions are soluble and prone to leaching [94,107]. Moreover, nitrate ions are not easily adsorbed in peat soils as they are negatively charged [33,34]. Nevertheless, the effectiveness of CZ treatments in T2, T3, and T4 in controlling the loss of nitrate at the soil depths could be attributed to the competing interactions between nitrate, ammonium, phosphate, cations, organic acids, and DOC, which resulted in the optimal and equilibrium sorption of nitrate ions on the actively charged sites of the zeolite. In contrast, the ineffectiveness of the treatments with CZ (T1 to T4) in controlling the loss of nitrate at the aerobic zone (0 cm to 30 cm) and from 0 cm to 60 cm during flower induction was unexpected. A satisfactory explanation for this anomalous finding is not available. Perhaps, the nitrate loss might have been influenced by the higher selectivity of CZ, particularly ammonium and K+ compared with anions, which affects nitrate adsorption capacity.
Throughout the pineapple plants’ growth and development, the differences in ammonium and nitrate losses following NPK fertilization across time in the peat soils with CZ (T1 to T4) are difficult to explain. Although the differences in ammonium and nitrate losses could be associated with the competitive adsorption between ammonium, nitrate, phosphate, and K+ from the peat soil solution onto the exchange sites of the CZ, the adsorption of root exudates such as amino acids, citric acids, malic acids, and phenolics onto the actively charged sites of the CZ might have influenced the sorption of ammonium and nitrate ions at the rhizosphere [111,112]. Furthermore, the losses of ammonium and nitrate could be affected by the mobilization of nutrients to the pineapple rooting zone via mass flow, thus leading to changes in the availability of ammonium and nitrate in the peat soil solution for sorption by the CZ [113]. Throughout the pineapple growth phases, the losses of ammonium and nitrate ions might have also been affected by the gradual release of nutrients with CZ, which influenced ammonium and nitrate ion movement in the peat soil solution and their uptake by pineapple roots at different growth stages.
During the pineapple plants’ growth and development, the different contents of exchangeable ammonium at different soil depths (0 cm to 30 cm, 0 cm to 60 cm, and 0 cm to 90 cm) could be attributed to peat oxidation, which is influenced by water table fluctuation (water table depth range: 36 cm to 87 cm) under low and high rainfall conditions in March 2017, June 2017, and September 2017. Peat oxidation improves organic N mineralization, particularly at the aerobic zone, because the biochemical process driven by many microorganism pathways occurs mainly at the upper part of the peat soils [105]. This process indirectly increases the availability of ammonium ions to be sorbed on the charged surfaces of the CZ. Additionally, the diffusion of CZ through peat macro- and micropores during contact with infiltrating rainwater throughout the dry and wet seasons might have influenced the sorption of ammonium ions at different soil layers [94,107]. Among the treatments, the effectiveness of T2 and T3 in enhancing exchangeable ammonium availability following NPK fertilization during the dry season (six months after planting: June 2017) regardless of soil depth was due to the accumulation of some of the CZ, which did not break down rapidly over time [44]. This finding further demonstrates the ability of CZ to diffuse into peat pore spaces at deeper soil layers to improve exchangeable ammonium retention via ion exchange. This also explains the effectiveness of T4 in improving exchangeable ammonium at a depth from 0 cm to 60 cm during the wet season (at three and nine months after planting). The buffering capacity of the CZ reduces peat soil acidity because of the accumulation of zeolite at the upper peat soil layer, which causes an increase in peat soil pH. This explains the higher soil pH for the peat soils treated with CZ (T1 to T4) throughout the pineapple growth phases. However, the soil pH of NPK-fertilized (T5) and non-treated (T6) peat soils remained acidic throughout the growth phases of the pineapple plants, and this might have impeded mineralization of organic N to ammonium ions [30,86]. This explains the low contents of the exchangeable ammonium ions in the control treatments (T5 and T6).
Throughout the pineapple growth and development, the differences in exchangeable ammonium retention following NPK fertilization across time (seven, fifteen, and thirty days after fertilization) in the peat soils with CZ (T1 to T4) is due to the development of the pineapple rooting system at various growth stages, root exudates, and organic N mineralization, N immobilization, and nitrification processes, which is influenced by the diversity of soil microorganisms and the soil pH at the rhizosphere [111,112,114]. Moreover, the soil exchangeable ammonium retention might have been influenced by the uptake of ammonium ions by pineapple plants and the movement of nutrients via mass flow into the pineapple rooting zone, which is influenced by the high preferential flow of the peat soil water under low and high rainfall conditions [94,111,113].
Among the treatments, the effectiveness of T1 in enhancing exchangeable ammonium availability following NPK fertilization throughout the growth phases of the pineapple plants relates to the amount of CZ used. The amounts of zeolite (25%) provided a sufficient number of actively charged surfaces for the optimal and equilibrium sorption of ammonium ions via ion exchange. Furthermore, the effectiveness of T1 in retaining exchangeable ammonium after NPK fertilization in pineapple-cultivated peat soils corroborated the results of the laboratory leaching experiments. This finding buttresses the fact of the ability of the CZ to adsorb ammonium ions onto their heterogeneously charged surfaces. These findings are consistent with the hypothesis of this present study. Again, these findings suggest that the significant retention of soil exchangeable ammonium with CZ was due to the accumulation of zeolite in the peat soils, which did not break down rapidly over time, but it instead remained in the soil to improve ammonium retention [44].
The increase in the leaf area of the pineapple plants with the treatments with CZ (T1 to T4) is related to the sufficient availability of exchangeable ammonium in the peat soil. The temporary retention of exchangeable ammonium on the active sites of the CZ resulted in the timely availability of these nutrients for optimum use by the pineapple plants during the vegetative phase. Likewise, the increase in the pineapple plant height with the treatments with CZ (T1) was because of the amounts of zeolite (25%) in this treatment, which concurred with the optimal and equilibrium sorption of ammonium onto their actively charged sites. This resulted in the sufficient availability of ammonium in the peat soils for utilization by pineapple plants. The increase in leaf, stem, and root N contents of the pineapple plants relates to the adsorption of soil exchangeable ammonium onto the actively charged sites of the CZ via ion exchange. However, differences in the N contents in the leaves, stems, and roots with the zeolite treatments could be attributed to the competitive interactions between ammonium, nitrate, P, and K+ ions on the charged sites of the zeolite and their ability to gradually desorb nutrients for plant uptake throughout the vegetative phase of the pineapple plants. This process is influenced by the preferential flow of the peat soil water, which affects the mobilization of ammonium, nitrate, phosphate, and K+ ions into the pineapple rooting zone for plant utilization [94,111,112].
The effectiveness of the CZ in increasing uptake and use efficiency of N by the pineapple plants gives credence to the ability of this mineral to adsorb and regulate exchangeable ammonium in the peat soils. The timely release of ammonium ions by CZ via diffusion according to plant needs led to the significant uptake and use efficiency of N. Additionally, the effectiveness of the CZ in enhancing N uptake and use efficiency could be associated with the buffering capacity of the CZ, which increases soil pH. As the surfaces of the CZ becomes more negatively charged with increasing pH, this process increases the affinity of this material for ammonium sorption. This process increases the availability of nutrients for pineapple plant uptake throughout the growth and development of the pineapple plants [115,116]. This observation is consistent with the increase in soil pH (pH range: 5.55 to 6.37) under the treatments with CZ (T1 to T4). This might explain the higher N uptake and use efficiency in treatment T4. In this present study, the effectiveness of CZ in improving N uptake and use efficiency of the pineapple plants due to the retention and release of ammonium in the peat soil for utilization by pineapple plants is consistent with the hypothesis of this present study and is in agreement with most previous studies, which demonstrate the ability of this mineral to improve soil and crop productivity in marginal soils, namely, rice and maize cultivated in mineral acid soils [47,55,56].
Moris is a medium-sized pineapple fruit weighing between 1.5 to 1.8 kg/fruit [4,28]. The higher fresh fruit weight with CZ (T1 to T4) indicates a good fresh fruit yield compared with the control (T5: compound NPK fertilizer). Again, the increase in the fruit weight with the treatments with CZ was because of the sufficient availability of ammonium in the peat soil. This also explains the increase in pineapple fruit yield per plot, and the improved fruit sweetness and fruit juice pH of the pineapples with the use of CZ. Moreover, the pure sucrose (TSS) contents expressed in °Brix of the pineapple fruits treated with CZ, which ranged between 13.32 and 13.75 °Brix, exceeded the 12.0 °Brix limit required by the international market (Codex Standard for Pineapples: Standard 182–1993: Revised 1–1999) [117]. Compared with NPK fertilization, the increase in fresh fruit weight, fruit yield, and fruit sweetness of the pineapple plants is consistent with the higher retention of nutrients, particularly exchangeable ammonium in the peat soils treated with CZ. These observations are in agreement with most previous studies, which demonstrate the importance of N in the production of good pineapple fruit yields and fruit quality [116,118]. In this present study, TSS, TA, and fruit juice pH of the Moris pineapples were determined at maturity index 5 (50% ripe) because of the preferred consumer acceptability for fresh fruit consumption [61].
From the foregoing discussion, the findings of this present study demonstrated that the application of CZ (T1 to T4: 5 to 20 g) in conjunction with the compound NPK 30:1:32 fertilizer (20 g) at three, six, and nine months after planting positively influenced pineapple growth attributes, yield, and fruit quality. This could be attributed to the accumulation of CZ in the peat soils, which did not break down rapidly over time, but instead, remained in the soil to improve nutrient retention, particularly of ammonium. The gradual build- up of inorganic N with CZ in the peat soil played an important role in promoting plant cell division, multiplication, and photosynthesis that resulted in increased plant height and leaf area, and improved plant N contents [37]. Additionally, the positive response of the pineapple yield and fruit quality to the adequate supply of N stimulated by CZ application could be attributed to the increased physiological activities resulting in assimilate production and their translocation and utilization for fruit development [37]. At present, taking into consideration the continuous increase in fertilizer price, the outcomes of this study suggest that monthly fertilization (at three, six, and nine months after planting) using compound NPK 30:1:32 fertilizers in conjunction with 25% CZ (T1: 5 g) promises to be effective in improving soil N availability and pineapple productivity through the regulation of the timely availability of exchangeable ammonium in drained peat soils.
In this present study, the use of compound NPK 30:1:32 fertilizer could not significantly improve the growth, uptake, and use efficiency of N, fruit yield, and fruit quality of the pineapple plants, not only because of the acidity of the peat soil, but also because of the leaching of ammonium and nitrate from the peat soils, particularly during the wet monsoon season. Thus, the split application of compound NPK 30:1:32 fertilizer and CZ during the wet season could be considered as a nutrient management strategy to provide time for N (particularly ammonium) to be absorbed and taken up by pineapple plants to ensure the production of good pineapple fruit yields.
5. Conclusions
The properties of CZ, such as high CEC, a heterogeneously structured framework, and functional groups, play an important role in nutrient exchange. Although laboratory leaching studies demonstrate the ability of CZ to improve ammonium and nitrate availability in peat soils, the field trials indicate that the use of CZ in conjunction with compound NPK fertilizer were more effective in improving exchangeable ammonium availability and pineapple productivity in drained tropical peat soil. The effectiveness of CZ in improving soil exchangeable ammonium availability in the peat soil was due to the sorption of this nutrient onto the actively charged sites and lattices of the zeolite via ion exchange. On the contrary, CZ was less effective in enhancing nitrate availability, which was due to the sorption of ammonium ions onto the zeolite and which protected ammonium ions from microbial nitrification that resulted in lower nitrate retention in peat soil. Exchangeable ammonium concentrations varied with soil depth because of the high preferential flow of the peat soil water throughout the wet and dry seasons. The combined use of CZ with NPK fertilizers also improves uptake and use efficiency of N, pineapple plant growth, fruit yield, and fruit quality because this mineral is able to synchronize the availability of N ions with the uptake of this nutrient by pineapple plants. A higher retention of soil exchangeable ammonium in pineapple cultivation treated with CZ suggests the build-up of this nutrient in the peat soil was due to the accumulation of zeolite in the soil which did not break down rapidly. The buffering capacity of the CZ decreased peat soil acidity, and this facilitated organic N mineralization, thus demonstrating that it is possible to use zeolite as a prospective liming material for the production agriculture on tropical peatlands. The outcomes of this study suggest that monthly fertilization (at three, six, and nine months after planting) using compound NPK 30:1:32 fertilizers (20 g) in combination with 25% CZ (5 g) could be embraced by pineapple farmers to improve N availability, soil acidity, fresh fruit yield, and fruit quality of pineapple cultivated in drained peat soils. Nevertheless, in-depth studies are required to enhance the formulation rate of CZ in combination with compound NPK fertilizers as a value-added soil amendment, not only to improve agronomic efficiency, but also to reduce farm operation costs for sustainable pineapple cultivation in tropical peat soils. Additionally, the long-term monitoring of macronutrient (N, P, and K) availability for pineapple cultivation in peat soils at a larger magnitude is required to verify the results of this study, because factors such as soil microbiota, organic matter heterogeneity, temperature, and rainfall distribution between planting cycles may influence the outcome of the present study.
Author Contributions
Conceptualization, L.N.L.K.C.; methodology, L.N.L.K.C. and O.H.A.; validation, L.N.L.K.C. and O.H.A.; formal analysis, L.N.L.K.C., O.H.A. and S.S.; investigation, L.N.L.K.C. and O.H.A.; resources, L.N.L.K.C. and O.H.A.; data curation, L.N.L.K.C. and O.H.A.; writing—original draft preparation, L.N.L.K.C.; writing—review and editing, L.N.L.K.C., O.H.A. and N.A.R.; visualization, L.N.L.K.C. and O.H.A.; supervision, L.N.L.K.C. and O.H.A.; project administration, L.N.L.K.C.; funding acquisition, L.N.L.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Government of Malaysia under the 12th Malaysia Plan Development Project and Fundamental Research Grant Scheme through the Malaysia Agricultural Research and Development Institute, grant number P-RP515 and FRGS/1/2015/WAB01/MOA/02/2.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to extend their special thanks and appreciation to Universiti Putra Malaysia Bintulu Campus Sarawak, Malaysia for the collaborative research. The facilities provided by MARDI Saratok and Universiti Putra Malaysia Bintulu Campus for this study are appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ismail, A.B.; Jamaludin, J. Land clearing techniques employed at MARDI Peat Research Station, Sessang, Sarawak and their immediate impacts. In A Case Study at MARDI Peat Research Station, Sessang, Sarawak, Malaysia; Ismail, A.B., Ong, H.K., Mohamad Hanif, M.J., Umi Kalsom, M.S., Eds.; MARDI: Serdang, Malaysia, 2007; pp. 1–8. [Google Scholar]
- Dolmat, M.T. Technology for Planting Oil Palm on Peat; Malaysian Palm Oil Board: Selangor, Malaysia, 2005; pp. 1–76. [Google Scholar]
- Kang, S.; Post, W.F.; Nichols, J.A.; Wang, D.; West, T.O.; Bandaru, V.; Izaurralde, R. Marginal lands: Concept, assessment and management. J. Agric. Sci. 2013, 5, 129–139. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Ahmad Husni, M.H.; Rahim, A.A.; Mohd Hanafi, M. Sustainable Production of Pineapples on Tropical Peat Soils; Universiti Putra Malaysia Press: Serdang, Malaysia, 2013; pp. 1–141. [Google Scholar]
- Bakar, I.A. Strategies for Mitigation of Carbon Emission in Peatland; MARDI Press: Serdang, Malaysia, 2010; pp. 1–35. [Google Scholar]
- IUSS. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, World Soil Reports No. 106; International Union of Soil Sciences: Vienna, Austria; FAO: Rome, Italy, 2015. [Google Scholar]
- Couwenberg, J. Greenhouse gas emissions from managed peat soils: Is the IPCC reporting guidance realistic? Mires Peat 2011, 8, 2. Available online: http://www.mires-and-peat.net/pages/volumes/map08/map0802.php (accessed on 31 August 2014).
- Huat, B.B.K. State of an art review of peat: General perspective. Int. J. Phys. Sci. 2011, 6, 1988–1996. [Google Scholar]
- Adon, R.; Bakar, I.; Wijeyesekera, D.C.; Zainorabidin, A. Overview of the sustainable uses of peat soil in Malaysia with some relevant geotechnical assessments. Int. J. Integr. Eng. 2012, 4, 38–46. Available online: https://publisher.uthm.edu.my/ojs/index.php/ijje/article/view/584 (accessed on 31 August 2014).
- Yuwati, T.W.; Rachmanadi, D.; Santosa, P.B.; Rusamana. Response of tropical peat swamp forest tree species seedlings to macro nutriens. J. Wetl. Environ. Manag. 2015, 3, 63–71. Available online: http://ijwem.unlam.ac.id/index/php/ijwem (accessed on 31 August 2014).
- Mutert, E.; Fairhurst, T.H.; von Uexküll, H.R. Agronomic management of oil palms on deep peat. Better Crops Int. 1999, 13, 22–27. [Google Scholar]
- Safford, L.; Maltby, E. Guidelines for Integrated Planning and Management of Tropical Lowland Peatlands with Special Reference to Southeast Asia; IUCN: Gland, Switzerland; Cambridge, UK, 1998; p. xvi-66. [Google Scholar]
- Mutalib, A.A.; Lim, J.S.; Wong, M.H.; Koonvai, L. Characterization, distribution, and utilization of peat in Malaysia. In Proceedings of the International Symposium on Tropical Peatland, Kuching, Malaysia, 6–10 May 1991; pp. 7–16. [Google Scholar]
- Funakawa, S.; Yonebayashi, K.; Shoon, J.F.; Khun, E.C.O. Nutritional environment of tropical peat soils in Sarawak, Malaysia based on soil solution composition. Soil Sci. Plant Nutr. 1996, 42, 833–843. [Google Scholar] [CrossRef]
- Balasundram, S.K.; Chong, Y.M.; Husni, M.H.A. Monitoring potassium levels in peat-grown pineapple using selected spectral ratios. In Proceedings of the 14th International Conference on Precision Agriculture, Montreal, QC, Canada, 24–27 June 2018. [Google Scholar]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat under stabilized land uses. Mires Peat 2015, 16, 8. Available online: http://www.mires-and-peat.net/pages/volumes/map16/map1608.php (accessed on 16 September 2016).
- Mohd Shukri, M.A.; Bastmasi, M.S.; Abdul Rahman, M.H.; Choo, L.N.L.K. Greenhouse gas emissions from agricultural sector in Malaysia. In Proceedings of the Greenhouse Gas Emissions from Peatland in Relation to Malaysia’s Commitment under the Paris Agreement on Climate Change, International Greentech and Ecoproducts Exchibition and Conference Malaysia, Kuala Lumpur, Malaysia, 30 September 2021. [Google Scholar]
- DOA. Crop Statistics Booklet (Food Crop Sub-Sector); Department of Agriculture Malaysia: Putrajaya, Malaysia, 2018; p. 49.
- Zainuddin, M.F.; Shamsudin, R.; Mokhtar, M.N.; Ismail, D. Physicochemical properties of pineapple plant waste fibres from the leaves and stems of different varieties. BioResources 2014, 9, 5311–5324. [Google Scholar] [CrossRef]
- DOA. Crop Statistics Booklet (Food Crop Sub-Sector); Department of Agriculture Malaysia: Putrajaya, Malaysia, 2021; p. 108.
- Salim, S. Transforming the Malaysian pineapple industry. In Proceedings of the 5th International Plantation Industry Conference and Exhibition, Johor, Malaysia, 2–4 November 2016. [Google Scholar]
- Lin, R.M.; Rahman, A.A. Status and impact of pineapple technology on mineral soil. Econ. Technol. Manag. Rev. 2010, 5, 11–19. [Google Scholar]
- Nik Masdek, N.H. Paratylenchus sp. associated with pineapple yield decline. J. Trop. Agric. Food Sci. 2007, 35, 191–199. [Google Scholar]
- Suhaimi, N.H.M.; Fatah, F.A. Profitability of pineapple production (Ananas comosus) among smallholders in Malaysia. Int. J. Recent Technol. Eng. 2019, 8, 4201–4207. [Google Scholar]
- FAOSTAT. Pineapple World Production 2020, Crops and Livestock Products Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; Available online: https://www.fao.org/faostat (accessed on 30 September 2021).
- Souza, L.F.S.; Reinhardt, D.H. Pineapple. In Fertilizing for High Yield and Quality Tropical Fruits of Brazil; IPI Bulletin 18; International Potash Institute: Zug, Switzerland, 2007; pp. 179–201. [Google Scholar]
- Malézieux, E.; Bartholomew, D.P. Plant nutrition. In The Pineapple: Botany, Production and Uses; Bartholomew, D.P., Paull, R.E., Rohrbach, K.G., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 143–165. [Google Scholar]
- Mohammed Selamat, M.; Abdul Rahman, H. Amalan kultur. In Penanaman Nanas—Nanas Makan Segar dan Nanas Kaleng; Mohammed Selamat, M., Ed.; MARDI: Serdang, Malaysia, 1996; pp. 22–34. [Google Scholar]
- Murtedza, M.; Padmanabhan, E.; Mei, B.L.H.; Siong, W.B. The Peat Soils of Sarawak, STRAPEAT Status Report; UNIMAS: Sarawak, Malaysia, 2002; pp. 1–16. [Google Scholar]
- Blodau, C.; Basiliko, N.; Mayer, B.; Moore, T.R. The fate of experimentally deposited nitrogen in mesocosms from two Canadian peatlands. Sci. Total Environ. 2006, 364, 215–228. [Google Scholar] [CrossRef]
- Maftu’ah, E.; Ma’as, A.; Purwanto, B.H. N, P and K storage efficiency on degraded peat soil through ameliorant application. J. Degrad. Min. Lands Manag. 2014, 1, 187–196. [Google Scholar] [CrossRef]
- Lappalainen, M.; Kukkonen, J.K.; Piirainen, S.; Sarjala, T.; Setälä, H.; Koivusalo, H.; Finér, L.; Laurén, A. Nitrogen release in decomposition of boreal mor and peat as affected by enchytraeid worms. Boreal Environ. Res. 2013, 18, 181–194. [Google Scholar]
- Lamb, J.A.; Fernandex, F.G.; Kaiser, D.E. Understanding Nitrogen in Soils (AG-FO-3770-B), University of Minnesota, USA. Available online: https://extension.umn.edu/nitrogen/understanding-nitrogen-soils (accessed on 20 July 2020).
- Walworth, J. Nitrogen in Soil and the Environment, University of Arizona, USA. Available online: https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1591.pdf (accessed on 20 July 2020).
- Tiemeyer, B.; Kahle, P. Nitrogen and dissolved organic carbon (DOC) losses from an artificially drained grassland on organic soils. Biogeosciences 2014, 11, 4123–4137. [Google Scholar] [CrossRef]
- Darnaudery, M.; Fournier, P.; Lechaudel, M. Low-input pineapple crops with high quality fruit: Promising impacts of locally integrated and organic fertilization compared to chemical fertilisers. Exp. Agric. 2018, 54, 286–302. [Google Scholar] [CrossRef]
- Tewodros, M.; Mesfin, S.; Getachew, W.; Ashenafi, A.; Neim, S. Effect of inorganic N and P fertilizers on fruit yield and yield components of pineapple (Ananas comosus Merr L. Var. Smooth cayenne) at Jimma, Southwest Ethiopia. Agrotechnology 2018, 7, 178. [Google Scholar] [CrossRef]
- Fangueiro, D.; Kidd, P.S.; Alvarenga, P.; Beesley, L. Chapter 10—Strategies for soil protection and remediation. In Soil Pollution from Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Elsevier Incorporated: Amsterdam, The Netherlands, 2017; pp. 251–281. [Google Scholar]
- Ramesh, K.; Reddy, K.S.; Rashmi, I.; Biswas, A.K.; Islam, K.R. Does particle size of clinoptilolite zeolite have a role in textural properties? Insight through differential pore-volume distribution of Barret, Joyner and Halenda model. Commun. Soil Sci. Plant Anal. 2015, 46, 2070–2078. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghavaland, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Belviso, C. Zeolite for potential toxic metal uptake from contaminated soil: A brief review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Kurniawan, T.; Nuryoto, N.; Firdaus, M.A. Zeolite for agriculture intensification and catalyst in agroindustry. World Chem. Eng. 2019, 3, 14–23. [Google Scholar]
- Jakkula, V.S.; Wani, S.P. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 1–15. [Google Scholar]
- Nur Aainaa, H.; Haruna Ahmed, O.; Ab Majid, N.M. Effects of clinoptilolite zeolite on phosphorus dynamics and yield of Zea Mays L. cultivated on an acid soil. PLoS ONE 2018, 13, e0204401. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Enhancing nitrogen availability from urea using clinoptilolite zeolite. Geoderma 2017, 306, 152–159. [Google Scholar] [CrossRef]
- Sangeetha, C.; Baskar, P. Zeolites and its potential uses in agriculture: A critical review. Agric. Rev. 2016, 37, 101–108. [Google Scholar] [CrossRef]
- Rabai, K.A.; Ahmed, O.H.; Kasim, S. Use of formulated nitrogen, phosphorus and potassium compound fertilizer using clinoptilolite zeolite in maize (Zea mays L.). Emir. J. Food Agric. 2013, 25, 712–722. [Google Scholar] [CrossRef]
- Abdi, G.H.; Khui, M.K.; Eshgi, S. Effects of natural zeolite on growth and flowering of strawberry (Fragaria x ananassa Duch.). Int. J. Agric. 2010, 5, 799–804. [Google Scholar]
- Kavoosi, M. Effects of zeolite application on rice yield, nitrogen recovery and nitrogen use efficiency. Commun. Soil Sci. Plant Anal. 2007, 38, 69–76. [Google Scholar] [CrossRef]
- Turk, M.; Bayram, G.; Budakli, E.; Celik, N. A study on effects of different mixtures of zeolite with soil rates on some yield parameters of Alfafa (Medicago sativa). J. Agron. 2006, 5, 118–121. [Google Scholar] [CrossRef][Green Version]
- Hernández-Espinosa, M.A.; Quiroz-Estrada, K.; Petranovskii, V.; Rojas, F.; Portillo, R.; Salgado, M.A.; Marcelo, M.; Efraín, R.; Felipe, C. Adsorption of N2, N2O and CO2 on epistilbite natural zeolite from Jalisco, Mexico after acid-treatment. Mineral 2018, 8, 196. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Chapter 4—Zeolites and their potential uses in agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 113, pp. 219–241. [Google Scholar]
- Zwingmann, N.; Mackinnon, I.D.R.; Gilkes, R.J. Use of zeolite synthesized from alkali treated kaolin as K fertilizer: Glasshouse experiments on leaching and uptake of K by wheat plants in sandy soil. Appl. Clay Sci. 2011, 53, 684–690. [Google Scholar] [CrossRef]
- Millán, G.; Agosto, F.; Vázquez, M.; Botto, L.; Lombardi, L.; Juan, L. Use of clinoptilolite zeolite as a carrier for nitrogen fertilizers in soils of the Pampean regions of Argentina. Cienc. Investig. Agrar. 2008, 35, 245–254. [Google Scholar]
- Palanivell, P.; Ahmed, O.H.; Ab Majid, N.M. Minimizing ammonia volatilization from urea, improving lowland rice (cv. MR219) seed germination, plant growth variables, nutrient uptake, and nutrient recovery using clinoptilolite zeolite. Arch. Agron. Soil Sci. 2016, 62, 708–724. [Google Scholar] [CrossRef]
- Omar, L.; Ahmed, O.H.; Jalloh, M.B.; Muhamad, A.M.N. Soil nitrogen fractions, nitrogen use efficiency and yield of Zea mays L. grown on a tropical acid soil treated with composts and clinoptilolite zeolite. Appl. Sci. 2020, 10, 4139. [Google Scholar] [CrossRef]
- Omar, L.; Ahmed, O.H.; Majid, N.M.A. Shot term enhancement of nutrients availability in Zea mays L. cultivation on an acid soil using compost and clinoptilolite zeolite. Compost Sci. Util. 2017, 25, 22–35. [Google Scholar] [CrossRef]
- Omar, L.; Ahmed, O.H.; Majid, M.M.A. Enhancing nutrient use efficiency of maize (Zea mays L.) from mixing urea with zeolite and peat soil water. Int. J. Phys. Sci. 2011, 6, 3330–3335. [Google Scholar]
- Aainaa, H.N.; Ahmed, O.H.; Kasim, S.; Majid, N.M. Reducing Eqypt rock phosphate use in zea mays cultivation on an acid soil using clinoptilolite zeolite. Sustain. Agric. Res. 2015, 4, 56–66. [Google Scholar] [CrossRef][Green Version]
- Ahmed, O.H.; Aainaa, H.N.; Majid, N.M.A. Zeolites to Unlock Phosphorus Fixation in Agriculture; Universiti Putra Malaysia Press: Serdang, Malaysia, 2017; pp. 1–188. [Google Scholar]
- Siti Rashima, R.; Wan Nur Hafzan, W.M.; Hazzeman, H. Physicochemical properties and sensory acceptability of pineapples of different varieties and stages of maturity. Food Res. 2019, 3, 491–500. [Google Scholar] [CrossRef]
- Lim, E.T. Peat Soils of Sarawak and the Analytical Methods; Department of Agriculture Sarawak: Kuching, Malaysia, 1991; pp. 25–28. [Google Scholar]
- Dugan, E.; Verhoef, A.; Robinson, S.; Saran, S. Bio-char from sawdust, maize stover and charcoal: Impact on water holding capacitites (WHC) from three soils from Ghana. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Ismail, A.B.; Asing, J.; Zulkefli, M. Residual impact of various land clearing techniques on peat chemical characteristics. In A Case Study at MARDI Peat Research Station, Sessang, Sarawak, Malaysia; Ismail, A.B., Ong, H.K., Mohamad Hanif, M.J., Umi Kalsom, M.S., Eds.; MARDI: Serdang, Malaysia, 2007; pp. 33–61. [Google Scholar]
- Harada, Y.; Inoko, A. The measurement of the cation exchange capacity of composts for the estimation of the degree of maturity. Soil Sci. Plant Nutr. 1980, 26, 127–134. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Bremner, J.M.; Keeney, D.R. Determination and isotope-ratio analysis of different forms of nitrogen in soils: 3. Exchangeable ammonium, nitrate and nitrite by extraction-distillation methods. Soil Sci. Soc. Am. J. 1966, 30, 577–582. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium and potassium. In Methods of soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Omar, L.; Ahmed, O.H.; Majid, N.M. Improving ammonium and nitrate release from urea using clinoptilolite zeolite and compost produced from agricultural wastes. Sci. World J. 2015, 2015, 574201. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.S.; Johnson, G.V. Fertilizer nutrient leaching and nutrient mobility. J. Nat. Resour. Life Sci. Educ. 1996, 25, 128–131. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Baker, D.E., Ellis, R., Jr., Keeney, D.R., Miller, R.H., Rhoades, J.D., Eds.; ASA-SSSA: Madison, WI, USA, 1982; pp. 643–693. [Google Scholar]
- Lehmann, J.; Kaiser, K.; Peter, I. Exchange resin cores for the estimation of nutrient fluxes in highly permeable tropical soil. J. Plant Nutr. Soil Sci. 2001, 164, 57–64. [Google Scholar] [CrossRef]
- Skogley, E.O.; Dobermann, A. Synthetic ion-exchange resins: Soil and environmental studies. J. Environ. Qual. 1996, 25, 13–24. [Google Scholar] [CrossRef]
- Chaudhary, P.; Godara, S.; Cheeran, A.N.; Chaudhari, A.K. Fast and accurate method for leaf area measurement. Int. J. Comput. Appl. 2012, 49, 22–25. [Google Scholar] [CrossRef]
- Malézieux, E.; Côte, F.; Bartholomew, D.P. Crop environment. In The Pineapple: Botany, Production and Uses; Bartholomew, D.P., Paull, R.E., Rohrbach, K.G., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 69–108. [Google Scholar]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. In Handbook of Reference Method for Plant Analysis; Soil and Plant Analysis Council; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 75–84. [Google Scholar]
- Pomares-Garcia, F.; Pratt, P.F. Recovery of 15N-labelled fertilizer from manured and sludged-amended soils. Soil Sci. Soc. Am. J. 1978, 42, 717–720. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, Y.; Fan, C.; Kuang, S.Z. The effects of composted pineapple residue return on soil properties and the growth and yield of pineapple. J. Soil Sci. Plant Nutr. 2013, 13, 433–444. [Google Scholar] [CrossRef]
- Hajar, N.; Zainal, S.; Nadzirah, K.Z.; Siti Roha, A.M.; Atikah, O.; Tengku Elida, T.Z.M. Physicochemical properties analysis of three indexes pineapple (Ananas comosus) peel extract variety N36. APCBEE Procedia 2012, 4, 115–121. [Google Scholar] [CrossRef]
- Othman, H.; Darus, F.M.; Mohammed, A.T. Experiences in peat development for oil palm planting in the MPOB Research Station at Sessang, Saratok. Oil Palm Bull. 2009, 58, 1–13. [Google Scholar]
- Abat, M.; McLaughlin, M.J.; Kriby, J.; Stacey, S.P. Adsorption and desorption of copper and zinc in tropical peat soils of Sarawak, Malaysia. Geoderma 2012, 175, 58–63. [Google Scholar] [CrossRef]
- Sim, A.K.F.; Ishak, C.F.; Hanif, A.H.M.; Melling, L. Effect of N fertilization on N2O emission from a tropical peat soil: A laboratory incubation study. In Proceedings of the 14th International Peat Congress: Peatlands in Balance, Stockholm, Sweden, 3–8 June 2012; p. 7. [Google Scholar]
- MARDI. Master Plan for Malaysian Agricultural Research and Development Institute Sessang Peat Research Station; MARDI: Serdang, Malaysia, 1996; pp. 16–22. [Google Scholar]
- Andriesse, J.P. Nature and Management of Tropical Peat Soils; FAO Soils Bulletin 59; FAO: Rome, Italy, 1988. [Google Scholar]
- Hashim, S.A.; Teh, C.B.S.; Ahmed, O.H. Influence of water table depths, nutrient leaching losses, subsidence of tropical peat soil and oil palm (Elaeis guineensis Jacq.) seedling growth. Malays. J. Soil Sci. 2019, 23, 13–30. [Google Scholar]
- Reeza, A.A.; Hussin, A.; Hanif, A.H.M.; Sukari, M.A.M. Effect of liming and fertilizer application in hemic and sapric of tropical peat: Phosphorus mineralization, infra-red spectroscopy and microscopy. Am. J. Agric. Biol. Sci. 2014, 9, 321–333. [Google Scholar] [CrossRef][Green Version]
- Ahmed, O.H.; Omar, L.; Majid, N.M.A. Enhancing Nitrogen Availability Using Compost and Clinoptilolite Zeolite; Universiti Putra Malaysia Press: Serdang, Malaysia, 2017; pp. 1–257. [Google Scholar]
- Idrus, A.; Titisari, A.D.; Sudiyo, R.; Soekrisno, R. Geology, characterization, quality improvement and recommended utilitzation of natural zeolite (zeolitic tuff) deposits from Gunung Kidul, Yogyakarta Special Territory, Indonesia. In Proceedings of the 2nd IASME/WSEAS International Conference on Geology and Seismology (GES’08), Cambridge, UK, 23–25 February 2008. [Google Scholar]
- Wahono, S.K.; Prasetyo, D.J.; Jatmiko, T.H.; Suwanto, A.; Pratiwi, D.; Hernawan; Vasilev, K. Transformation of modernite-clinoptilolite natural zeolite at different calcination temperatures. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012009. [Google Scholar] [CrossRef]
- Hernawan; Wahono, S.K.; Maryana, R.; Pratiwi, D. Modification of Gunungkidul natural zeolite as bioethanol dehydrating agents. Energy Procedia 2015, 65, 116–120. [Google Scholar] [CrossRef][Green Version]
- Lambert, K.; Vanderdeelen, J. Iron interaction in a peat soil from West-Kalimantan. In Proceedings of the International Symposium on Tropical Peatland, Kuching, Malaysia, 6–10 May 1991; pp. 144–150. [Google Scholar]
- Liu, H.; Lennartz, B. Hydraulic functions of peat soils and ecosystem service. Front. Environ. Sci. 2019, 7, 92. [Google Scholar] [CrossRef]
- Tajuddin, S.A.M.; Rahman, J.A.; Rahim, N.H.A.; Mohamed, R.M.S.R.; Algheethi, A.A.S.A. Influence of potassium on sapric peat under different environmental conditions. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012073. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sánchez, E.; Milán, Z.; Cortés, I.; de la la Rubia, M.A. Application of natural zeolites in anaerobic digestion process: A review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef]
- Colella, C. Natural zeolites. In Studies in Surface Science and Catalysis; Čejka, J., Bekkum, H.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 157, pp. 13–40. [Google Scholar]
- Król, M. Natural vs. synthethic zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and modified zeolite—Alginate composites. Application for removal of heavy metal cations from contaminated water solutions. Minerals 2018, 8, 11. [Google Scholar] [CrossRef]
- Natpinit, P.; Chantrawongphaisal, B.; Anuwattana, R.; Ditkaew, T.; Kongsomboon, C.; Thapnui, P.; Hongcharoensri, P. Reduction of greenhouse emissions using zeolite 4A under different fertilizer usages in rice cultivation. Appl. Environ. Res. 2019, 4, 70–82. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Giuseppe, D.D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstren, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx, and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Murkani, M.; Nasrollahi, M.; Ravanbakhsh, M.; Bahrami, P.; Jaafarzadeh, N.; Fard, H. Evaluation of natural zeolite clinoptilolite efficiency for the removal of ammonium and nitrate from aquatic solutions. Environ. Health Eng. Manag. 2015, 2, 17–22. [Google Scholar]
- Mumpton, F. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 33463–33470. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.K.; Hayashi, S. Modification on natural clinoptilolite zeolite for its NH4+ retention capacity. J. Hazard. Mater. 2009, 169, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, J.; Silvennoinen, H.; Hämäläinen, R.; Limin, S.; Raison, R.J.; Vasander, H. Nitrous oxide fluxes from tropical peat with different disturbance history and management. Biogeosciences 2012, 9, 1337–1350. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Sapek, A.; Sapek, B.; Chrzanowski, S.; Jaszczyński, J. Mobilization of substances in peat soils and their transfer within the groundwater and into surface water. Agron. Res. 2007, 5, 155–163. [Google Scholar]
- Hussain, A.; Saiyudi, N.K.W.M. Introduction to Surface and Colloid Chemistry; Universiti Teknologi Malaysia Press: Johor, Malaysia, 2005; pp. 1–195. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, M.; Niquette, P.; Mimeault, M.; Hausler, R. Advances in principal factors influencing carbon dioxide adsorption on zeolites. Sci. Technol. Adv. Mater. 2008, 9, 013007. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between plant roots, rhizosphere microorganisms, and nitrogen, and its special focus on rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Goss, M.J.; Miller, M.H.; Bailey, L.D.; Grant, C.A. Root growth and distribution in relation to nutrient availability and uptake. Eur. J. Agron. 1993, 2, 56–67. [Google Scholar] [CrossRef]
- McNear, D.H., Jr. The rhizosphere—Roots, soil, and everything in between. Nat. Educ. Know. 2013, 4, 1. [Google Scholar]
- Ramadhani, W.S.; Nuraini, Y. The use of pineapple liquid waste and cow dung compost to improve the availability of soil N, P, and K growth of pineapple plant in an ultisol of Central Lampung. J. Degrad. Min. Lands Manag. 2018, 6, 1457–1465. [Google Scholar] [CrossRef]
- Pegoraro, R.F.; de Souza, B.A.M.; Maia, V.M.M.; da Silva, D.F.; Medeiros, A.C.; Sampaio, R.A. Macronutrient uptake, accumulation and export by the irrigated ‘VICTORIA’ pineapple plant. Rev. Bras. Cienc. Solo 2014, 38, 896–904. [Google Scholar] [CrossRef]
- Hepton, A.; Hodgson, A.S. Processing. In The Pineapple: Botany, Production and Uses; Bartholomew, D.P., Paull, R.E., Rohrbach, K.G., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 281–290. [Google Scholar]
- Zubir, M.N.; Sam, N.S.M.; Ghani, N.S.A.; Ismail, A.A. Growth performance of pineapple (Ananas comosus var. MD2) with different application of granular fertilizer on tropical peat soil. Int. J. Agric. For. Plant 2020, 10, 89–95. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).