Long-Term Field Evaluation of Conventional vs. Micropropagated Plants of Chrysanthemum cinerariifolium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
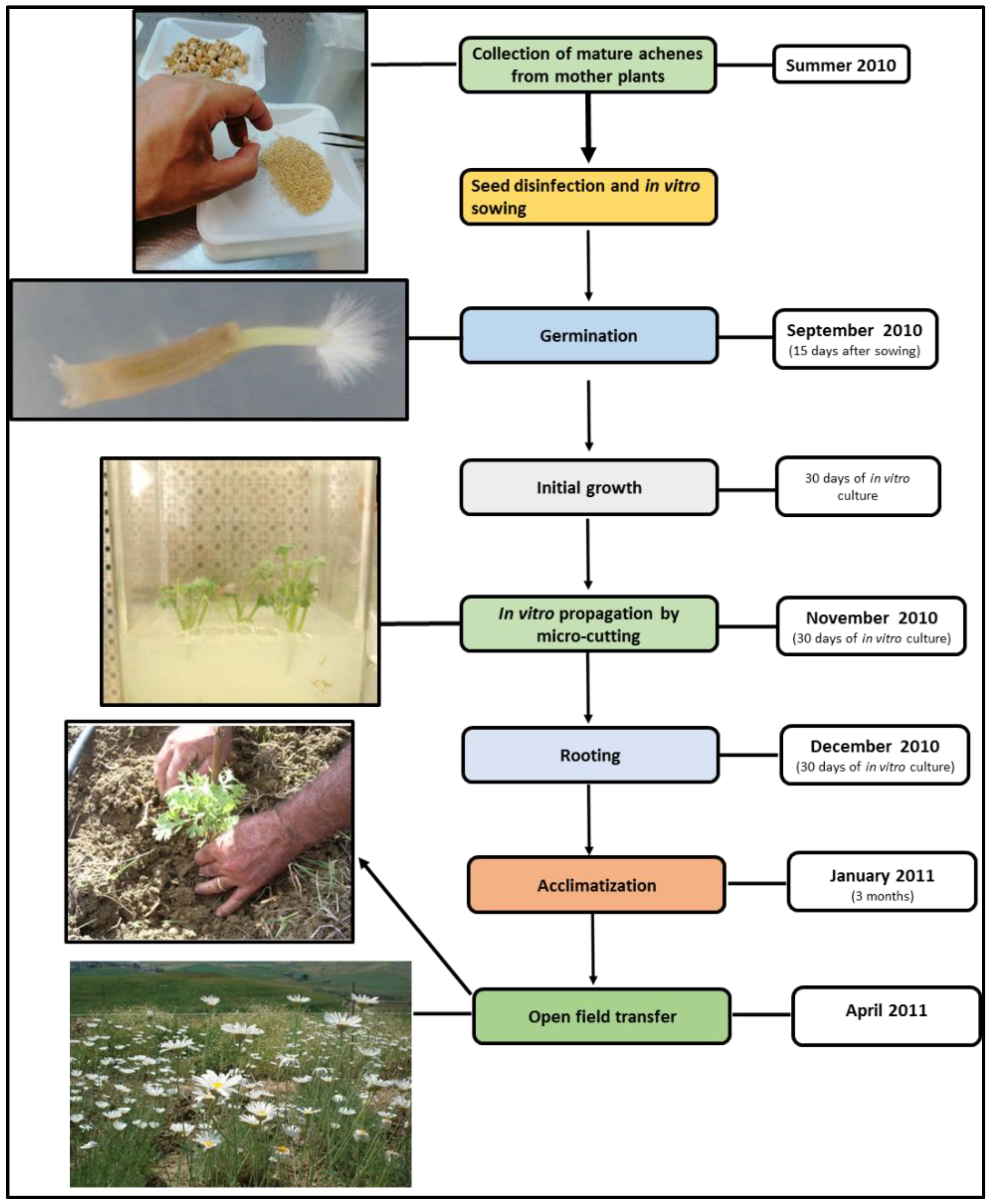
2.2. In Vitro Culture and Acclimatization
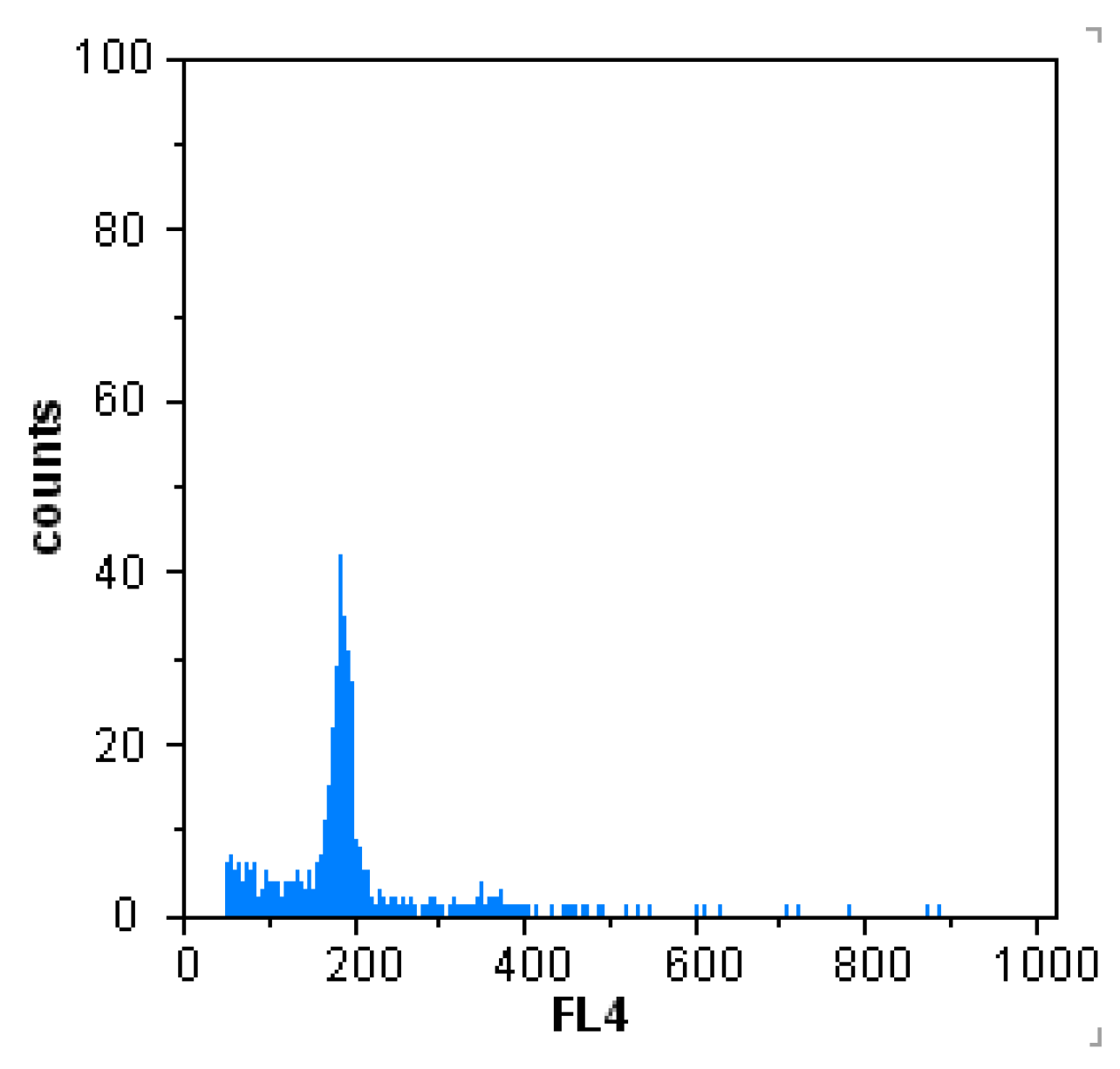
2.3. Assessment of Ploidy through Flow Cytometry
2.4. Conventional Cuttings
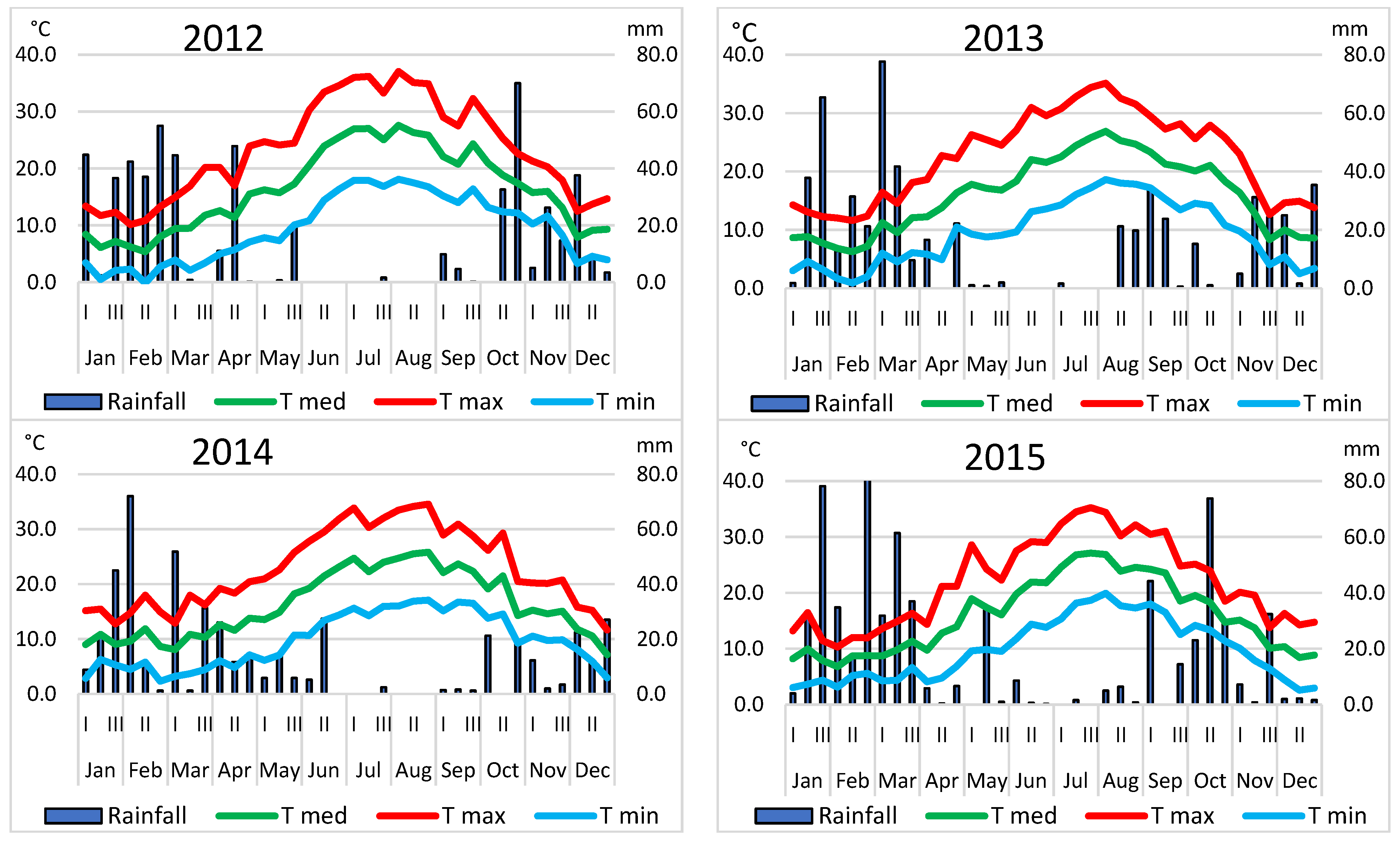
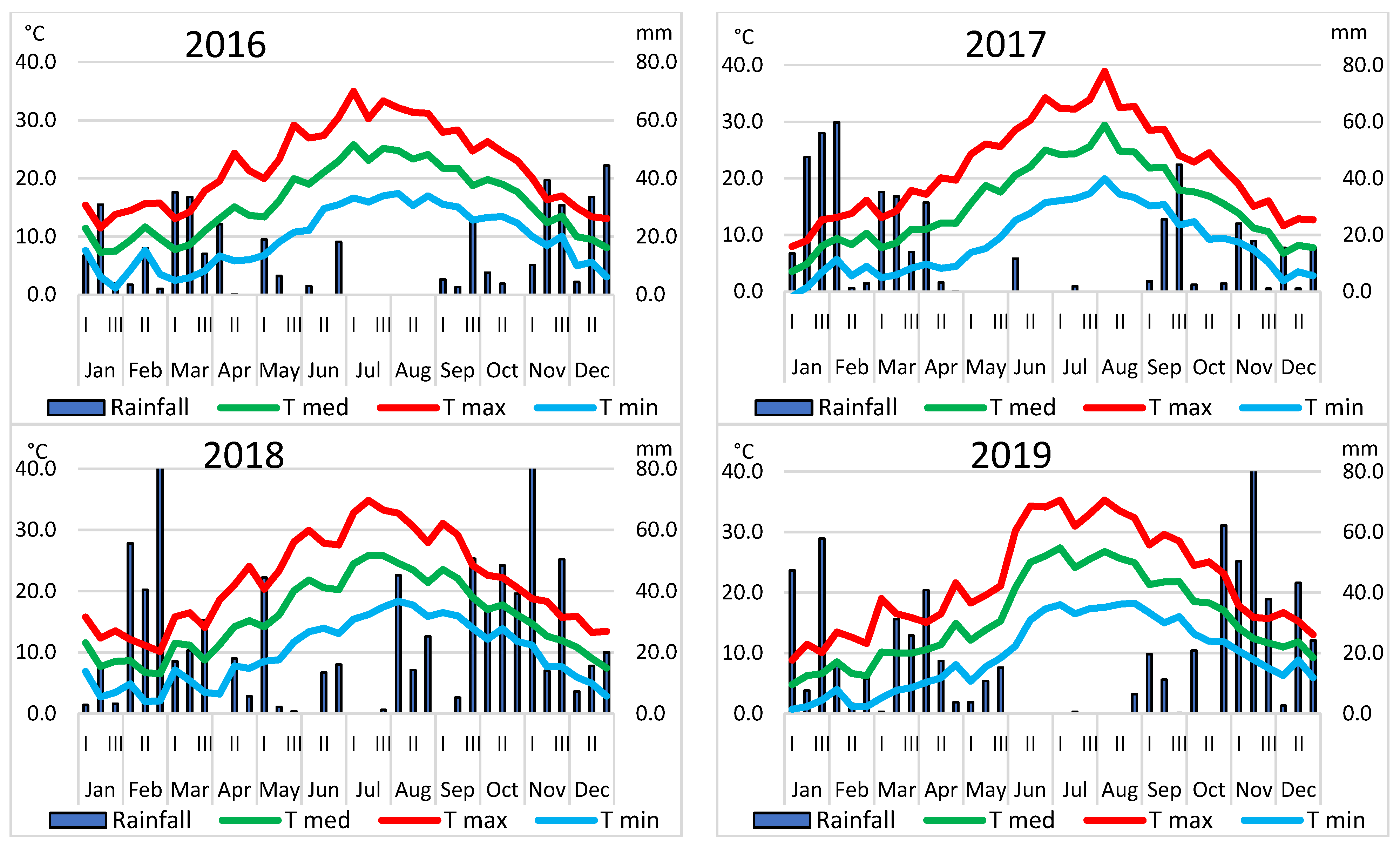
2.5. Open Field Management and Calculation of Thermal Sums
2.6. Statistical Treatment of Data
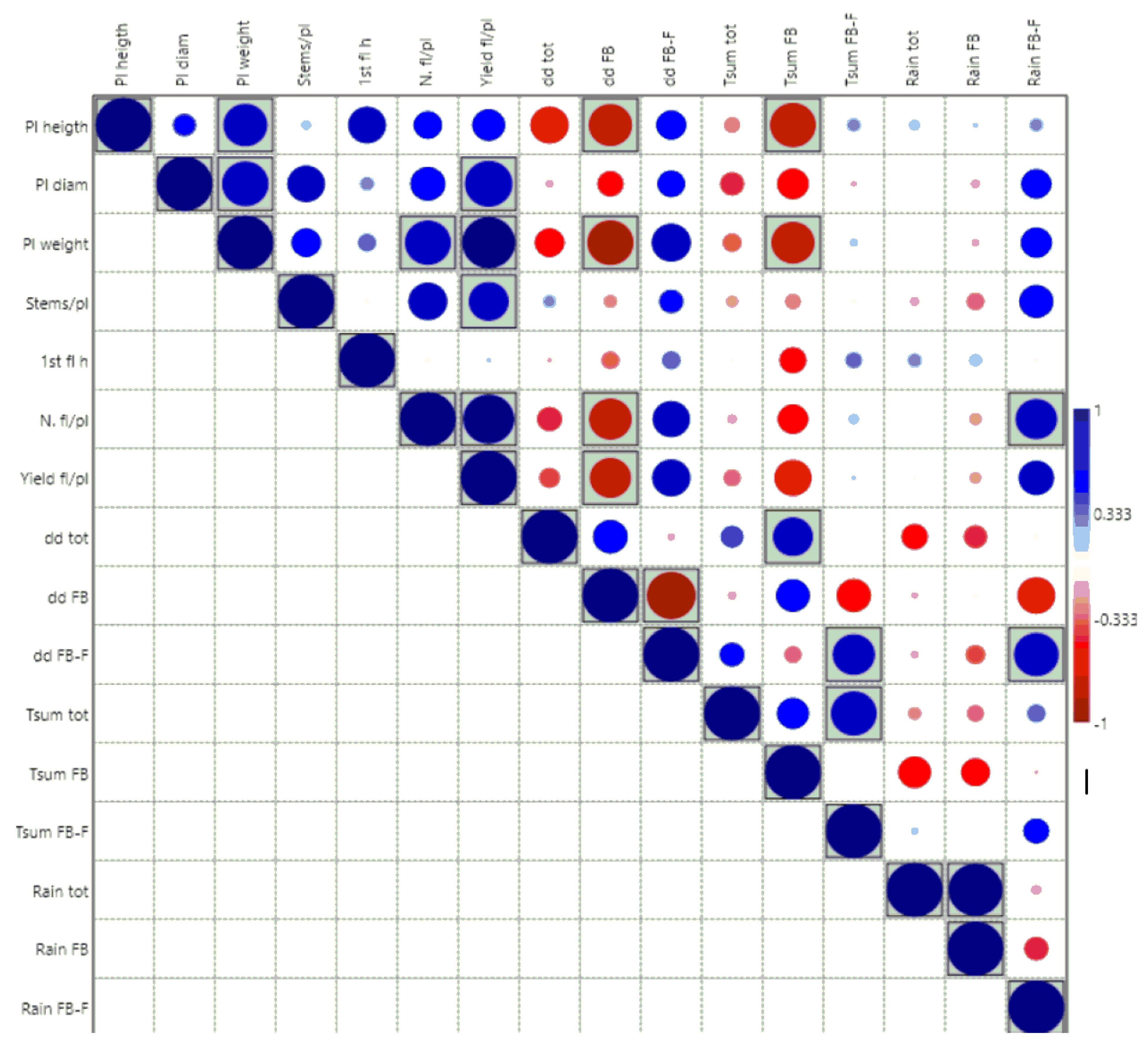
3. Results
3.1. In Vitro Culture and Acclimatization
3.2. Analysis of Ploidy Level
3.3. Field Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- RBGK (Royal Botanic Gardens of Kew). Tanacetum cinerariifolium (Trevis.) Sch. Bip. 2022. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:252275-1#source-KBD (accessed on 3 October 2022).
- Maciver, D.R. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses; Constituents of Pyrethrum Extract; Casida, J.E., Quistad, G.B., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 108–122. ISBN 01-950-82109. [Google Scholar]
- Cantele, A. Coltivazioni Erbacee—Piante Oleifere, da Zucchero, da Fibra, Orticole e Aromatiche; Piretro (Tanacetum cinerariaefolium o Chrisanthemum cinerariaefolium L.); Pàtron Editore: Bologna, Italy, 2001; pp. 449–452. ISBN 9788855526227. [Google Scholar]
- Bruneton, J. Pharmacognosy, Phytochemistry, Medicinal Plants; Lavoiser Publishing: Paris, France, 1995; pp. 493–497. ISBN 9781898298137. [Google Scholar]
- Catalano, C.; Abbate, L.; Fatta del Bosco, S.; Motisi, A.; Carrubba, A. Natural Products: Research Reviews; Micropropagation and in Vitro Culture of Pyrethrum [Chrysanthemum cinerariifolium (Trev.) Vis.]; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2014; Volume 2, pp. 189–212. ISBN 9789351301059. [Google Scholar]
- Grdiša, M.; Jeran, N.; Varga, F.; Klepo, T.; Ninčević, T.; Šatović, Z. Accumulation Patterns of Six Pyrethrin Compounds across the Flower Developmental Stages—Comparative Analysis in Six Natural Dalmatian Pyrethrum Populations. Agronomy 2022, 12, 252. [Google Scholar] [CrossRef]
- Jeran, N.; Grdiša, M.; Varga, F.; Šatović, Z.; Liber, Z.; Dabić, D.; Biošić, M. Pyrethrin from Dalmatian pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch. Bip.): Biosynthesis, biological activity, methods of extraction and determination. Phytochem. Rev. 2021, 20, 875–905. [Google Scholar] [CrossRef]
- Brewer, J.K. Flowering and seedsetting in pyrethrum (Chrisanthemum cinerariaefolium Vis.). A review. Pyrethrum Post 1968, 9, 18–21. [Google Scholar]
- Bhat, B.K. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses; Breeding Methodologies Applicable to Pyrethrum; Casida, J.E., Quistad, G.B., Eds.; Oxford University Press: New York, NY, USA, 1995; pp. 67–95. ISBN 01-950-82109. [Google Scholar]
- Bhat, B.K.; Menary, R.C. Scanning electron microscopic study of oil glands in pyrethrum flowers. Pyrethrum Post 1986, 15, 11–15. [Google Scholar]
- Levy, L.W. A large-scale application of tissue culture: The mass propagation of pyrethrum clones in Ecuador. Environ. Exp. Bot. 1981, 21, 389–395. [Google Scholar] [CrossRef]
- McCown, B.H. Special symposium: In vitro plant recalcitrance of woody and herbaceous perennial plants: Dealing with genetic predeterminism. In Vitro Cell. Dev. Biol. 2000, 36, 149–154. [Google Scholar] [CrossRef]
- Sadat-Hosseini, M.; Vahdati, K.; Leslie, C.A. Germination of Persian walnut somatic embryos and evaluation of their genetic stability by ISSR fingerprinting and flow cytometry. HortScience 2019, 54, 1576–1580. [Google Scholar] [CrossRef]
- Sahijram, L.; Soneji, J.R.; Bollamma, K. Invited review: Analyzing somaclonal variation in micropropagated bananas (Musa spp.). Plant 2003, 39, 551–556. [Google Scholar]
- Viehmannova, I.; Bortlova, Z.; Vitamvas, J.; Cepkova, P.H.; Eliasova, K.; Svobodova, E.; Travnickova, M. Assessment of somaclonal variation in somatic embryo-derived plants of yacon [Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson] using inter simple sequence repeat analysis and flow cytometry. Electron. J. Biotechnol. 2014, 17, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mohandass, S.; Sampath, V.; Gupta, R. Response of ambient temperatures on production of flowers in pyrethrum at Kodaikanal (India). Acta Hortic. 1986, 188, 163–168. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Hammer, Ø. PAST 4.04. 2022. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 3 October 2022).
- Khan, I.; Khan, M.A.; Shehzad, M.A.; Ali, A.; Mohammad, S.; Ali, H.; Alymeni, M.N.; Ahmad, P. Micropropagation and production of health promoting Lignans in Linum usitatissimum. Plants 2020, 9, 728. [Google Scholar] [CrossRef]
- Lubell-Brand, J.D.; Kurtz, L.E.; Brand, M.H. An in vitro—Ex vitro micropropagation system for hemp. Horttechnology 2021, 31, 199–207. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Sheng Gerald, L.T.; Teixeira da Silva, J.A. Micropropagation in the twenty-first century. Plant Cell Cult. Protoc. 2018, 1815, 17–46. [Google Scholar]
- Hassankhah, A.; Vahdati, K.; Lotfi, M.; Mirmasoumi, M.; Preece, J.; Assareh, M.H. Effects of ventilation and sucrose concentrations on the growth and plantlet anatomy of micropropagated Persian walnut plants. Int. J. Hortic. Sci. Technol. 2014, 1, 111–120. [Google Scholar]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Sliwinska, E.; Zimny, J.; Drozdowska, L. Instability of ploidy level during regeneration of transformed and non-transformed tobacco (Nicotiana tabacum L.). Biotechnologia 2003, 3, 260–266. (In Polish) [Google Scholar]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- Sliwinska, E.; Thiem, B. Genome size stability in six medicinal plant species propagated in vitro. Biol. Plant 2007, 51, 556–558. [Google Scholar] [CrossRef]
- Loureiro, J.; Capelo, A.; Brito, G.; Rodriguez, E.; Silva, S.; Pinto, G.; Santos, C. Micropropagation of Juniperus phoenicea from adult plant explants and analysis of ploidy stability using flow cytometry. Biol. Plant 2007, 51, 7–14. [Google Scholar] [CrossRef]
- Thiem, B.; Sliwinska, E. Flow cytometric analysis of nuclear DNA content in cloudberry (Rubus chamaemorus L.) in vitro cultures. Plant Sci. 2003, 164, 129–134. [Google Scholar] [CrossRef]
| Source | DF | Plant Height (cm) | Plant Diameter (cm) | Plant Weight (g) | N. of Stems/Plant | Height 1st Flower (cm) | N. Flowers/Plant | Yield of Flowers/Plant (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 7 | ||||||||||||||
| 2012 | 61.13 ± 2.1 | AB | 36.46 ± 2.7 | BC | 116.14 ± 4.0 | CD | 10.17 ± 1.1 | D | 40.37 ± 1.9 | AB | 50.30 ± 6.6 | B | 18.93 ± 2.7 | B | |
| 2013 | 62.08 ± 2.6 | AB | 32.49 ± 1.3 | C | 123.19 ± 5.4 | CD | 31.62 ± 3.7 | BC | 38.95 ± 1.6 | AB | 90.50 ± 11.1 | AB | 34.91 ± 4.1 | B | |
| 2014 | 68.52 ± 2.1 | A | 47.86 ± 4.2 | A | 293.5 ± 23.5 | A | 54.63 ± 5.2 | A | 31.32 ± 2.6 | B | 153.4 ± 18.8 | A | 96.80 ± 14.2 | A | |
| 2015 | 65.35 ± 1.9 | A | 43.55 ± 1.5 | AC | 199.67 ± 9.2 | B | 30.41 ± 4.3 | BC | 46.10 ± 1.5 | A | 78.80 ± 10.3 | B | 55.84 ± 7.5 | AB | |
| 2016 | 60.10 ± 3.2 | AB | 41.72 ± 2.1 | AC | 178.7 ± 11.1 | BC | 30.24 ± 2.4 | BC | 42.18 ± 2.2 | AB | 68.96 ± 9.9 | B | 48.19 ± 3.3 | B | |
| 2017 | 39.47 ± 1.9 | C | 35.39 ± 2.9 | BC | 61.16 ± 8.1 | D | 24.33 ± 2.8 | CD | 17.63 ± 1.8 | C | 60.33 ± 9.2 | B | 24.63 ± 3.3 | B | |
| 2018 | 50.50 ± 4.9 | BC | 42.48 ± 2.0 | AC | 131.9 ± 14.5 | BD | 46.29 ± 6.8 | AB | 38.32 ± 3.7 | AB | 85.30 ± 13.6 | AB | 41.95 ± 4.0 | B | |
| 2019 | 57.84 ± 2.2 | AB | 43.64 ± 0.9 | AC | 162.49 ± 8.9 | BC | 64.05 ± 2.9 | A | 41.44 ± 2.2 | AB | 87.20 ± 10.3 | AB | 55.10 ± 1.9 | AB | |
| F-value (7, 52) | 11.08 *** | 4.85 *** | 29.99 *** | 18.71 *** | 15.35 *** | 7.28 *** | 12.55 *** | ||||||||
| Errora | 52 | ||||||||||||||
| Propagation method (PM) | 1 | ||||||||||||||
| Cuttings | 61.40 ± 1.8 | A | 39.20 ± 1.3 | 181.1 ± 12.4 | A | 34.12 ± 2.5 | 38.99 ± 1.7 | A | 88.69 ± 6.1 | 51.16 ± 3.9 | |||||
| In vitro micropropagated | 56.03 ± 1.7 | B | 41.20 ± 1.5 | 134.86 ± 7.7 | B | 32.07 ± 3.3 | 35.40 ± 1.6 | B | 74.30 ± 8.0 | 40.35 ± 5.3 | |||||
| F-value (1, 24) | 8.10 ** | 2.11 n.s. | 5.05 * | 1.40 n.s. | 10.82 ** | <1 n.s. | <1 n.s. | ||||||||
| PM *Y | 7 | ||||||||||||||
| F-value (7, 24) | 2.30 n.s. | <1 n.s. | <1 n.s. | 1.30 n.s. | 2.33 n.s. | 1.54 n.s. | <1 n.s. | ||||||||
| Errorb | 24 | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalano, C.; Carra, A.; Carimi, F.; Motisi, A.; Abbate, L.; Sarno, M.; Carrubba, A. Long-Term Field Evaluation of Conventional vs. Micropropagated Plants of Chrysanthemum cinerariifolium. Agronomy 2022, 12, 2756. https://doi.org/10.3390/agronomy12112756
Catalano C, Carra A, Carimi F, Motisi A, Abbate L, Sarno M, Carrubba A. Long-Term Field Evaluation of Conventional vs. Micropropagated Plants of Chrysanthemum cinerariifolium. Agronomy. 2022; 12(11):2756. https://doi.org/10.3390/agronomy12112756
Chicago/Turabian StyleCatalano, Caterina, Angela Carra, Francesco Carimi, Antonio Motisi, Loredana Abbate, Mauro Sarno, and Alessandra Carrubba. 2022. "Long-Term Field Evaluation of Conventional vs. Micropropagated Plants of Chrysanthemum cinerariifolium" Agronomy 12, no. 11: 2756. https://doi.org/10.3390/agronomy12112756






