Effect of Light Intensity on Gene Expression in Hypocotyl during the Elongation in a Leaf-Yellowing Mutant of Pepper (Capsicum annuum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Treatments
2.2. Determination of Phenotypic Data
2.3. RNA Extraction and Library Preparation
2.4. Transcriptome Analysis
2.5. Quantitative Real-Time PCR
2.6. Data Analysis
2.7. Accession Numbers
3. Results
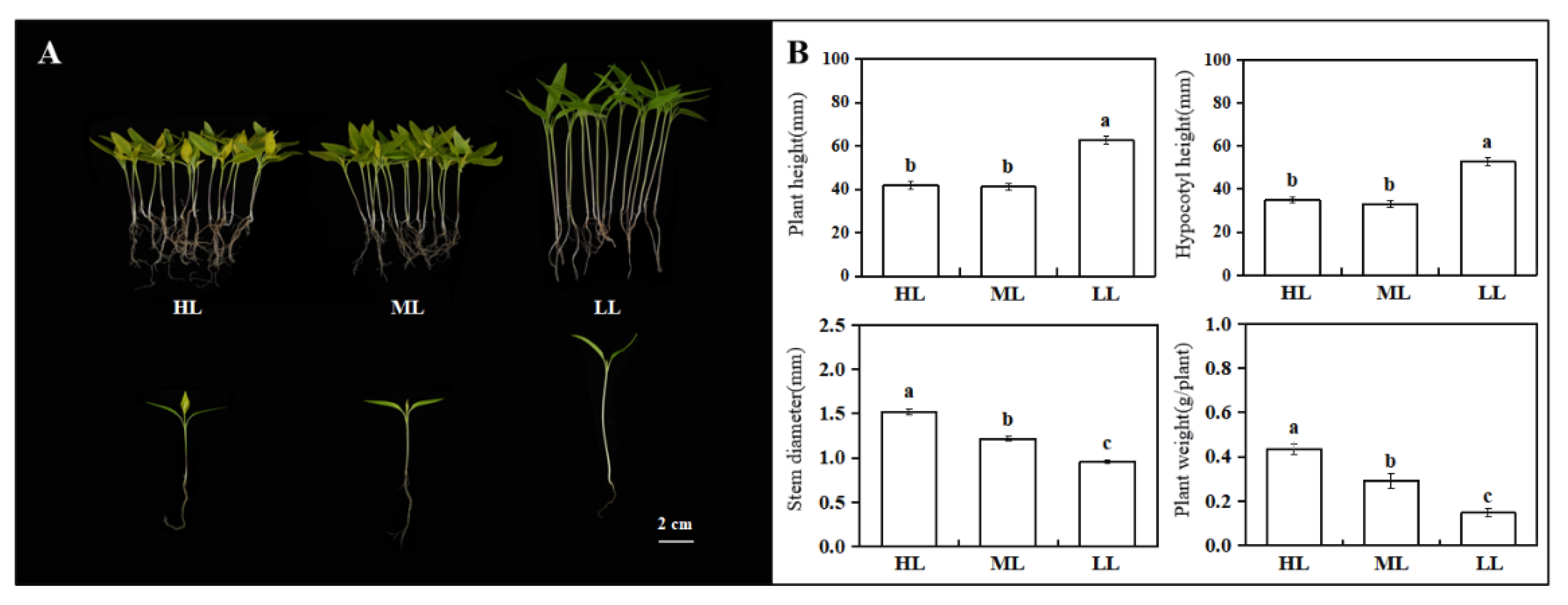
3.1. Plant Phenotypic Index Statistics
3.2. Specificity of Gene Accumulation in Tissue Types of Pepper
3.3. Identification of Differentially Expressed Genes (DEGs)
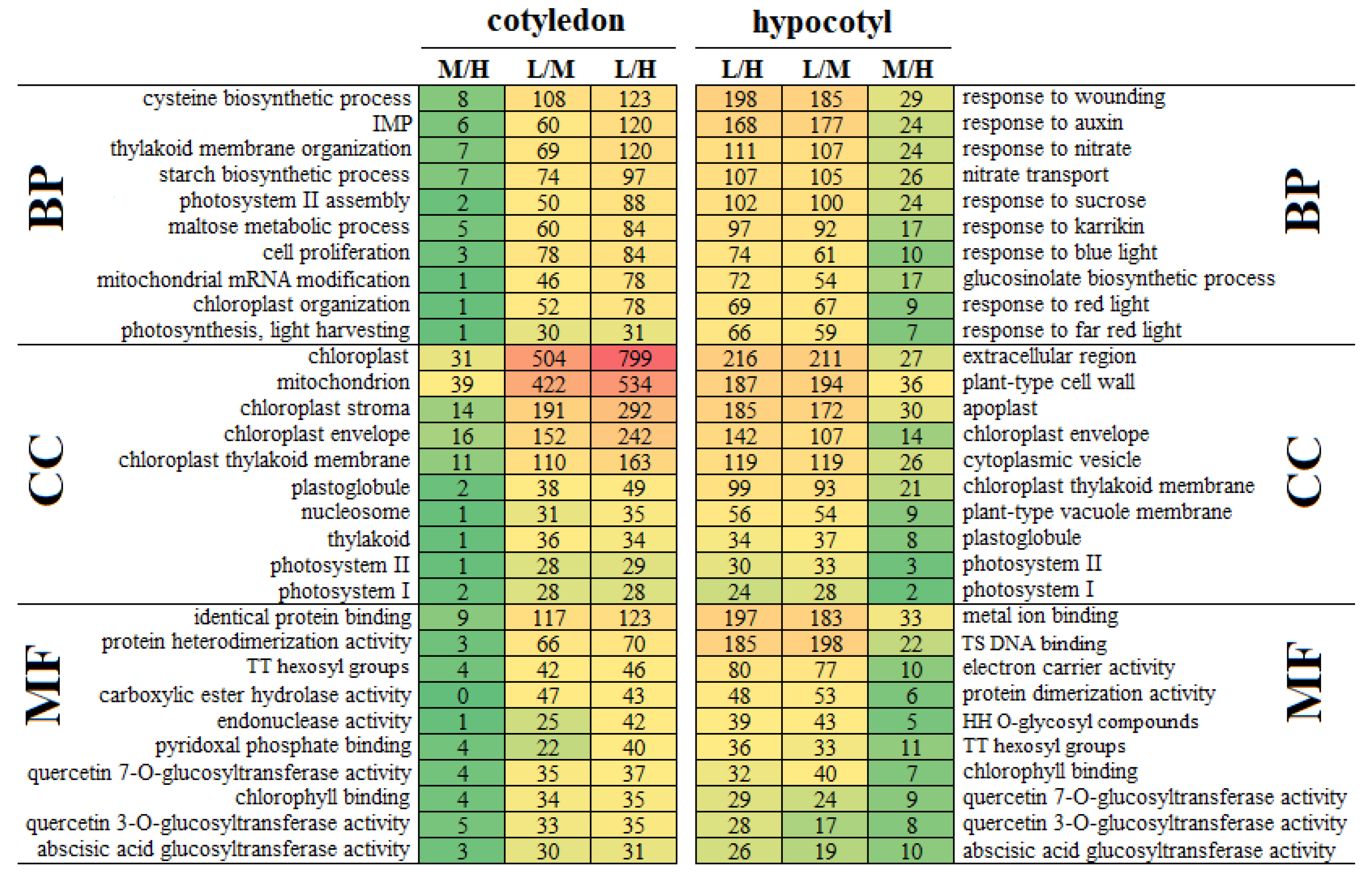
3.4. GO Analysis of DEGs
3.5. Aux/IAA Expression Analysis
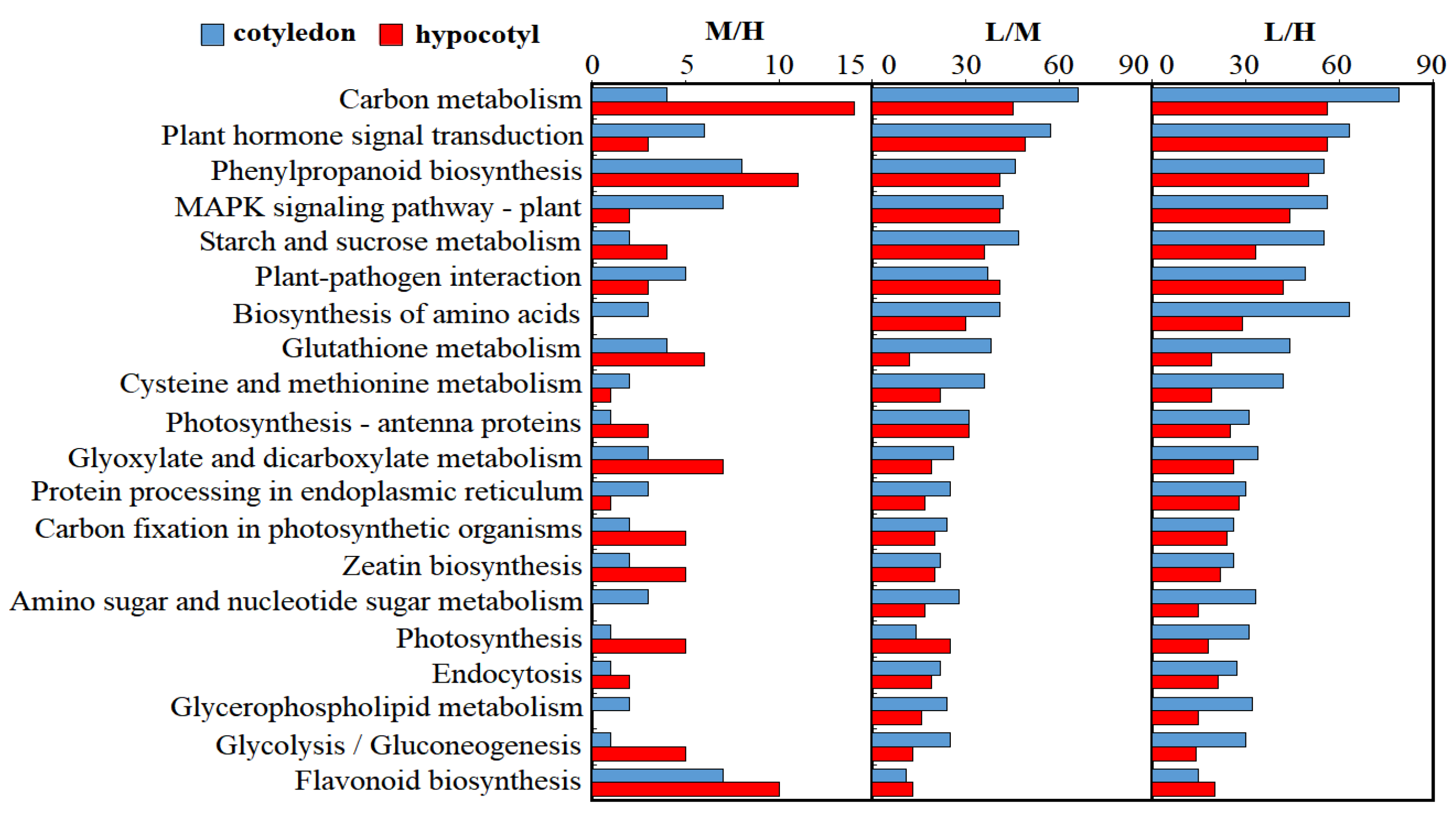
3.6. KEGG Enrichment
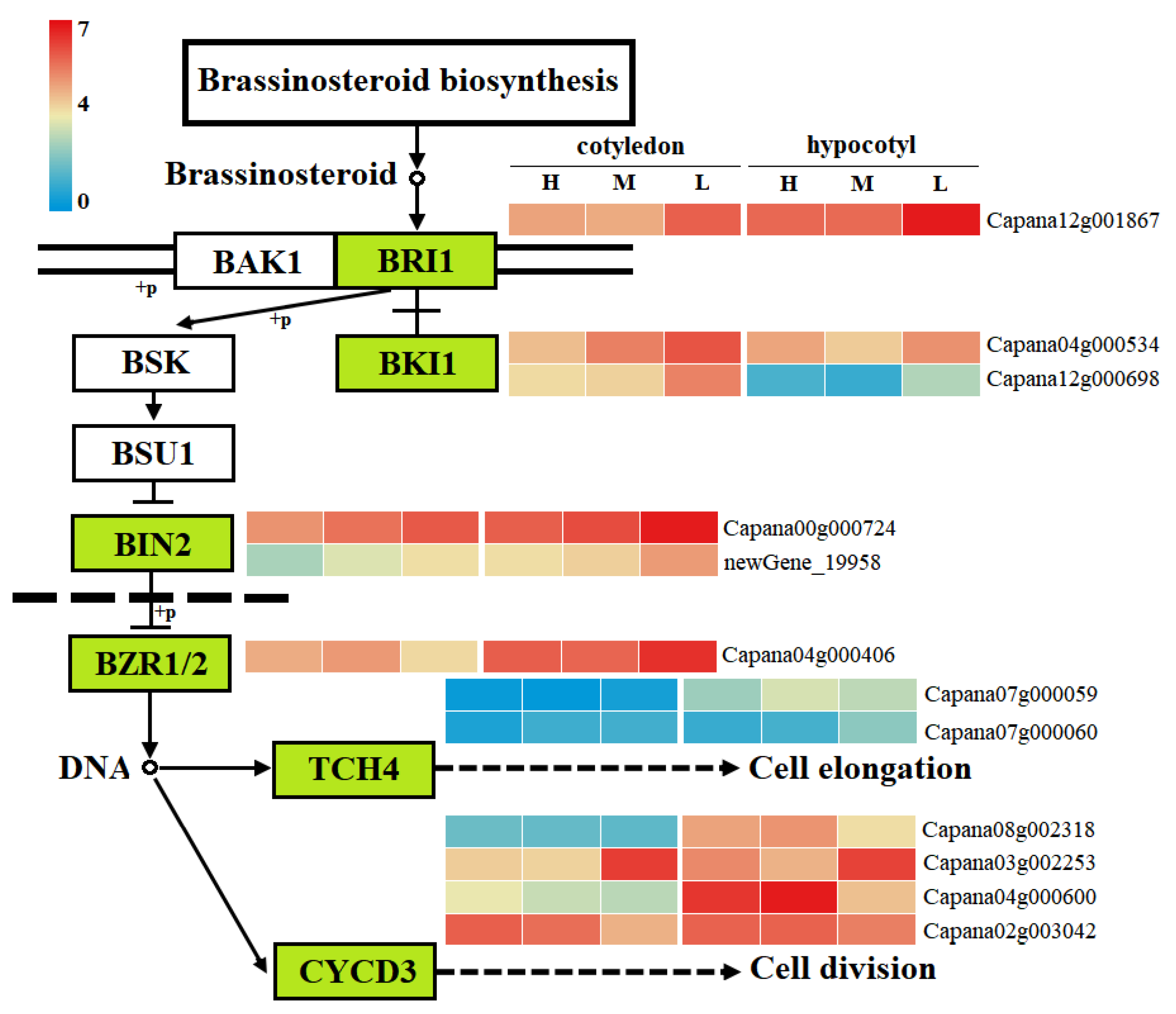
3.7. Gene Expression Network Analysis of Plant Hormone Signal Transduction Pathway
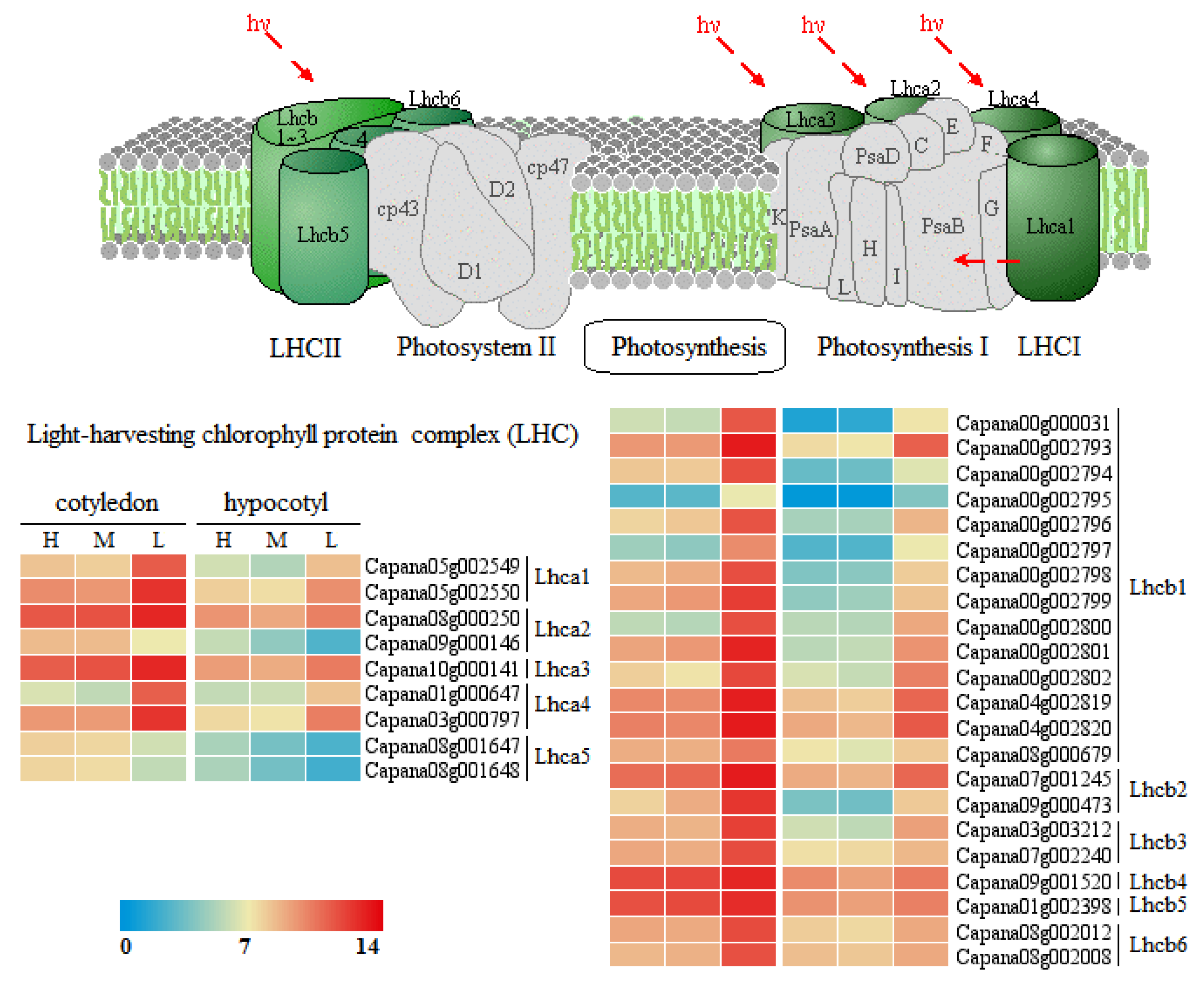
3.8. Gene Expression Network Analysis of Photosynthesis-Antenna Proteins Pathway
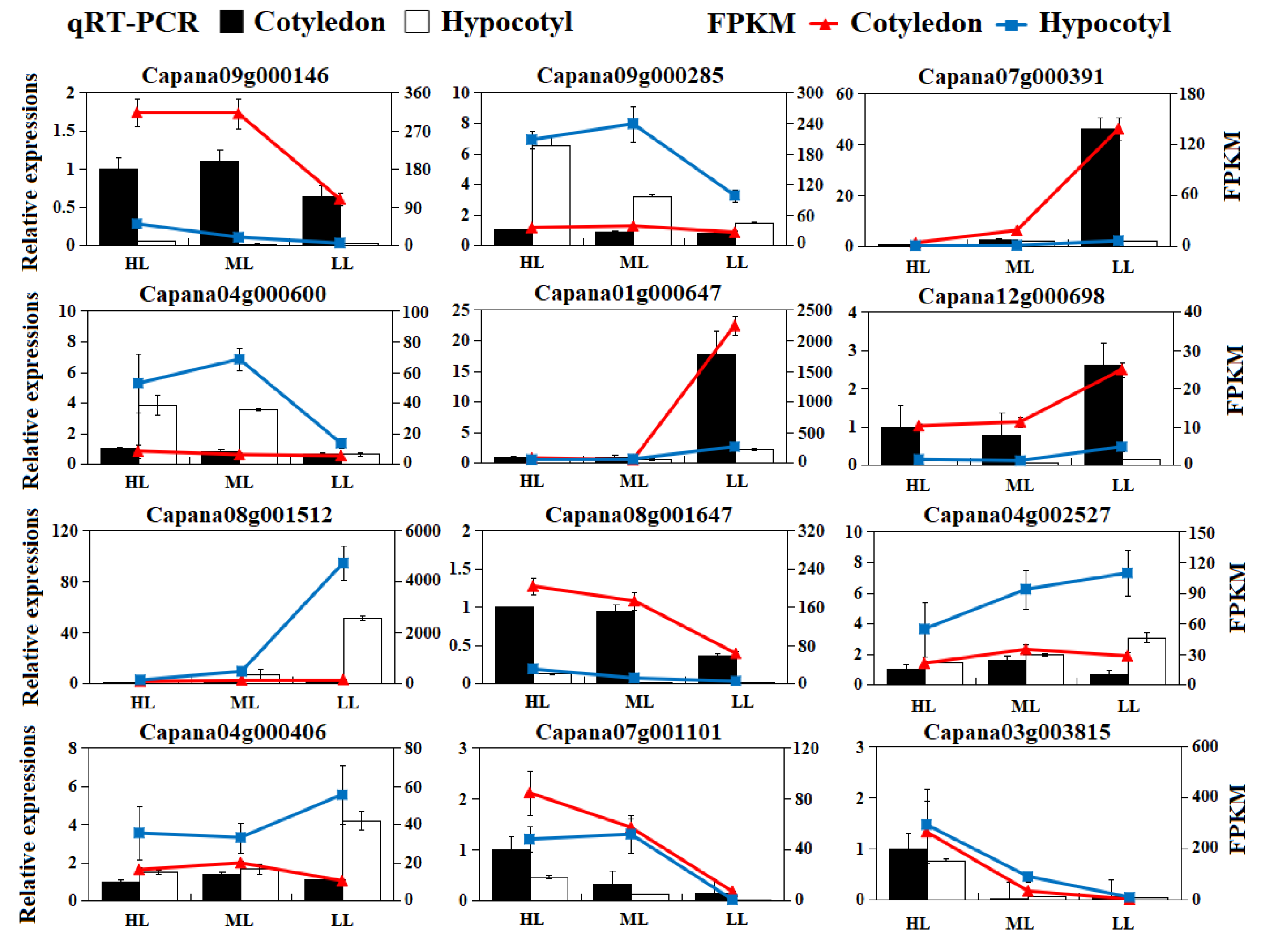
3.9. RT-qPCR Validation of Gene Expression Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.S.; Sun, B.M.; Cai, W.; Zhou, X.; Mao, Y.H.; Chen, C.J.; Wei, J.L.; Cao, B.H.; Chen, C.M.; Chen, G.J.; et al. Natural vari-ations in the MYB transcription factor MYB31 determine the evolution of extremely pungent peppers. New Phytol. 2019, 223, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.M.; Zhou, X.; Chen, C.M.; Chen, C.J.; Chen, K.H.; Chen, M.X.; Liu, S.J.; Chen, G.J.; Cao, B.H.; Cao, F.R.; et al. Coexpression network analysis reveals an MYB transcriptional activator involved in capsaicinoid biosynthesis in hot peppers. Hortic. Res. 2020, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 29, 66–74. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.; Toru, M.; Masahumi, J.; Masaaki, H.; Satoru, T.; Yoshikazu, I.; Takuya, I.; Yutaka, S. Effects of Supplemental Lighting with Light-Emitting Diodes (LEDs) on Tomato Yield and Quality of Single-Truss Tomato Plants Grown at High Planting Density. Seibutsu Kankyo Chosetsu 2012, 50, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wei, H.B.; Ma, M.D.; Li, Q.Q.; Kong, D.X.; Sun, J.; Ma, X.J.; Wang, B.B.; Chen, C.X.; Xie, Y.R.; et al. Arabidopsis FHY3 and FAR1 Proteins Regulate the Balance between Growth and Defense Responses Under Shade Conditions. Plant Cell 2019, 31, 2089–2106. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Kim, K.; Kang, H.; Zulfugarov, I.S.; Bae, G.; Lee, C.H.; Lee, D.; Choi, G. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2009, 106, 7660–7665. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Li, B.S.; Demone, J. Photoreceptor partner FHY1 has an independent role in gene modulation and plant development under far-red light. Proc. Natl. Acad. Sci. USA 2014, 111, 11888–11893. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chory, J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef]
- Procko, C.; Crenshaw, C.M.; Ljung, K.; Noel, J.P.; Chory, J. Cotyledon-Generated Auxin Is Required for Shade-Induced Hypocotyl Growth in Brassica rapa. Plant Physiol. 2014, 165, 1285–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.Q.; Wang, X.H.; Sun, J.B.; Mao, T.L. Coordinated Regulation of Hypocotyl Cell Elongation by Light and Ethylene through a microtubule destabilizing protein. Plant Physiol. 2018, 176, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.Q.; Qi, L.L.; Li, Y.N.; Chu, J.F.; Li, C.Y. PIF4–Mediated Activation of YUCCA8 Expression Integrates Temperature into the Auxin Pathway in Regulating Arabidopsis Hypocotyl Growth. PLoS Genet. 2012, 8, e1002594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.H.; Yoon, G.M. Light-induced stabilization of ACS contributes to hypocotyl elongation during the dark-to-light transition in Arabidopsis seedlings. Plant J. 2019, 98, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.Q.; Chen, W.C.; Liang, C.L.; Zou, X.X. Fine-mapping and transcriptome analysis of the photosensitive leaf -yellowing gene CaLY1 in pepper (Capsicum annuum L.). Hortic. Plant J. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Osorio, S.; Alba, R.; Nikoloski, Z.; Kochevenko, A.; Fernie, A.; Giovannoni, J. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 2012, 159, 1713–1729. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Kubota, K.H.; Burton-Smith, R.N.; Tokutsu, R.; Song, C.H.; Akimoto, S.; Yokono, M.; Ueno, Y.; Kim, E.; Watanabe, A.; Murata, K.; et al. Ten antenna proteins are associated with the core in the supramolecular organization of the photosystem I supercomplex in Chlamydomonas reinhardtii. J. Biol. Chem. 2019, 294, 4304–4314. [Google Scholar] [CrossRef]
- Gerotto, C.; Franchin, C.; Arrigoni, G.; Morosinotto, T. In Vivo Identification of Photosystem II Light Harvesting Complexes Interacting with photosystem ii subunits. Plant Physiol. 2015, 168, 1747–1761. [Google Scholar] [CrossRef] [Green Version]
- Pötter, E.; Kloppstech, K. Effects of light stress on the expression of early light-inducible proteins in barley. Eur. J. Biochem. 1993, 214, 779–786. [Google Scholar] [CrossRef]
- Klimmek, F.; Sjödin, A.; Noutsos, C.; Leister, D.; Jansson, S. Abundantly and rarely expressed Lhc protein genes exhibit distinct regulation patterns in plants. Plant Physiol. 2006, 140, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Ganeteg, U.; Klimmek, F.; Jansson, S. Lhca5—An LHC-type protein associated with photosystem I. Plant Mol. Biol. 2004, 54, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Halliday, K.J.; Martinez-Garcı’a, J.F.; Josse, E.M. Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.J.; Gao, Z.X.; Dong, J.; He, H.; Terzaghi, W. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; He, S.; Zhang, J.; Mao, Z.; Wang, W.; Li, T. Photoactivated CRY1 and phyB Interact Directly with AUX/IAA Proteins to Inhibit Auxin Signaling in Arabidopsis. Mol. Plant. 2017, 17, 30373–30378. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Xie, F.; Jiang, Y.; Li, Z.; Huang, X.; Li, L. Phytochrome A Negatively Regulates the Shade Avoidance Response by Increasing Auxin/Indole Acidic Acid Protein Stability. Dev. Cell 2018, 44, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Kloosterman, B.; Visser, R.G.; Bachem, C.W. Isolation and characterization of a novel potato Auxin/Indole-3-Acetic Acid family member (StIAA2) that is involved in petiole hyponasty and shoot morphogenesis. Plant Physiol. Biochem. 2006, 44, 766–775. [Google Scholar] [CrossRef]
- Deng, W.; Fang, Y.; Liu, M.; Wang, X.Y.; Li, Z.G. Down-regulation of SlIAA15 in tomato altered stem xylem de-velopment and production of volatile compounds in leaf exudates. Plant Signal. Behav. 2012, 7, 911–913. [Google Scholar] [CrossRef] [Green Version]
- Oh, E.; Zhu, J.Y.; Bai, M.Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife 2014, 3, e03031. [Google Scholar] [CrossRef]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 1, 164–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Yang, Y.; Yang, J.; Zhang, X.; Pan, Y.; Guo, H. IAA3-mediated repression of PIF proteins coordinates light and auxin signaling in Arabidopsis. PLoS Genet. 2021, 17, e1009384. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, P.; Walker, L.M.; Young, J.C.; Sonawala, A.; Timpte, C.; Estelle, M.; Reed, J.W. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000, 123, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcriptionfactors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- García, B.S.; Lucas, M.D.; Martínez, C.; Ruiz, A.E.; Davière, J.M.; Prat, S. BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes Dev. 2014, 28, 1681–1694. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nam, K.H.; Vafeados, D.; Chory, J. BIN2, a new brassinosteroidinsensitive locus in Arabidopsis. Plant Physiol. 2001, 127, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, Z.; Zeng, Q.W.; Wang, S.B.; Luo, Y.W.; Huang, Y.; Xin, Y.C.; He, N.J. Normal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- He, J.X.; Gendron, J.M.; Sun, Y.; Gampala, S.L.; Gendron, N.; Sun, C.Q.; Wang, Z.Y. BZR1 is a transcriptional re-pressor with dual roles in brassinosteroid homeostasis and growth responses. Science 2005, 307, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- He, G.H.; Liu, J.; Dong, H.X.; Sun, J.Q. The Blue Light Receptor CRY1 Interacts with BZR1and BIN2 to Modulate the Phosphorylation and Nuclear Function of BZR1 in Repressing BR Signaling in Arabidopsis. Mol. Plant. 2019, 12, 689–703. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, Y.J.; Corvalán, C.; Fujioka, S.; Cho, S.; Park, T.; Choe, S. Darkness and gulliver2/phyB mutation decrease the abundance of phosphorylated BZR1 to activate brassinosteroid signaling in Arabidopsis. Plant J. 2014, 77, 737–747. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Dai, Y.; Huang, Y.; Sun, H.; Li, Z.; Yang, B.; Zhang, Z.; Chen, W.; Ou, L.; Liu, Z.; et al. Effect of Light Intensity on Gene Expression in Hypocotyl during the Elongation in a Leaf-Yellowing Mutant of Pepper (Capsicum annuum L.). Agronomy 2022, 12, 2762. https://doi.org/10.3390/agronomy12112762
Mao L, Dai Y, Huang Y, Sun H, Li Z, Yang B, Zhang Z, Chen W, Ou L, Liu Z, et al. Effect of Light Intensity on Gene Expression in Hypocotyl during the Elongation in a Leaf-Yellowing Mutant of Pepper (Capsicum annuum L.). Agronomy. 2022; 12(11):2762. https://doi.org/10.3390/agronomy12112762
Chicago/Turabian StyleMao, Lianzhen, Yunhua Dai, Yu Huang, Hao Sun, Ziyu Li, Bozhi Yang, Zhuqing Zhang, Wenchao Chen, Lijun Ou, Zhoubin Liu, and et al. 2022. "Effect of Light Intensity on Gene Expression in Hypocotyl during the Elongation in a Leaf-Yellowing Mutant of Pepper (Capsicum annuum L.)" Agronomy 12, no. 11: 2762. https://doi.org/10.3390/agronomy12112762







