Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experiment 1: The Effect of Temperature on In Vitro Seed Germination
2.3. Experiment 2: Effect of Temperature and Sucrose Concentration on Bulblet In Vitro Production
2.4. Experimental Design and Statistical Analysis
3. Results
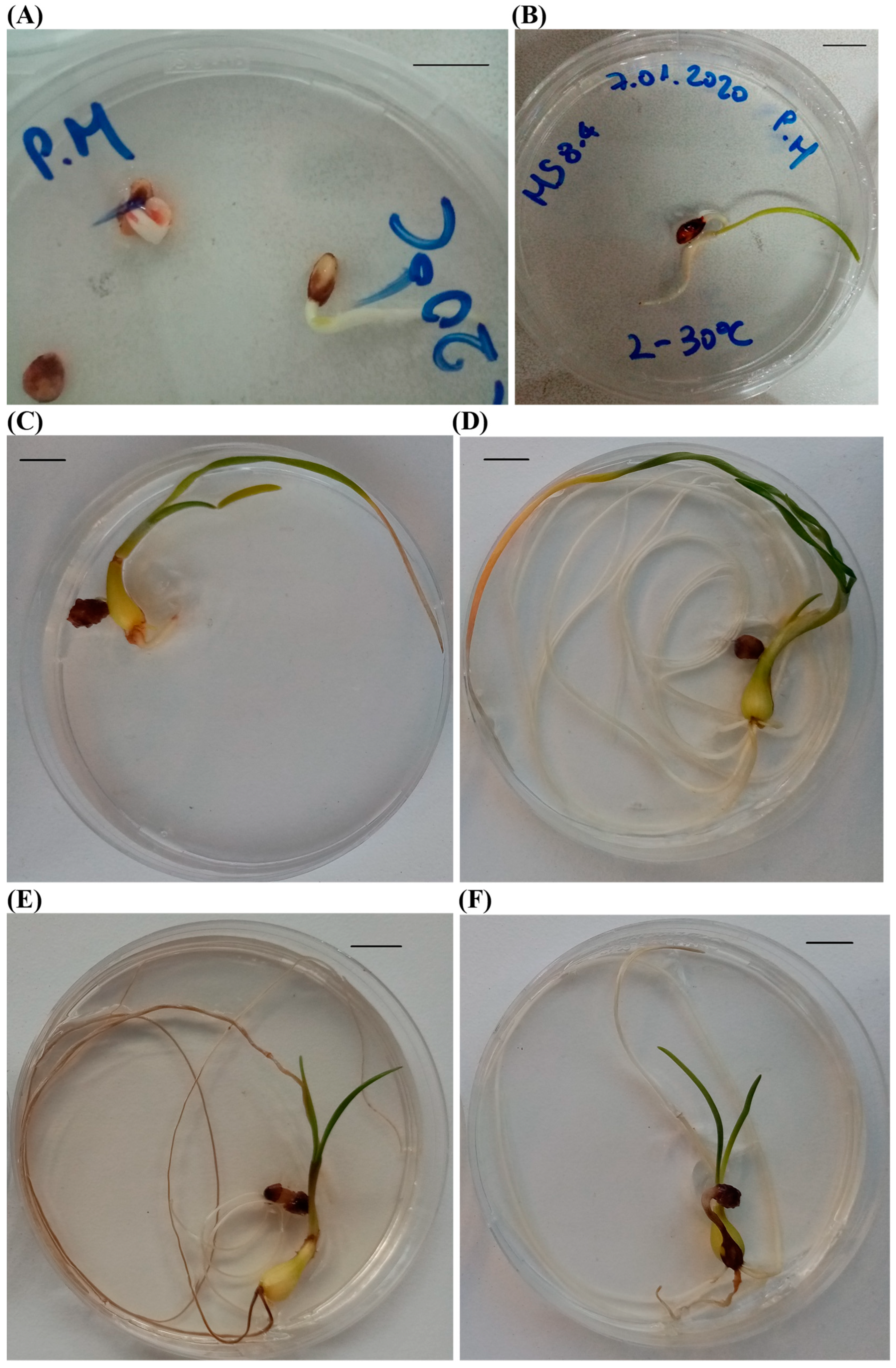
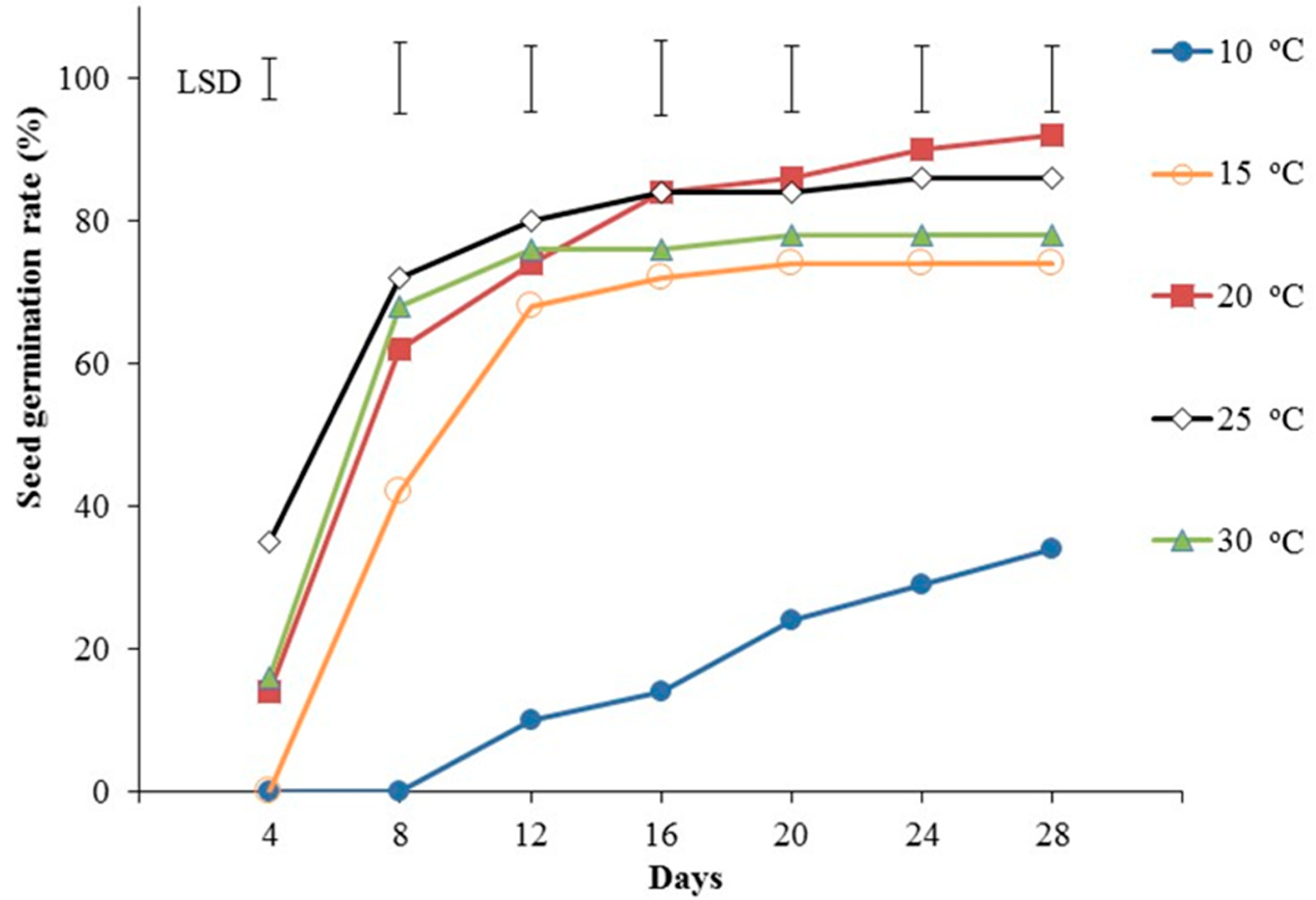
3.1. Experiment 1
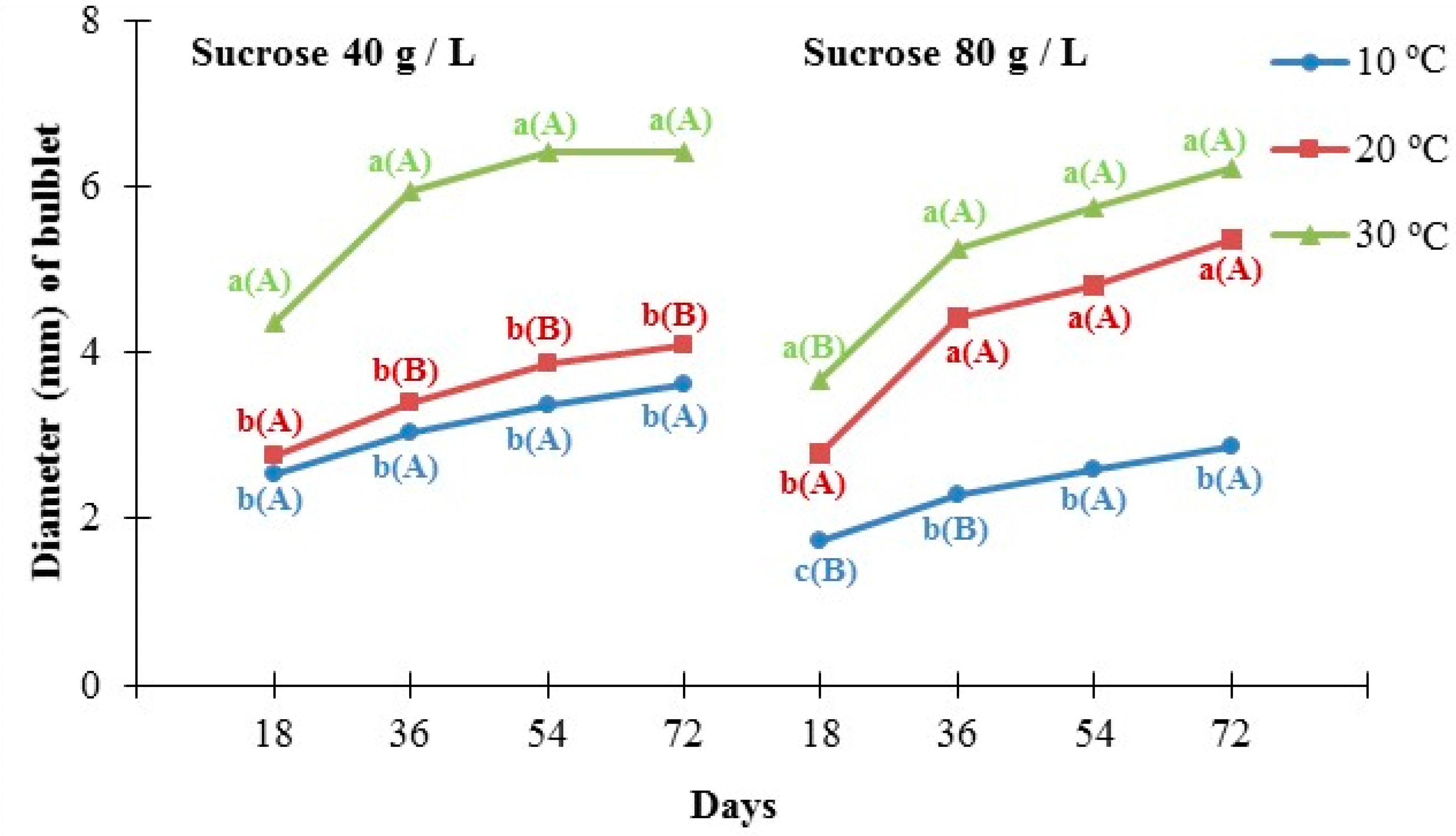
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkanis, A.; Polyzos, N.; Kompocholi, M.; Petropoulos, S.A. Rock Samphire, a Candidate Crop for Saline Agriculture: Cropping Practices, Chemical Composition and Health Effects. Appl. Sci. 2022, 12, 737. [Google Scholar] [CrossRef]
- Petropoulos, S.; Ntatsi, G.; Levizou, E.; Barros, L.; Ferreira, I. Nutritional profile and chemical composition of Cichorium spinosum ecotypes. LWT Food Sci. Technol. 2016, 73, 95–101. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Differential regulation of photorespiratory gene expression by moderate and severe salt and drought stress in relation to oxidative stress. Plant Sci. 2011, 180, 540–547. [Google Scholar] [CrossRef]
- Blamey, M.; Grey-Wilson, C. Mediterranean Wild Flowers; Blamey, M., Grey-Wilson, C., Eds.; HarperCollins: New York, NY, USA, 1993. [Google Scholar]
- Giovino, A.; Domina, G.; Bazan, G.; Campisi, P.; Scibetta, S. Taxonomy and conservation of Pancratium maritimum (Amaryllidaceae) and relatives in the Central Mediterranean. Acta Bot. Gall. 2015, 162, 289–299. [Google Scholar] [CrossRef]
- Zahreddine, H.; Clubbe, C.; Baalbaki, R.; Ghalayini, A.; Talhouk, S.N. Status of native species in threatened Mediterranean habitats: The case of Pancratium maritimum L. (sea daffodil) in Lebanon. Biol. Conserv. 2004, 120, 11–18. [Google Scholar] [CrossRef]
- Nikopoulos, D.; Alexopoulos, A.A. In vitro propagation of an endangered medicinal plant: Pancratium maritimum L. J. Food Agric. Environ. 2008, 6, 393–398. [Google Scholar]
- Grassi, F.; Cazzaniga, E.; Minuto, L.; Peccenini, S.; Barberis, G.; Basso, B. Evaluation of biodiversity and conservation strategies in Pancratium maritimum L. for the Northern Tyrrhenian Sea. Biodivers. Conserv. 2005, 14, 2159–2169. [Google Scholar] [CrossRef]
- Sanaa, A.; Fadhel, N. Ben Genetic diversity in mainland and island populations of the endangered Pancratium maritimum L. (Amaryllidaceae) in Tunisia. Sci. Hortic. 2010, 125, 740–747. [Google Scholar] [CrossRef]
- De Castro, O.; Avino, M.; Di Maio, A.; Menale, B.; Guida, M. Sanger and next generation sequencing in the characterisation of arbuscular mycorrhizal fungi (AMF) in Pancratium maritimum L. (Amaryllidaceae), a representative plant species of Mediterranean sand dunes. Planta 2018, 248, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Pavlov, A.; Georgiev, V.; Weber, J.; Bley, T.; Viladomat, F.; Bastida, J.; Codina, C. Changes in apolar metabolites during in vitro organogenesis of Pancratium maritimum. Plant Physiol. Biochem. 2010, 48, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Catinella, G.; Bruno, M.; Falco, T.; Tundis, R.; Loizzo, M.R. Investigating the Antiproliferative and Antioxidant Properties of Pancratium maritimum L. (Amaryllidaceae) Stems, Flowers, Bulbs, and Fruits Extracts. Evid. Based Complement. Altern. Med. 2018, 2018, 9301247. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, G.A.; Shaala, L.A.; Youssef, D.T.A. Non-alkaloidal compounds from the Bulbs of the Egyptian plant Pancratium maritimum. Z. Für Nat. C 2014, 69, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.; Kekre, N.; Naderi, J.; McNulty, J. Induction of apoptotic cell death specifically in rat and human cancer cells by pancratistatin. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Cedrón, J.C.; Ravelo, Á.G.; León, L.G.; Padrón, J.M.; Estévez-Braun, A. Antiproliferative and structure activity relationships of Amaryllidaceae alkaloids. Molecules 2015, 20, 13854–13863. [Google Scholar] [CrossRef] [Green Version]
- Rokbeni, N.; M’rabet, Y.; Cluzet, S.; Richard, T.; Krisa, S.; Boussaid, M.; Boulila, A. Determination of phenolic composition and antioxidant activities of Pancratium maritimum L. from Tunisia. Ind. Crops Prod. 2016, 94, 505–513. [Google Scholar] [CrossRef]
- Sanaa, A.; Boulila, A.; Boussaid, M.; Fadhel, N. Ben Alginic acid and derivatives, new polymers from the endangered Pancratium maritimum L. Ind. Crops Prod. 2013, 44, 290–293. [Google Scholar] [CrossRef]
- Tayoub, G.; Al-Odat, M.; Amer, A.; Aljapawe, A.; Ekhtiar, A. Antiproliferative effects of Pancratium maritimum extracts on normal and cancerous cells. Iran. J. Med. Sci. 2018, 43, 52–64. [Google Scholar]
- Nair, J.J.; Van Staden, J. Antifungal activity based studies of Amaryllidaceae plant extracts. Nat. Prod. Commun. 2017, 12, 1953–1956. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, R.C.G.; Di Gioia, F.; Ferreira, I.; Petropoulos, S.A. Halophytes for future horticulture: The case of small-scale farming in the mediterranean basin. In Halophytes for Future Horticulture: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–28. ISBN 9783030178543. [Google Scholar]
- Juan Vicedo, J. Pancratium Maritimum. The IUCN Red List of Threatened Species 2018. Available online: https://www.iucnredlist.org/species/18990540/57467022 (accessed on 20 October 2022).
- Sirin, U.; Kanmaz, E. In vitro propagation of sea daffodil (Pancratium maritimum L.) using seedling explants. Fresenius Environ. Bull. 2017, 25, 7710–7716. [Google Scholar]
- Georgiev, V.; Ivanov, I.; Pavlov, A. Obtaining and selection of Pancratium maritimum L. in vitro cultures with acetylcholinesterase inhibitory action. Biotechnol. Biotechnol. Equip. 2010, 24, 149–154. [Google Scholar] [CrossRef]
- Georgiev, V.; Ivanov, I.; Berkov, S.; Pavlov, A. Alkaloids biosynthesis by Pancratium maritimum L. shoots in liquid culture. Acta Physiol. Plant. 2011, 33, 927–933. [Google Scholar] [CrossRef]
- Bogdanova, Y.; Pandova, B.; Yanev, S.; Stanilova, M. Biosynthesis of lycorine by in vitro cultures of Pancratium maritimum L. (Amaryllidaceae). Biotechnol. Biotechnol. Equip. 2009, 23, 919–922. [Google Scholar] [CrossRef]
- Yasemin, S.; Köksal, N.; Büyükalaca, S. Effects of Disinfection Conditions and Culture Media on in vitro Germination of Sea Daffodil (Pancratium maritimum). J. Biol. Environ. Sci. 2018, 12, 13–22. [Google Scholar]
- Dragassaki, M.; Economou, A.S.; Vlahos, J.C. Bulblet formation in vitro and plantlet survival extra vitrum in Pancratium maritimum L. Acta Hortic. 2003, 616, 347–352. [Google Scholar] [CrossRef]
- George, E.F. Plant tissue culture procedure—Background. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., De Klerk, G.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 1, pp. 1–28. ISBN 9781402050046. [Google Scholar]
- Slabbert, M.M.; de Bruyn, M.H.; Ferreira, D.I.; Pretorius, J. Regeneration of bulblets from twin scales of Crinum macowanii in vitro. Plant Cell Tissue Organ Cult. 1993, 33, 133–141. [Google Scholar] [CrossRef]
- Nikopoulos, D.; Nikopoulou, D.; Alexopoulos, A.A. Methods for the preservation of genetic material of Pancratium maritimum (Amaryllidaceae). J. Food Agric. Environ. 2008, 6, 538–546. [Google Scholar]
- Santos, A.; Fidalgo, F.; Santos, I.; Salema, R. In vitro bulb formation of Narcissus asturiensis, a threatened species of the Amaryllidaceae. J. Hortic. Sci. Biotechnol. 2002, 77, 149–152. [Google Scholar] [CrossRef]
- Panayotova, L.G.; Ivanova, T.A.; Bogdanova, Y.Y.; Gussev, C.V.; Stanilova, M.I.; Bosseva, Y.Z.; Stoeva, T.D. In vitro cultivation of plant species from sandy dunes along the Bulgarian Black Sea Coast. Phytol. Balc. 2008, 14, 119–123. [Google Scholar]
- Paradiso, R.; Buonomo, R.; De Pascale, S.; Cardarelli, M. Evaluation of spontaneous species for the innovation in floriculture: Pancratium maritimum L. as ornamental plant. Acta Hortic. 2010, 881, 563–566. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.; Murphy, A. Plant Physiology and Development; Sinauer Associates Publishers: Sunderland, MA, USA, 2015; p. 855. [Google Scholar]
- Cheesman, L.; Finnie, J.F.; Van Staden, J. Eucomis zambesiaca baker: Factors affecting in vitro bulblet induction. South Afr. J. Bot. 2010, 76, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Benvenuti, S. Seed ecology of Mediterranean hind dune wildflowers. Ecol. Eng. 2016, 91, 282–293. [Google Scholar] [CrossRef]
- Balestri, E.; Cinelli, F. Germination and early-seedling establishment capacity of Pancratium maritimum L. (Amaryllidaceae) on coastal dunes in the north-western Mediterranean. J. Coast. Res. 2004, 20, 761–770. [Google Scholar] [CrossRef]
- Delipetrou, P. Ecophysiological Study of Seed Germination in Maritime Plants with Special Emphasis on the Effect of Light. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 1996. [Google Scholar]
- Saadon, S.; Zaccai, M. Lilium candidum bulblet and meristem development. Vitr. Cell. Dev. Biol. Plant 2013, 49, 313–319. [Google Scholar] [CrossRef]
- Ascough, G.D.; Rice, L.J.; Van Staden, J. Considerations for evaluating flower abscission in potted plants with multiple inflorescences—Plectranthus as a case study. South Afr. J. Bot. 2008, 74, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Ptak, A.; Simlat, M.; Kwiecień, M.; Laurain-Mattar, D. Leucojum aestivum plants propagated in in vitro bioreactor culture and on solid media containing cytokinins. Eng. Life Sci. 2013, 13, 261–270. [Google Scholar] [CrossRef]
- Varshney, A.; Sharma, M.P.; Adholeya, A.; Dhawan, V.; Srivastava, P.S. Enhanced growth of micropropagated bulblets of Lilium sp. inoculated with arbuscular mycorrhizal fungi at different P fertility levels in an alfisol. J. Hortic. Sci. Biotechnol. 2002, 77, 258–263. [Google Scholar] [CrossRef]
- Sellés, M.; Viladomat, F.; Bastida, J.; Codina, C. Callus induction, somatic embryogenesis and organogenesis in Narcissus confusus: Correlation between the state of differentiation and the content of galanthamine and related alkaloids. Plant Cell Rep. 1999, 18, 646–651. [Google Scholar] [CrossRef]
- Sultana, J.; Sultana, N.; Siddique, M.N.A.; Islam, A.K.M.A.; Hossain, M.M.; Hossain, T. In vitro bulb production in Hippeastrum (Hippeastrum hybridum). J. Cent. Eur. Agric. 2010, 11, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Azadi, P.; Khosh-Khui, M. Micropropagation of Lilium ledebourii (Baker) Boiss as affected by plant growth regulator, sucrose concentration, harvesting season and cold treatments. Electron. J. Biotechnol. 2007, 10, 582–591. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in Sucrose Cleavage Pattern and Rapid Starch Accumulation Govern Lily Shoot-to-Bulblet Transition in vitro. Front. Plant Sci. 2021, 11, 564713. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Zhou, L.; Fu, X.; Lei, T.; Chen, Q.; Yu, X.; Zhou, Y.; Li, W.; Hu, J.; et al. Sucrose signaling function on the formation and swelling of bulblets of Lilium sargentiae E.H. Wilson. Plant Cell Tissue Organ Cult. 2018, 135, 143–153. [Google Scholar] [CrossRef]
- Pouris, J.; Meletiou-Christou, M.S.; Chimona, C.; Rhizopoulou, S. Seasonal functional partitioning of carbohydrates and proline among plant parts of the sand daffodil. Agronomy 2020, 10, 539. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexopoulos, A.A.; Mavrommati, E.; Kartsonas, E.; Petropoulos, S.A. Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L. Agronomy 2022, 12, 2786. https://doi.org/10.3390/agronomy12112786
Alexopoulos AA, Mavrommati E, Kartsonas E, Petropoulos SA. Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L. Agronomy. 2022; 12(11):2786. https://doi.org/10.3390/agronomy12112786
Chicago/Turabian StyleAlexopoulos, Alexios A., Eleni Mavrommati, Epaminondas Kartsonas, and Spyridon A. Petropoulos. 2022. "Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L." Agronomy 12, no. 11: 2786. https://doi.org/10.3390/agronomy12112786
APA StyleAlexopoulos, A. A., Mavrommati, E., Kartsonas, E., & Petropoulos, S. A. (2022). Effect of Temperature and Sucrose on In Vitro Seed Germination and Bulblet Production of Pancratium maritimum L. Agronomy, 12(11), 2786. https://doi.org/10.3390/agronomy12112786






