Sugarcane Ratoon Yield and Soil Phosphorus Availability in Response to Enhanced Efficiency Phosphate Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Treatments
2.2. Weather Conditions
2.3. Soil Phosphorus
2.4. Plant Analysis
2.5. Statistical Analysis
3. Results
3.1. Phosphorus Availability in the Soil
3.2. Plant Nutritional Status
3.3. Effects on Sugarcane Technological Quality and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2022–2031; OECD: Paris, France, 2022; ISBN 978-92-64-58870-7. [Google Scholar]
- de A Conab, C.N. Acompanhamento da safra brasileira cana-de-açúcar. Acompan. Safra Bras. 2021, 8, 59. [Google Scholar]
- Heinrichs, R.; Otto, R.; Magalhães, A.; Meirelles, G. Importance of Sugarcane InBrazilian and World Bioconomy. In Springer Nature; S. Dabber: Cham, Switzerland, 2017. [Google Scholar]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, A.F.; Flores, R.A.; Casaroli, D.; Bueno, A.M.; Pessoa-de-Souza, M.A.; de Lima, F.S.R.; Marques, E.P. K Dynamics in the Soil–Plant System for Sugarcane Crops: A Current Field Experiment Under Tropical Conditions. Sugar Tech 2021, 23, 1247–1257. [Google Scholar] [CrossRef]
- Gonçalves, V.; Meurer, E.; Tatsch, F.; Carvalho, S.; Neto, O. Biodisponibilidade de Cádmio Em Fertilizantes Fosfatados. Rev. Bras. Ciência Solo 2008, 32, 2871–2875. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Prague, Czech Republic, 2017; ISBN 978-1-60535-255-8. [Google Scholar]
- Sanders, J.L.; Murphy, L.S.; Noble, A.; Melgar, R.J.; Perkins, J. Improving Phosphorus Use Efficiency with Polymer Technology. Procedia Eng. 2012, 46, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Zanão, L.A., Jr.; Arf, O.; dos Reis, R.A., Jr.; Pereira, N. Phosphorus Fertilization with Enhanced Efficiency in Soybean and Corn Crops. Aust. J. Crop Sci. 2020, 14, 78–84. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bayer, C.; Torrent, J.; Barrón, V. Mineralogy and Phosphorus Adsorption in Soils of South and Central-West Brazil under Conventional and No-Tillage Systems. Acta Sci. Agron. 2014, 36, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Gopalasundaram, P.; Bhaskaran, A.; Rakkiyappan, P. Integrated Nutrient Management in Sugarcane. Sugar Tech 2012, 14, 3–20. [Google Scholar] [CrossRef]
- Costa, D.B.D.; Neto, D.E.S.; Freire, F.J.; De, E.C.A. Adubação fosfatada em cana planta e soca em argissolos do nordeste de diferentes texturas. Rev. Caatinga 2014, 27, 10. [Google Scholar]
- Zambrosi, F.C.B. Phosphorus Fertilizer Reapplication on Sugarcane Ratoon: Opportunities and Challenges for Improvements in Nutrient Efficiency. Sugar Tech 2020, 23, 704–708. [Google Scholar] [CrossRef]
- Guelfi, D.R. Tecnologias e Inovações Para Fertilizantes Fosfatados. NPTC 2021, 10, 14–33. [Google Scholar]
- AAPFCO Association of American Plant Food Control Officials (AAPFCO). Available online: http://www.aapfco.org/publications.html (accessed on 18 February 2021).
- Santos, E.F. dos Mecanismos de Interação Fósforo-Zinco no Sistema Solo-Planta: Disponibilidade No Solo, Avaliações Fisiológicas e Expressão de Transportadores de Fosfato. Ph. D. Thesis, em Biologia na Agricultura e no Ambiente, Universidade de São Paulo, Piracicaba, Brazil, 2018. [Google Scholar]
- Soil Survey Division Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação Da Fertilidade de Solos Tropicais; Instituto Agronômico de Campinas: Campinas, Brazil, 2001.
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; Embrapa Solos: Brasilia, DF, Brazil, 2017; Volume 3, ISBN 978-85-7035-771-7. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Gonçalves, J.L.M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia-INMET. Available online: https://portal.inmet.gov.br/ (accessed on 15 April 2021).
- Tedesco, M.J.; Gianello, C.; Anghinoni, I.; Bissani, C.A.; Camargo, F.A.O.; Wiethölter, S. Manual de Adubação e Calagem.; Comissão de Química e Fertilidade do Solo-RS/SC: Porto Alegre, Brazil, 2004. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Malavolta, E. Avaliação Do Estado Nutricional Das Plantas: Princípios e Aplicações/Eurípedes Malavolta, Godofredo Cesar Vitti, Sebastião Alberto de Oliveira. Piracicaba Potafos. 1997. Available online: https://books.google.com.sg/books/about/Avalia%C3%A7%C3%A3o_do_estado_nutricional_das_pl.html?hl=pt-BR&id=Lu9EAAAAYAAJ&redir_esc=y (accessed on 18 October 2022).
- Fernandes, A.C. Cálculos na Agroindústria da Cana-de-açúcar; Sociedade dos Técnicos Açucareiros e Alcooleiros do Brasil: Piracicaba, Brazil, 2011. [Google Scholar]
- SAS® SAS OnDemand for Academics. Available online: https://welcome.oda.sas.com/home (accessed on 9 February 2022).
- Pimentel-Gomes, F. Curso de Estatistica Experimental, 15th ed.; ESALQ: Piracicaba, Brazil, 2009; ISBN 978-85-7133-055-9. [Google Scholar]
- van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, Â.M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo; IAC: Campinas, Brazil, 1997; pp. 173–251. [Google Scholar]
- de Gazola, R.N.; Buzetti, S.; Dinalli, R.P.; Teixeira Filho, M.C.M.; de Celestrino, T.S. Efeito residual da aplicação de fosfato monoamônio revestido por diferentes polímeros na cultura de milho. Rev. Ceres 2013, 60, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Bortolon, L.; Gianello, C. Simultaneous Multielement Extraction with the Mehlich-1 Solution for Southern Brazilian Soils Determined by ICP-OES and the Effects on the Nutrients Recommendations to Crops. Rev. Bras. Ciência Solo 2010, 34, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Chagas, W.F.T.; Emrich, E.B.; Guelfi, D.R.; Caputo, A.L.C.; Faquin, V. Productive characteristics, nutrition and agronomic efficiency of polymer-coated MAP in lettuce crops. Rev. Ciência Agronômica 2015, 46, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Schlindwein, J.A.; Gianello, C. Nível de Suficiência e Índice de Equivalência Entre o Fósforo Determinado Pelos Métodos da Resina de Troca Iônica e Mehilch-1. Curr. Agric. Sci. Technol. 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Schlindwein, J.A.; Bortolon, L. Soil Phosphorus Available for Crops and Grasses Extracted with Three Soil-Test Methods in Southern Brazilian Soils Amended with Phosphate Rock. Commun. Soil Sci. Plant Anal. 2011, 42, 283–292. [Google Scholar] [CrossRef]
- de Freitas, I.F.; Novais, R.F.; de Villani, E.M.A.; Novais, S.V. Phosphorus Extracted by Ion Exchange Resins and Mehlich-1 from Oxisols (Latosols) Treated with Different Phosphorus Rates and Sources for Varied Soil-Source Contact Periods. Rev. Bras. Ciênc. Solo 2013, 37, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Mumbach, G.L.; de Oliveira, D.A.; Warmling, M.I.; Gatiboni, L.C. Phosphorus Extraction by Mehlich 1, Mehlich 3 and Anion Exchange Resin in Soils with Different Clay Contents. Rev. Ceres 2018, 65, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Crusciol, C.A.C.; Mancuso, M.A.C.; Garcia, R.A.; Castro, G.S.A. Crescimento radicular e aéreo de cultivares de arroz de terras altas em função da calagem. Bragantia 2012, 71, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Lefèvre, I.; Ziebel, J.; Guignard, C.; Hausman, J.-F.; Rosales, R.O.G.; Bonierbale, M.; Hoffmann, L.; Schafleitner, R.; Evers, D. Drought Impacts Mineral Contents in Andean Potato Cultivars. J. Agron. Crop Sci. 2012, 198, 196–206. [Google Scholar] [CrossRef]
- Carneiro, F.M.; Furlani, C.E.A.; Ormond, A.T.S.; Kazama, E.H.; Silva, R.P. da Mechanized Fertilization: Individual Application of Nitrogen, Phosphorus and Potassium in Sugarcane. Rev. Ciênc. Agron. 2017, 48, 278–287. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.M.; Dionardo-Miranda, L. Dinâmica do desenvolvimento radicular da cana-de-açúcar e implicações no controle de nematóides, 2nd ed.; Revista e Ampliada: Campinas, Brazil, 2011; ISBN 978-85-87645-48-7. [Google Scholar]
- Simões, W.L.; Calgaro, M.; Guimarães, M.J.M.; de Oliveira, A.R.; Pinheiro, M.P.M.A. Sugarcane crops with controlled water deficit in the submiddle são francisco valley, Brazil. Rev. Caatinga 2018, 31, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants-3rd Edition. Available online: https://www.elsevier.com/books/marschners-mineral-nutrition-of-higher-plants/marschner/978-0-12-384905-2 (accessed on 22 May 2020).
- Calheiros, A.; Oliveira, M.; Ferreira, V.; Barbosa, G.; Costa, J.; Lima, G.; Aristides, E. Acúmulo de Nutrientes e Produção de Sacarose de Duas Variedades de Cana-de-Açúcar Na Primeira Rebrota, Em Função de Doses de Fósforo. STAB 2011, 29, 4. [Google Scholar]
- Inman-Bamber, N.G. Sugarcane Water Stress Criteria for Irrigation and Drying Off. Field Crops Res. 2004, 89, 107–122. [Google Scholar] [CrossRef]
- Araújo, R.; Alves Junior, J.; Casaroli, D.; Evangelista, A.W.P. Variation in the sugar yield in response to drying-off of sugarcane before harvest and the occurrence of low air temperatures. Bragantia 2016, 75, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Simões Neto, D.E.; de Oliveira, A.C.; Freire, F.J.; dos Freire, M.B.G.S.; do Nascimento, C.W.A.; Rocha, A.T. da Extração de fósforo em solos cultivados com cana-de-açúcar e suas relações com a capacidade tampão. Rev. Bras. Eng. Agríc. Ambient. 2009, 13, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, A.W.; de Sá, L.A.; Rodrigues, W.A.R.; Moura, A.B.; dos Oliveira, M.S. Growth and Yield of Sugarcane as a Function of Phosphorus Doses and Forms of Application. Rev. Bras. Eng. Agríc. Ambient. 2016, 20, 29–35. [Google Scholar] [CrossRef]
- de Gava, G.J.C.; de Silva, M.A.; da Silva, R.C.; Jeronimo, E.M.; Cruz, J.C.S.; Kölln, O.T. Produtividade de três cultivares de cana-de-açúcar sob manejos de sequeiro e irrigado por gotejamento. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 250–255. [Google Scholar] [CrossRef] [Green Version]
- de Abreu, M.L.; de Silva, M.A.; Teodoro, I.; de Holanda, L.A.; Sampaio Neto, G.D. Crescimento e produtividade de cana-de-açúcar em função da disponibilidade hídrica dos Tabuleiros Costeiros de Alagoas. Bragantia 2013, 72, 262–270. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, 2nd ed.; International Fertilizer Industry Association (IFA): Paris, France, 2010; ISBN 978-2-9523139-7-1. [Google Scholar]
- Briassoulis, D.; Dejean, C. Critical Review of Norms and Standards for Biodegradable Agricultural Plastics Part Ι. Biodegradation in Soil. J. Polym. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Guelfi, D.R.; Chagas, W.F.T.; Lacerda, J.R.; Chagas, R.M.R.; de Souza, T.L.; Andrade, A.B. Monoammonium Phosphate Coated with Polymers and Magnesium for Coffee Plants. Ciênc. Agrotec. 2018, 42, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Pelá, A.; Ribeiro, M.A.; Bento, R.U.; Cirino, L.H.; Reis Júnior, R.A. Enhanced-Efficiency Phosphorus Fertilizer: Promising Technology for Carrot Crop. Hortic. Bras. 2018, 36, 492–497. [Google Scholar] [CrossRef]
- Pelá, A.; Bento, R.U.; Crispim, L.B.R.; dos Reis, R.A., Jr. Enhanced efficiency of Phosphorus fertilizer in Soybean and Maize. Aust. J. Crop Sci. 2019, 13, 1638–1642. [Google Scholar] [CrossRef]
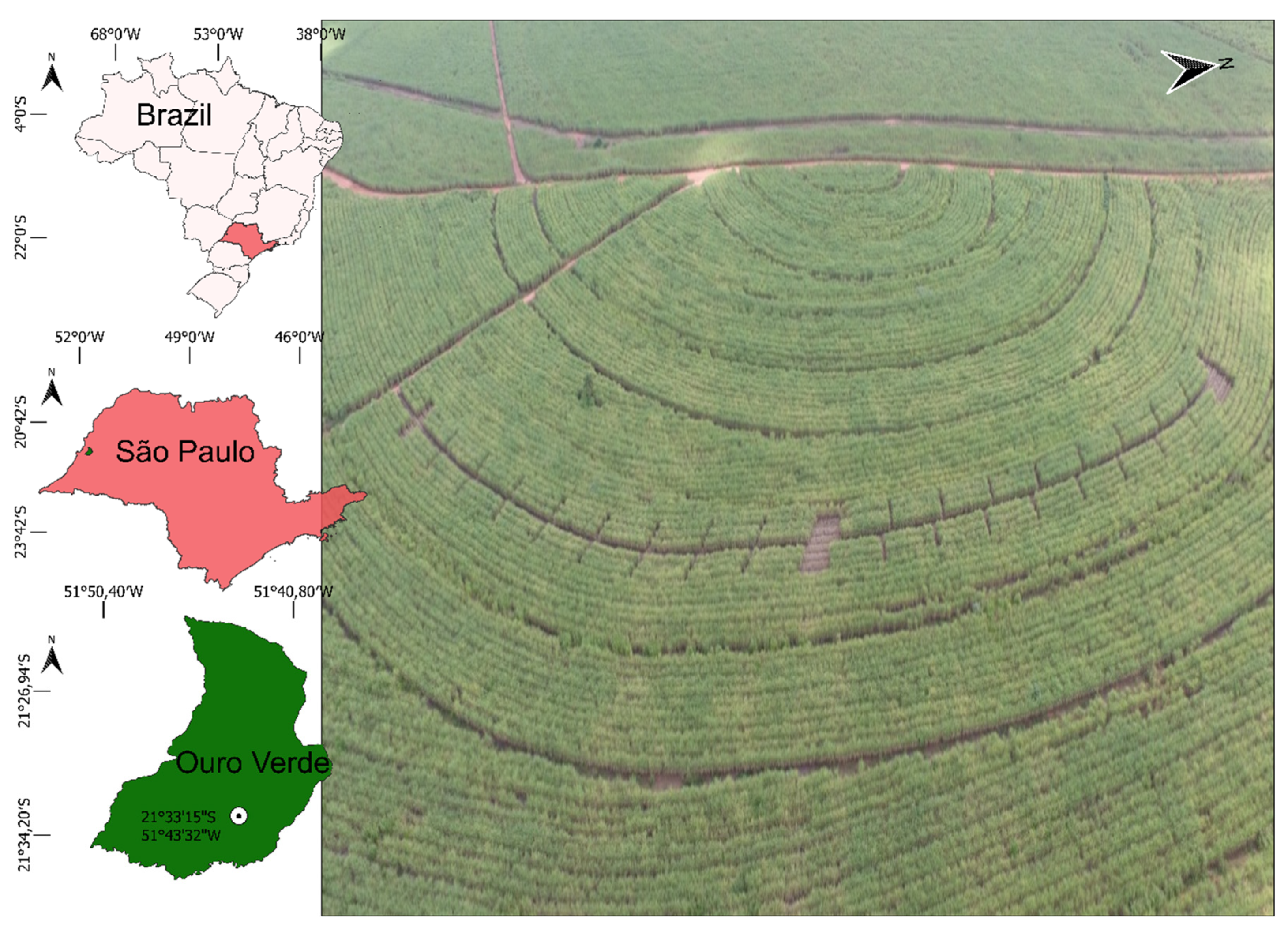
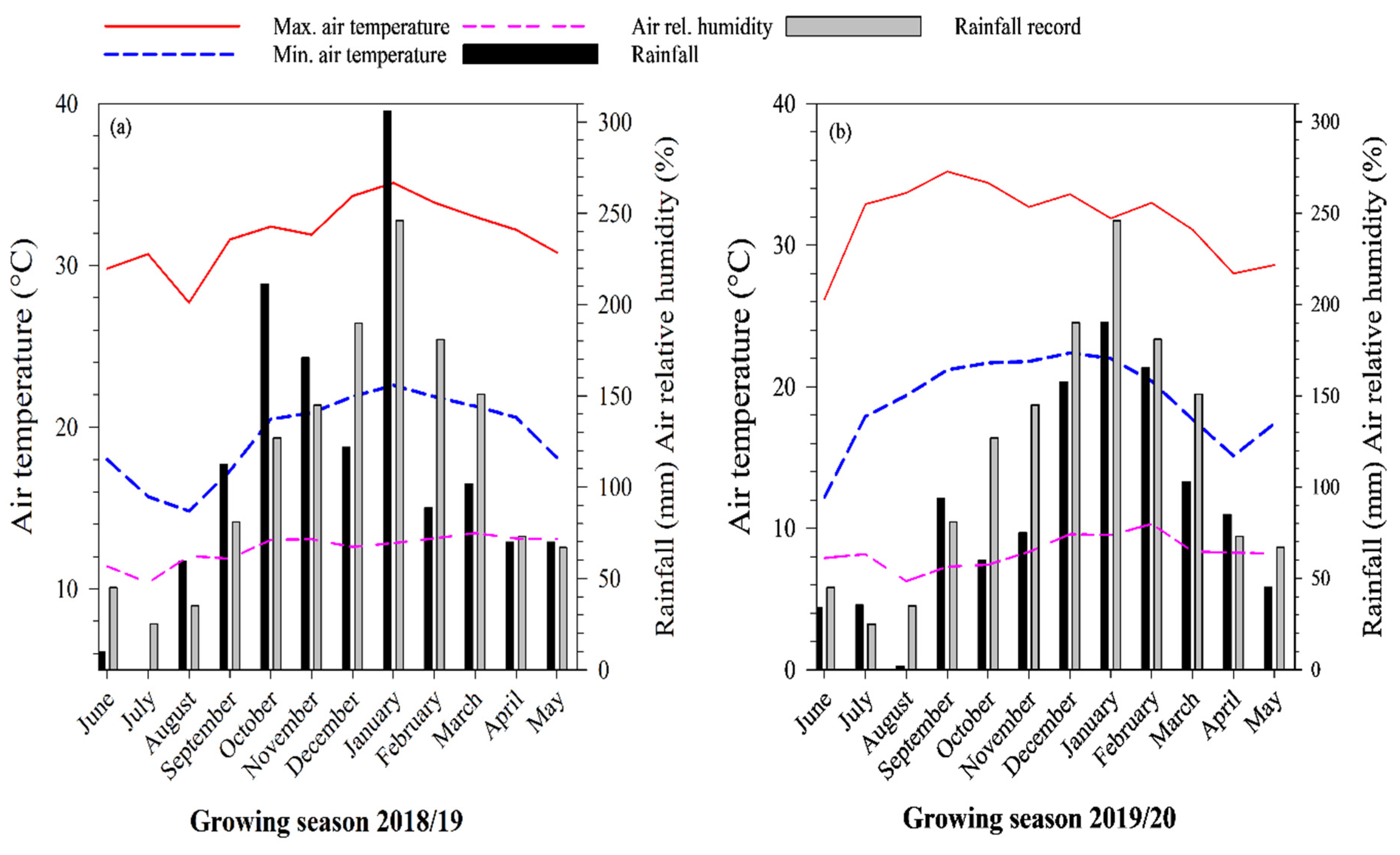

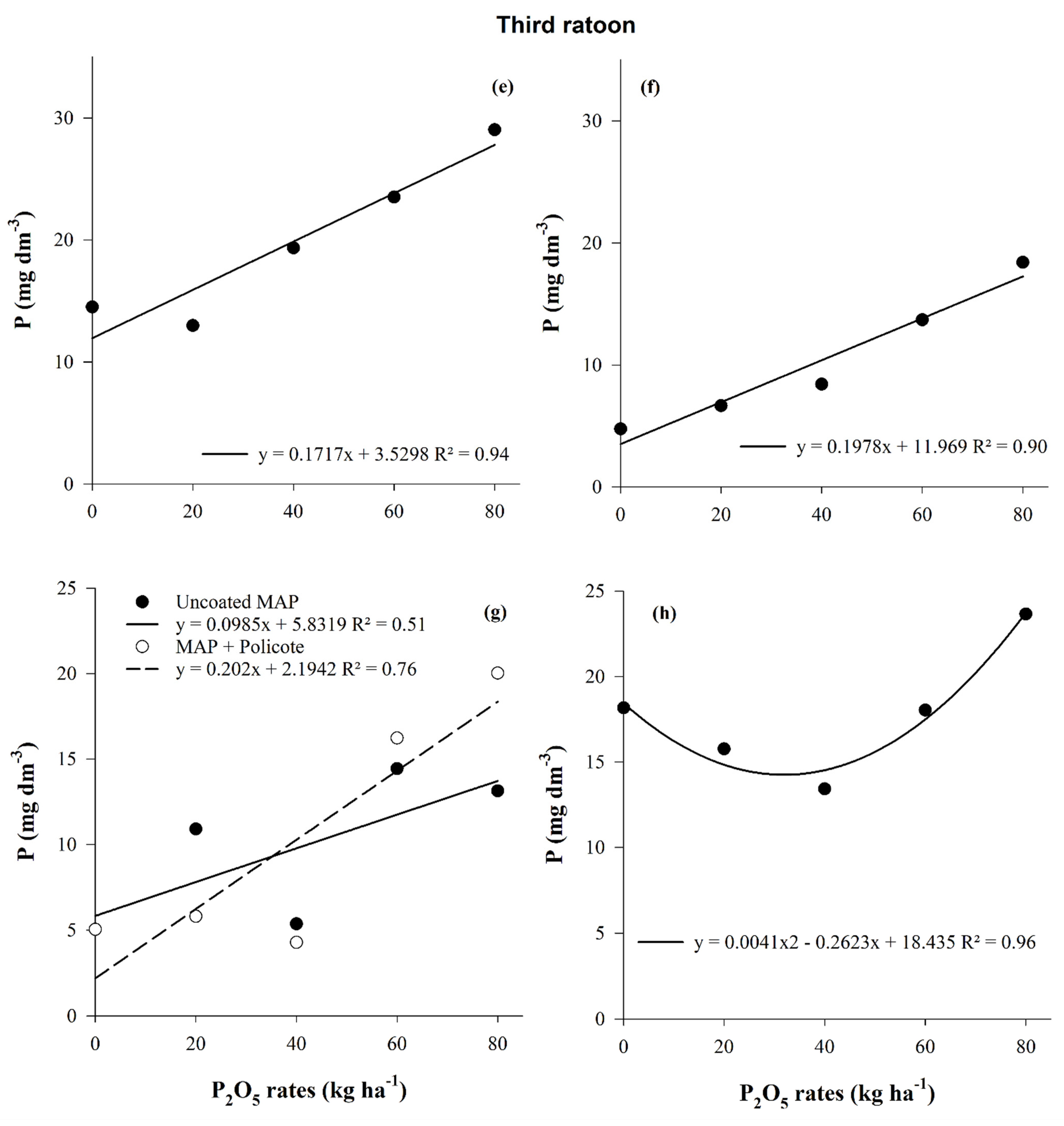

| Layer | pH CaCl2 | OM | P | K | Ca | Mg | Al | H + Al | BS | CEC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| m | g kg−1 | mg dm−3 | mmolc dm−3 | ||||||||
| 0–0.10 | 4.99 | 10.0 | 5.19 | 1.03 | 10.91 | 3.82 | 1 | 15 | 15.76 | 30.76 | |
| 0.10–0.20 | 4.86 | 7.02 | 7.02 | 0.36 | 9.98 | 3.92 | 1 | 15 | 14.26 | 29.26 | |
| 0.20–0.40 | 5.59 | 7.23 | 6.97 | 0.08 | 10.65 | 6.23 | 0 | 12 | 16.96 | 28.96 | |
| Sand | Silt | Clay | V | m | S | B | Cu | Fe | Mn | Zn | |
| g kg−1 | % | mg dm−3 | |||||||||
| 0–0.10 | 822 | 46 | 132 | 51.2 | 5.97 | 3.88 | 0.18 | 0.77 | 34.62 | 8.50 | 0.58 |
| 0.10–0.20 | 846 | 32 | 122 | 48.7 | 6.55 | 5.28 | 0.17 | 0.81 | 43.98 | 8.91 | 0.69 |
| 0.20–0.40 | 804 | 73 | 123 | 65.6 | 0 | 6.62 | 0.12 | 0.81 | 27.20 | 5.66 | 0.53 |
| Fertilizer Sources | P-Resin | Mehlich-1 | |||
|---|---|---|---|---|---|
| Third Ratoon | Second Ratoon | Third Ratoon | |||
| 0–0.10 m | 0–0.10 m | 0.10–0.20 m | 0–0.10 m | 0.10–0.20 m | |
| mg dm−3 | |||||
| Uncoated MAP | 10.89 b | 17.24 | 17.36 b | 18.19 b | 16.65 b |
| MAP + Policote | 12.72 a | 18.61 | 19.90 a | 24.26 a | 18.79 a |
| p-value | 0.0149 * | 0.332 ns | 0.0018 ** | 0.0001 *** | 0.0001 *** |
| Ratoon | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| g kg−1 | ||||||
| Second | 17.87 a | 1.68 b | 14.62 a | 6.98 b | 1.43 b | 1.08 |
| Third | 12.65 b | 2.41 a | 7.35 b | 7.95 a | 2.00 a | 1.15 |
| p-value | 0.0002 *** | 0.003 ** | 0.0001 *** | 0.0132 * | 0.005 ** | 0.5682 ns |
| Ratoon | Brix | Fiber | Moisture | POL | PC | Purity | RS | TRS |
|---|---|---|---|---|---|---|---|---|
| % | kg t−1 | |||||||
| Second | 15.06 b | 11.64 b | 73.31 a | 15.40 b | 13.13 b | 87.17 ns | 0.55 ns | 131.47 b |
| Third | 16.49 a | 12.71 a | 70.80 b | 17.53 a | 14.66 a | 88.91 | 0.50 | 145.72 a |
| p-value | 0.003 ** | 0.001 ** | 0.0008 *** | 0.003 ** | 0.06 ** | 0.1262 ns | 0.073 ns | 0.006 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.L.B.d.; Cassimiro, J.B.; Lira, M.V.d.S.; Boni, A.d.S.; Donato, N.d.L.; Reis, R.d.A., Jr.; Heinrichs, R. Sugarcane Ratoon Yield and Soil Phosphorus Availability in Response to Enhanced Efficiency Phosphate Fertilizer. Agronomy 2022, 12, 2817. https://doi.org/10.3390/agronomy12112817
Oliveira CLBd, Cassimiro JB, Lira MVdS, Boni AdS, Donato NdL, Reis RdA Jr., Heinrichs R. Sugarcane Ratoon Yield and Soil Phosphorus Availability in Response to Enhanced Efficiency Phosphate Fertilizer. Agronomy. 2022; 12(11):2817. https://doi.org/10.3390/agronomy12112817
Chicago/Turabian StyleOliveira, Clayton Luís Baravelli de, Juliana Bonfim Cassimiro, Maikon Vinicius da Silva Lira, Ariele da Silva Boni, Natália de Lima Donato, Roberto dos Anjos Reis, Jr., and Reges Heinrichs. 2022. "Sugarcane Ratoon Yield and Soil Phosphorus Availability in Response to Enhanced Efficiency Phosphate Fertilizer" Agronomy 12, no. 11: 2817. https://doi.org/10.3390/agronomy12112817
APA StyleOliveira, C. L. B. d., Cassimiro, J. B., Lira, M. V. d. S., Boni, A. d. S., Donato, N. d. L., Reis, R. d. A., Jr., & Heinrichs, R. (2022). Sugarcane Ratoon Yield and Soil Phosphorus Availability in Response to Enhanced Efficiency Phosphate Fertilizer. Agronomy, 12(11), 2817. https://doi.org/10.3390/agronomy12112817




_Qin.png)



