Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of HRW
2.3. Plants, Growth Conditions, and Treatments
2.4. ALS Activity Assay
2.4.1. Extraction of the ALS Enzyme
2.4.2. Measurement of ALS Enzyme Activity
2.5. Antioxidant Enzyme Assays
2.6. Determination of the BS Content in Rice Leaves
2.7. Statistical Analysis
3. Results
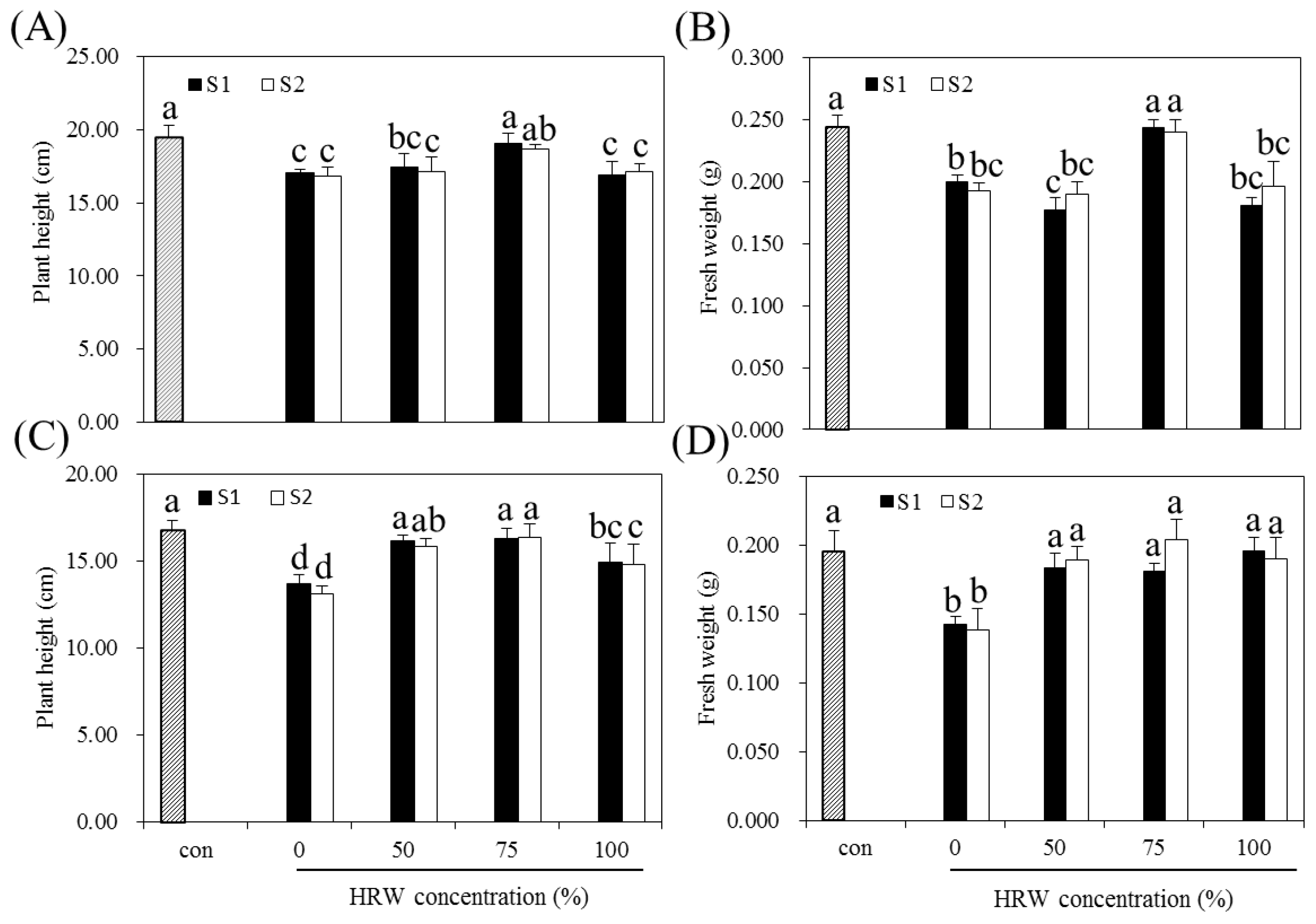
3.1. Effects of HRW and BS on the Growth of Indica and Japonica Rice
3.2. Effect of HRW on BS-Induced Growth Inhibition
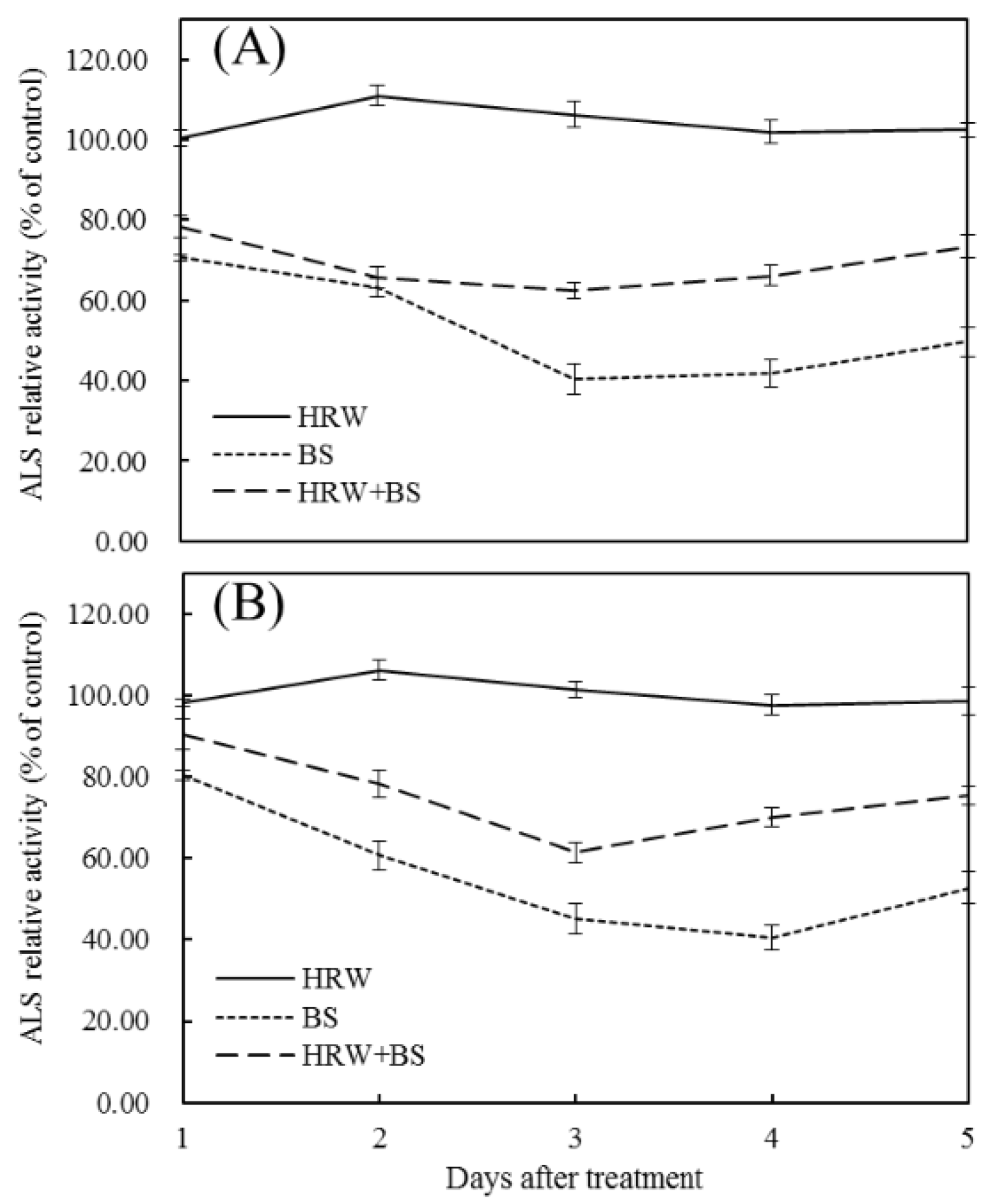
3.3. Effects of HRW and BS on ALS Enzyme Activity
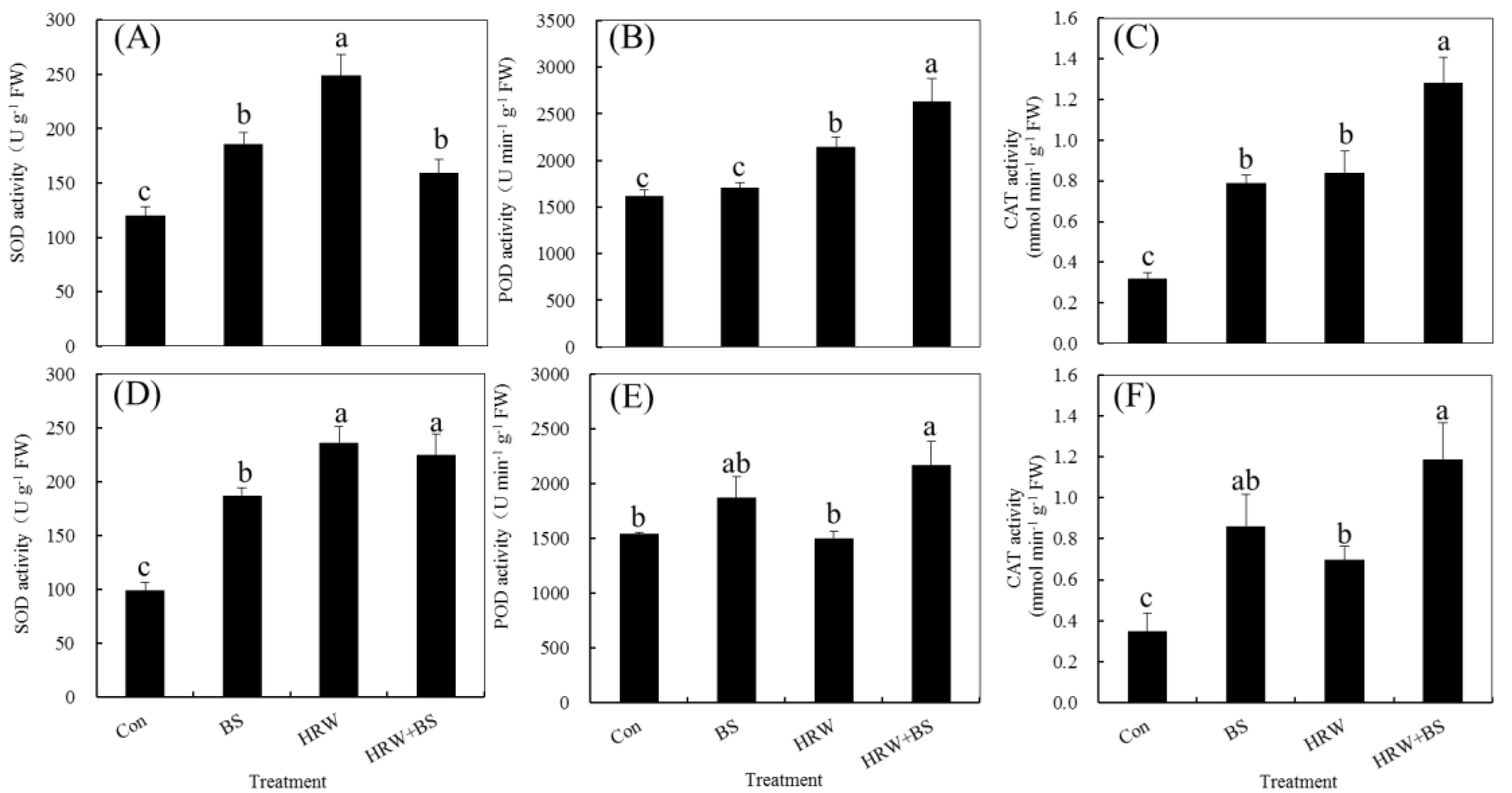
3.4. Changed Activity of Antioxidant Enzymes
3.5. HRW Enhances BS Metabolism in Rice Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rajcan, I.; Swanton, C.J. Understanding maize weed competition: Resource competition, light quality and the whole plant. Field Crops Res. 2001, 71, 139–150. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, T.; Zhao, B.; Yang, X.; Peng, Q.; Li, Y.; Bai, L. Effects of common Echinochloa varieties on grain yield and grain quality of rice. Field Crops Res. 2017, 203, 163–172. [Google Scholar] [CrossRef]
- Lee, N.; Thierfelder, C. Weed control under conservation agriculture in dryland smallholder farming systems of southern Africa. A review. Agron. Sustain. Dev. 2017, 37, 48. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.L. Encyclopedia of World Pesticides: Herbicide Volume; Chemical Industry Press: Beijing, China, 2002; pp. 86–88. [Google Scholar]
- Chauhan, B.S.; Abugho, S.B. Effect of Growth Stage on the Efficacy of postemergence herbicides on four weed species of direct-seeded rice. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.J.; Bayer, D.E.; Carriere, M.D.; Ateh, C.M.; Yim, K.-O. Mechanisms of resistance to bispyribac-Sodium in an Echinochloa phyllopogon accession. Pestic. Biochem. Physiol. 2000, 68, 156–165. [Google Scholar] [CrossRef]
- Ding, G.; Li, X.; Zhang, F.; Chen, J.; Huang, L.; Qiao, X. Mechanism-based quantitative structure–activity relationships on toxicity of selected herbicides to Chlorella vulgaris and Raphidocelis subcapitata. Bull. Environ. Contam. Toxicol. 2009, 83, 520–524. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kawata, M.; Ando, I.; Shimizu, T.; Ohshima, M. Selecting genetic transformants of indica and indica-derived rice cultivars using bispyribac sodium and a mutated ALS gene. Plant Cell Rep. 2010, 29, 1287–1295. [Google Scholar] [CrossRef]
- Martini, L.F.D.; Burgos, N.R.; Noldin, J.A.; de Avila, L.A.; Salas, R.A. Absorption, translocation and metabolism of bispyribac-sodium on rice seedlings under cold stress. Pest Manag. Sci. 2014, 71, 1021–1029. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Conclusion on the pear review of the pesticide risk assessment of the active substance bispyribac. EFSA J. 2010, 8, 1692. [Google Scholar]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.-I.; Ishii, T.; Konno, T.; Nakamura, I. Comparison study of allelochemicals and bispyribac-sodium on the germination and growth response of Echinochloa crus-galli L. J. Plant Growth Regul. 2018, 38, 501–512. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Z.; Shen, W.; Lou, Y. Increasing tolerance to bispyribac-sodium is able to allow glutathione homeostasis to recover in indica rice compared with japonica rice. Pestic. Biochem. Physiol. 2018, 153, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Saika, H.; Horita, J.; Taguchi-Shiobara, F.; Nonaka, S.; Nishizawa-Yokoi, A.; Iwakami, S.; Hori, K.; Matsumoto, T.; Tanaka, T.; Itoh, T.; et al. A novel rice cytochrome P450 gene, CYP72A31, confers tolerance to acetolactate synthase-inhibiting herbicides in rice and Arabidopsis. Plant Physiol. 2014, 166, 1232–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubitz, W.; Reijerse, E.J.; Messinger, J. Solar water-splitting into H2 and O2: Design principles of photosystem II and hydrogenases. Energy Environ. Sci. 2008, 1, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Sun, X.; Xia, Z.-F. Hydrogen resuscitation, a new cytoprotective approach. Clin. Exp. Pharmacol. Physiol. 2011, 38, 155–163. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother. Pharmacol. 2009, 64, 753–761. [Google Scholar] [CrossRef]
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, K.-I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674. [Google Scholar] [CrossRef]
- Liu, S.; Liu, K.; Sun, Q.; Liu, W.; Xu, W.; Denoble, P.; Tao, H.; Sun, X. Consumption of hydrogen water reduces paraquat-induced acute lung injury in rats. J. Biomed. Biotechnol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golding, A.-L.; Dong, Z. Hydrogen production by nitrogenase as a potential crop rotation benefit. Environ. Chem. Lett. 2010, 8, 101–121. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, M.; Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in Plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef] [PubMed]
- Su, N.N.; Wu, Q.; Liu, Y.Y.; Cai, J.T.; Shen, W.B.; Xia, K. Hydrogen rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesisin two varieties of radish sprouts under UV-A irradiation. J. Agri. Food Chem. 2014, 62, 6454–6462. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Wang, Y.; Gu, R. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Cai, J.; Shen, Z.; Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 2015, 175, 174–182. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Wang, Y.; Shen, Z.; Shen, W.; Xu, X. Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol. Environ. Saf. 2017, 144, 369–379. [Google Scholar] [CrossRef]
- Cui, W.; Fang, P.; Zhu, K.; Mao, Y.; Gao, C.; Xie, Y.; Wang, J.; Shen, W. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol. Environ. Saf. 2014, 105, 103–111. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, M.; Lai, D.; Wei, Z.; Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhu, S.; Jiang, Y.; Wang, N.; Wang, R.; Shen, W.; Yang, J. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 2013, 370, 47–57. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2012, 36, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Wang, Z.; Shen, W.; Xu, X. Protective effects of hydrogen-rich water on the photosynthetic apparatus of maize seedlings (Zea mays L.) as a result of an increase in antioxidant enzyme activities under high light stress. Plant Growth Regul. 2015, 77, 43–56. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, X.; Lei, D.; Hu, S.; Shen, Z.; Shen, W.; Xu, X. Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul. 2017, 83, 69–82. [Google Scholar] [CrossRef]
- Cui, W.; Gao, C.; Fang, P.; Lin, G.; Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Vila-Aiub, M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2007, 177, 155–166. [Google Scholar] [CrossRef]
- Huang, B.-K.; Xu, S.; Xuan, W.; Li, M.; Cao, Z.-Y.; Liu, K.-L.; Ling, T.-F.; Shen, W.-B. Carbon monoxide alleviates salt-induced oxidative damage in wheat seedling leaves. J. Integr. Plant Biol. 2006, 48, 249–254. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Yang, R.B.; Guo, Z.Y.; Gong, D.X. Determination of bispyribac-sodium residues in water, soil and rice tissues by high performance liquid chromatography (HPLC). J. Agro-Environ. Sci. 2005, 24, 407–409. (In Chinese) [Google Scholar]
- Li, L.; Zeng, Y.; Cheng, X.; Shen, W. The Applications of molecular hydrogen in horticulture. Horticulturae 2021, 7, 513. [Google Scholar] [CrossRef]
- Zein, A.A.; Baky, M.; Hassan, S.M.; Derbalah, A.S.; Hamza, A.M. Evolution and mechanism of rice weeds resistance to herbicides I. resistance of Echinochloa colonum to bispyribac-soduim with respect to its effect on chlorophyll content. J. Agric. Res. Kafer El-Sheikh Univ. 2010, 36, 480–494. [Google Scholar]
- Marchezan, M.; Avila, L.; Agostinetto, D.; Schaedler, C.; Langaro, A.; Oliveira, C.; Zimmer, M.; Schreiber, F. Morphological and biochemical alterations of paddy rice in response to stress caused by herbicides and total plant submersion. Planta Daninha 2017, 35, e017139830. [Google Scholar] [CrossRef]
- Begum, M.; Juraimi, A.; Omar, S.S.; Rajan, A.; Azmi, M. Effect of herbicides for the control of Fimbristylis miliacea (L.) Vahl. in cice. J. Agron. 2008, 7, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sharma, N.; Pant, R.; Tandon, S. Terminal residue analysis of acephate and methamidofos in rice. Ind. J. Plant Prot. 2017, 45, 249–253. [Google Scholar]
- Singh, R.; Pal, R.; Singh, T.; Singh, A.P.; Yadaw, S.; Singh, J. Management of weeds in direct-seeded rice by bispyribac-sodium. Indian J. Weed Sci. 2014, 46, 126–128. [Google Scholar]
- Singh, N.; Singh, S.B. Adsorption and leaching behaviour of bispyribac-sodium in soils. Bull. Environ. Contam. Toxicol. 2014, 94, 125–128. [Google Scholar] [CrossRef]
- Pradhan, D.; Singh, R.K.; Verma, S.K. Genotoxic potential assessment of the herbicide bispyribac-sodium in a fresh water fish Clarias batrachus (Linn.). Bull. Environ. Contam. Toxicol. 2020, 105, 715–720. [Google Scholar] [CrossRef]
- Ning, H.S.; Xiao, L.Y.; Guo, F.C.; Hong, Y. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 2007, 68, 1779–1787. [Google Scholar]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- McCarthy-Suárez, I. Role of reactive oxygen species in auxin herbicide phytotoxicity: Current information and hormonal implications-are gibberellins, cytokinins, and polyamines involved? Botany 2017, 95, 369–385. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; McCarthy, I.; Gómez, M.; Sandalio, L.M.; Corpas, F.J.; Del Río, L.A. ROS-mediated enzymatic systems involved in the oxidative action of 2,4-D. Plant Cell Environ. 2004, 27, 1135–1148. [Google Scholar] [CrossRef]
- Sunohara, Y.; Matsumoto, H. Quinclorac-induced cell death is accompanied by generation of reactive oxygen species in maize root tissue. Phytochemistry 2008, 69, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- López-García, P.; Moreira, D. The Syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 2020, 5, 655–667. [Google Scholar] [CrossRef]
- Russell, G.; Zulfiqar, F.; Hancock, J.T. Hydrogenases and the role of molecular hydrogen in plants. Plants 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wu, L.; Kettlewell, B.; Caldwell, C.D.; Layzell, D.B. Hydrogen fertilization of soils-Is this a benefit of legumes in rotation? Plant Cell Environ. 2003, 26, 1875–1879. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Russell, G.; Hancock, J.T. Molecular hydrogen in agriculture. Planta 2021, 254, 1–14. [Google Scholar] [CrossRef]
- Jia, L.; Jin, X.-Y.; Zhao, L.-X.; Fu, Y.; Ye, F. Research progress in the design and synthesis of herbicide safeners: A Review. J. Agric. Food Chem. 2022, 70, 5499–5515. [Google Scholar] [CrossRef]
| Treatments | Response Variables | ||
|---|---|---|---|
| Root Length (cm) | Biomass (g/FW) | Plant Height (cm) | |
| CK | 6.07 ± 0.25a | 0.243 ± 0.010a | 19.47 ± 0.54a |
| 50% HRW alone | 6.25 ± 0.40a | 0.246 ± 0.021a | 19.24 ± 0.25a |
| 75% HRW alone | 6.31 ± 0.23a | 0.253 ± 0.013a | 19.04 ± 0.07a |
| 100% HRW alone | 5.91 ± 0.22a | 0.231 ± 0.004a | 18.98 ± 0.62a |
| S1 | 6.31 ± 0.06a | 0.200 ± 0.002b | 17.04 ± 0.23b |
| S2 | 6.25 ± 0.08a | 0.193 ± 0.003b | 16.81 ± 0.20b |
| Treatments | Response Variables | ||
|---|---|---|---|
| Root Length (cm) | Biomass (g/FW) | Plant Height (cm) | |
| CK | 5.31 ± 0.23a | 0.195 ± 0.013ab | 16.74 ± 0.42ab |
| 50% HRW alone | 5.24 ± 0.17ab | 0.179 ± 0.005b | 17.00 ± 0.23a |
| 75% HRW alone | 5.26 ± 0.42ab | 0.206 ± 0.016a | 16.21 ± 0.19b |
| 100% HRW alone | 5.57 ± 0.14ab | 0.193 ± 0.008ab | 17.24 ± 0.14a |
| S1 | 4.95 ± 0.07ab | 0.142 ± 0.001c | 13.71 ± 0.17c |
| S2 | 4.62 ± 0.46b | 0.138 ± 0.002c | 13.11 ± 0.17c |
| Treatments | 9311 (Indica Rice) | Nipponbare (Japonica Rice) | ||
|---|---|---|---|---|
| Residue (mg/kg) | Degradation (%) | Residue (mg/kg) | Degradation (%) | |
| Control | 1.278 ± 0.018 | - | 1.024 ± 0.191 | - |
| BS | 0.445 ± 0.072 | 65.18 ± 5.63b | 0.557 ± 0.059 | 45.61 ± 5.71b |
| 50% HRW + BS | 0.271 ± 0.015 | 78.79 ± 1.18a | 0.273 ± 0.035 | 73.34 ± 3.38a |
| 75% HRW + BS | 0.243 ± 0.032 | 80.99 ± 2.54a | 0.294 ± 0.052 | 71.29 ± 5.05a |
| 100% HRW + BS | 0.304 ± 0.048 | 76.21 ± 3.74a | 0.241 ± 0.028 | 76.46 ± 2.74a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, T.; Wang, Y.; Cao, J.; Zhang, Z.; Li, G.; Shen, W.; Lou, Y.; Wang, H. Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation. Agronomy 2022, 12, 2821. https://doi.org/10.3390/agronomy12112821
Gu T, Wang Y, Cao J, Zhang Z, Li G, Shen W, Lou Y, Wang H. Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation. Agronomy. 2022; 12(11):2821. https://doi.org/10.3390/agronomy12112821
Chicago/Turabian StyleGu, Tao, Yaxiong Wang, Jingjing Cao, Zichang Zhang, Gui Li, Wenbiao Shen, Yuanlai Lou, and Hongchun Wang. 2022. "Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation" Agronomy 12, no. 11: 2821. https://doi.org/10.3390/agronomy12112821
APA StyleGu, T., Wang, Y., Cao, J., Zhang, Z., Li, G., Shen, W., Lou, Y., & Wang, H. (2022). Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation. Agronomy, 12(11), 2821. https://doi.org/10.3390/agronomy12112821







