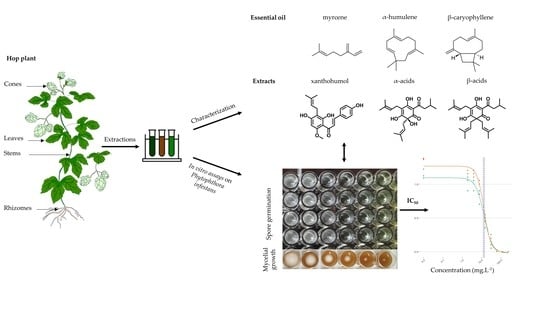
Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extracts, Sub-Extracts, and Purified Compounds of Hop
2.1.1. Extraction
2.1.2. Purification of Xanthohumol and the Mixtures of α- and αβ-Acids by CPC for Bioassays
2.1.3. Purification and Structural Identification of Major Acylphloroglucinols Obtained by Preparative HPLC for Characterization in Hop Extracts and Quantification
2.2. Phytochemical Analyses
2.2.1. Characterization of Hop Commercial Essential Oil by GC-MS
2.2.2. UHPLC-UV-MS Analyses and Quantification
2.3. In Vitro Anti-Oomycete Activity
2.3.1. Phytophthora infestans Culture Condition
2.3.2. Activity on Mycelial Growth
2.3.3. Activity on Spore Germination
2.3.4. Microscopical Observations
2.3.5. IC50 and Statistical Data Analysis
3. Results
3.1. Phytochemical Analyses
3.1.1. Characterization of the Essential Oil from Hop Cones by GC-MS Analysis
3.1.2. Characterization and Quantification of Hop Phenolic Compounds from Cones, Leaves, Stems, and Rhizomes
3.1.3. Characterization of xanthohumol, α-Acids, and αβ-Acids Mix by UHPLC-UV-MS
3.2. In Vitro Anti-Oomycete Activity on Mycelial Growth and Spore Germination
3.2.1. Hop Cone Essential Oil Activity
3.2.2. Extracts and Sub-Extracts Activity
3.2.3. Prenylated Phenolic Compound Activity
3.2.4. Morphological Modifications of the Mycelium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerhäuser, C. Broad Spectrum Anti-Infective Potential of Xanthohumol from Hop (Humulus lupulus L.) in Comparison with Activities of Other Hop Constituents and Xanthohumol Metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Ceh, B.; Kac, M.; Košir, I.; Abram, V.; Ceh, B.; Kac, M.; Košir, I.J.; Abram, V. Relationships between Xanthohumol and Polyphenol Content in Hop Leaves and Hop Cones with Regard to Water Supply and Cultivar. Int. J. Mol. Sci. 2007, 8, 989–1000. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a Very Popular Beer Ingredient and Medicinal Plant: Overview of Its Phytochemistry, Its Bioactivity, and Its Biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An Overview of the Antimicrobial Properties of Hop. In Natural Antimicrobial Agents; Mérillon, J.-M., Riviere, C., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–54. ISBN 978-3-319-67045-4. [Google Scholar]
- Bocquet, L.; Rivière, C.; Dermont, C.; Samaillie, J.; Hilbert, J.-L.; Halama, P.; Siah, A.; Sahpaz, S. Antifungal Activity of Hop Extracts and Compounds against the Wheat Pathogen Zymoseptoria tritici. Ind. Crops Prod. 2018, 122, 290–297. [Google Scholar] [CrossRef]
- Schiller, H.; Forster, A.; Vonhoff, C.; Hegger, M.; Biller, A.; Winterhoff, H. Sedating Effects of Humulus lupulus L. Extracts. Phytomedicine 2006, 13, 535–541. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a Potent Phytoestrogen in Hops (Humulus lupulus L.) and Beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.-N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; et al. Pharmacokinetics of Prenylated Hop Phenols in Women Following Oral Administration of a Standardized Extract of Hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [Green Version]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, L.; et al. A Comparison of Antioxidant and Antimicrobial Activity between Hop Leaves and Hop Cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Gill, C.; Rowland, I. A Comparison of the Anticancer Properties of Isoxanthohumol and 8-Prenylnaringenin Using in Vitro Models of Colon Cancer. Biofactors 2013, 39, 441–447. [Google Scholar] [CrossRef]
- Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Anti-Inflammatory and Vascularprotective Properties of 8-Prenylapigenin. Eur. J. Pharmacol. 2009, 620, 120–130. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-Derived Bitter Acids as Multipotent Bioactive Compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. A New Flavanone with Antifungal Activity Isolated from Hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In Vitro Evaluation of Antibacterial, Anticollagenase, and Antioxidant Activities of Hop Components (Humulus lupulus) Addressing Acne Vulgaris. Phytomedicine 2009, 16, 369–377. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic Compounds from Humulus lupulus as Natural Antimicrobial Products: New Weapons in the Fight against Methicillin Resistant Staphylococcus Aureus, Leishmania Mexicana and Trypanosoma Brucei Strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [Green Version]
- Buckwold, V.E.; Wilson, R.J.H.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W.; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral Activity of Hop Constituents against a Series of DNA and RNA Viruses. Antiviral Res. 2004, 61, 57–62. [Google Scholar] [CrossRef]
- Lou, S.; Zheng, Y.-M.; Liu, S.-L.; Qiu, J.; Han, Q.; Li, N.; Zhu, Q.; Zhang, P.; Yang, C.; Liu, Z. Inhibition of Hepatitis C Virus Replication in Vitro by Xanthohumol, a Natural Product Present in Hops. Planta Med. 2014, 80, 171–176. [Google Scholar] [CrossRef]
- Engelson, M.; Solberg, M.; Karmas, E. Antimycotic Properties of Hop Extract in Reduced Water Activity Media. J. Food Sci. 1980, 45, 1175–1178. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langezaal, C.R.; Chandra, A.; Scheffer, J.J.C. Antimicrobial Screening of Essential Oils and Extracts of Some Humulus lupulus L. Cultivars. Pharm. Weekbl. Sci. Ed. 1992, 14, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Sato, Y. Antifungal Activities of Hop Bitter Resins and Related Compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar] [CrossRef]
- Schmalreck, A.F.; Teuber, M. Structural Features Determining the Antibiotic Potencies of Natural and Synthetic Hop Bitter Resins, Their Precursors and Derivatives. Can. J. Microbiol. 1975, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of Hop Extract as Antifungal Ingredient for Bread Making and Selection of Autochthonous Resistant Starters for Sourdough Fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef]
- Srinivasan, V.; Goldberg, D.; Haas, G.J. Contributions to the Antimicrobial Spectrum of Hop Constituents. Econ. Bot. 2004, 58, S230–S238. [Google Scholar] [CrossRef]
- Arruda, T.R.; Pinheiro, P.F.; Silva, P.I.; Bernardes, P.C. A New Perspective of a Well-Recognized Raw Material: Phenolic Content, Antioxidant and Antimicrobial Activities and α- and β-Acids Profile of Brazilian Hop (Humulus lupulus L.) Extracts. LWT 2021, 141, 110905. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.; Hejazi, M.; Tiemann, K.; Parsons, J.G.; Duarte-Gardea, M.; Henning, J. Use of Hop (Humulus lupulus) Agricultural by-Products for the Reduction of Aqueous Lead(II) Environmental Health Hazards. J. Hazard. Mater. 2002, 91, 95–112. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Properties of Natural Cellulose Fibers from Hop Stems. Carbohydr. Polym. 2009, 77, 898–902. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Pereira, E.L.; Rodrigues, M.Â. Recycling Nutrient-Rich Hop Leaves by Composting with Wheat Straw and Farmyard Manure in Suitable Mixtures. J. Environ. Manag. 2021, 284, 112105. [Google Scholar] [CrossRef]
- Hooker, W.J. Compendium of Potato Diseases; International Potato Center: Lima, Peru, 1981; ISBN 978-0-89054-027-5. [Google Scholar]
- Cooke, L.R.; Schepers, H.T.A.M.; Hermansen, A.; Bain, R.A.; Bradshaw, N.J.; Ritchie, F.; Shaw, D.S.; Evenhuis, A.; Kessel, G.J.T.; Wander, J.G.N.; et al. Epidemiology and Integrated Control of Potato Late Blight in Europe. Potato Res. 2011, 54, 183–222. [Google Scholar] [CrossRef] [Green Version]
- Fry, W. Phytophthora Infestans: The Plant (and R Gene) Destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Wu, E.-J.; Yang, L.-N.; Zhu, W.; Chen, X.-M.; Shang, L.-P.; Zhan, J. Diverse Mechanisms Shape the Evolution of Virulence Factors in the Potato Late Blight Pathogen Phytophthora infestans Sampled from China. Sci. Rep. 2016, 6, 26182. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 Oomycete Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2014, 16, 413–434. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Visser, R.G.F.; van der Vossen, E.A.G. Societal Costs of Late Blight in Potato and Prospects of Durable Resistance Through Cisgenic Modification. Potato Res. 2008, 51, 47–57. [Google Scholar] [CrossRef]
- Tsedaley, B. Late Blight of Potato (Phytophthora Infestans) Biology, Economic Importance and Its Management Approaches. J. Biol. Agric. Healthc. 2014, 4, 215–226. [Google Scholar]
- Schepers, H.T.A.M.; Kessel, G.J.T.; Lucca, F.; Förch, M.G.; van den Bosch, G.B.M.; Topper, C.G.; Evenhuis, A. Reduced Efficacy of Fluazinam against Phytophthora Infestans in the Netherlands. Eur. J. Plant Pathol. 2018, 151, 947–960. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen Decades of Antimicrobial Copper Compounds Applied in Agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef] [Green Version]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial Activities of the Essential Oils of Various Plants against Tomato Late Blight Disease Agent Phytophthora Infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef]
- Villa-Ruano, N.; Pacheco-Hernández, Y.; Rubio-Rosas, E.; Lozoya-Gloria, E.; Mosso-González, C.; Ramón-Canul, L.G.; Cruz-Durán, R. Essential Oil Composition and Biological/Pharmacological Properties of Salmea scandens (L.) DC. Food Control 2015, 57, 177–184. [Google Scholar] [CrossRef]
- Thanh, V.M.; Bui, L.M.; Bach, L.G.; Nguyen, N.T.; Thi, H.L.; Thi, T.T.H. Origanum majorana L. Essential Oil-Associated Polymeric Nano Dendrimer for Antifungal Activity against Phytophthora infestans. Materials 2019, 12, 1446. [Google Scholar] [CrossRef] [PubMed]
- Brennan, N.J.; Larsen, L.; Lorimer, S.D.; Perry, N.B.; Chapin, E.L.; Werk, T.L.; Henry, M.J.; Hahn, D.R. Fungicidal Sesquiterpene Dialdehyde Cinnamates from Pseudowintera axillaris. J. Agric. Food Chem. 2006, 54, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Forrer, H.-R.; Vogelgsang, S.; Musa, T. Botanicals and Phosphonate Show Potential to Replace Copper for Control of Potato Late Blight. J. Fungi 2017, 3, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clerck, C.D.; Maso, S.D.; Parisi, O.; Dresen, F.; Zhiri, A.; Jijakli, M.H. Screening of Antifungal and Antibacterial Activity of 90 Commercial Essential Oils against 10 Pathogens of Agronomical Importance. Foods 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Harmonised Tripartite Guideline. Validation of Analytical procedures: Text and methodology Q2-R1. In International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; ICH Working Group: Geneva, Switzerland, 2014. [Google Scholar]
- Andrivon, D. Inhibition by Aluminum of Mycelial Growth and of Sporangial Production and Germination In Phytophthora infestans. Eur. J. Plant Pathol. 1995, 101, 527–533. [Google Scholar] [CrossRef]
- Sharma, N.; Gruszewski, H.A.; Park, S.-W.; Holm, D.G.; Vivanco, J.M. Purification of an Isoform of Patatin with Antimicrobial Activity against Phytophthora infestans. Plant Physiol. Biochem. 2004, 42, 647–655. [Google Scholar] [CrossRef]
- Muchembled, J.; Deweer, C.; Sahmer, K.; Halama, P. Antifungal Activity of Essential Oils on Two Venturia inaequalis Strains with Different Sensitivities to Tebuconazole. Environ. Sci. Pollut. Res. Int. 2018, 25, 29921–29928. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Matsukura, Y.; Ozaki, H.; Nishimura, K.; Shindo, K. Identification and Quantification of the Oxidation Products Derived from α-Acids and β-Acids During Storage of Hops (Humulus lupulus L.). J. Agric. Food Chem. 2013, 61, 3121–3130. [Google Scholar] [CrossRef]
- Hao, J.; Speers, R.; Fan, H.; Deng, Y.; Dai, Z. A Review of Cyclic and Oxidative Bitter Derivatives of Alpha, Iso-Alpha and Beta-Hop Acids. J. Am. Soc. Brew. Chem. 2020, 78, 1–14. [Google Scholar] [CrossRef]
- Macchioni, V.; Picchi, V.; Carbone, K. Hop Leaves as an Alternative Source of Health-Active Compounds: Effect of Genotype and Drying Conditions. Plants 2022, 11, 99. [Google Scholar] [CrossRef]
- Morcol, T.B.; Wysocki, K.; Sankaran, R.P.; Matthews, P.D.; Kennelly, E.J. UPLC-QTof-MSE Metabolomics Reveals Changes in Leaf Primary and Secondary Metabolism of Hop (Humulus lupulus L.) Plants under Drought Stress. J. Agric. Food Chem. 2020, 68, 14698–14708. [Google Scholar] [CrossRef]
- Morcol, T.B.; Matthews, P.D.; Kennelly, E.J. Differences in Leaf Chemistry and Glandular Trichome Density between Wild Southwestern American Hop (Humulus neomexicanus) and Commercial Hop Cultivars. J. Agric. Food Chem. 2021, 69, 7798–7814. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kocábek, T.; Sukumari Nath, V.; Awasthi, P.; Shrestha, A.; Kumar Killi, U.; Jakse, J.; Patzak, J.; Krofta, K.; Matoušek, J. Dissection of Dynamic Transcriptome Landscape of Leaf, Bract, and Lupulin Gland in Hop (Humulus lupulus L.). Int. J. Mol. Sci. 2019, 21, 233. [Google Scholar] [CrossRef] [Green Version]
- Berne, S.; Kovačević, N.; Kastelec, D.; Javornik, B.; Radišek, S. Hop Polyphenols in Relation to Verticillium Wilt Resistance and Their Antifungal Activity. Plants 2020, 9, 1318. [Google Scholar] [CrossRef]
- Maissa, B.J.; Walid, H. Antifungal Activity of Chemically Different Essential Oils from Wild Tunisian Thymus Spp. Nat. Prod. Res. 2015, 29, 869–873. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Zoghroban, A.A.M.; El-Bakry, A.M.; Kassem, S.M. Insecticidal and Antifungal Activities of Crude Extracts and Pure Compounds from Rhizomes of Curcuma longa L. (Zingiberaceae). J. Agric. Sci. Technol. 2019, 21, 1049–1061. [Google Scholar]
- Rogozhin, E.A.; Vasilchenko, A.S.; Barashkova, A.S.; Smirnov, A.N.; Zavriev, S.K.; Demushkin, V.P. Peptide Extracts from Seven Medicinal Plants Discovered to Inhibit Oomycete Phytophthora infestans, a Causative Agent of Potato Late Blight Disease. Plants 2020, 9, 1294. [Google Scholar] [CrossRef]
- van de Mortel, J.E.; Tran, H.; Govers, F.; Raaijmakers, J.M. Cellular Responses of the Late Blight Pathogen Phytophthora infestans to Cyclic Lipopeptide Surfactants and Their Dependence on G Proteins. AEM 2009, 75, 4950–4957. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.J.; Mohanta, T.K.; Chung, J.Y.; Ryu, M.; Park, G.; Shim, S.; Hong, S.B.; Seo, H.; Bae, D.W.; Bae, I.; et al. Trichoderma Metabolites as Biological Control Agents against Phytophthora Pathogens. Biol. Control 2016, 92, 128–138. [Google Scholar] [CrossRef]
- Di Francesco, A.; Milella, F.; Mari, M.; Roberti, R. A Preliminary Investigation into Aureobasidium Pullulans as a Potential Biocontrol Agent against Phytophthora Infestans of Tomato. Biol. Control 2017, 114, 144–149. [Google Scholar] [CrossRef]
- Messgo-Moumene, S.; Li, Y.; Bachir, K.; Houmani, Z.; Bouznad, Z.; Chemat, F. Antifungal Power of Citrus Essential Oils against Potato Late Blight Causative Agent. J. Essent. Oil Res. 2015, 27, 169–176. [Google Scholar] [CrossRef]






| Products | LOD (ng.mL−1) | LOQ (ng.mL−1) | Linearity Range (µg.mL−1) | Equation | R2 |
|---|---|---|---|---|---|
| Xanthohumol | 2.5 | 10 | 0.010–10 | y = 41129.80 x + 159.37 | 0.9974 |
| Humulone | 10 | 25 | 0.025–100 | y = 7319.23 x + 41.38 | 0.9975 |
| Lupulone | 10 | 100 | 0.025–10 | y = 9747.26 x + 46.84 | 0.9979 |
| Compound | Molar Mass (g.mol−1) | Chemical Formula | Retention Time (min) | Percentage in the EO |
|---|---|---|---|---|
| Myrcene | 136 | C10H16 | 16.8 | 16.4% |
| Coapene | 204 | C15H24 | 42.8 | 1.0% |
| trans-caryophyllene | 204 | C15H24 | 45.3 | 33.0% |
| α-humulene | 204 | C15H24 | 47.3 | 31.4% |
| γ-muurolene | 204 | C15H24 | 48.6 | 1.3% |
| α-selinene | 204 | C15H24 | 49.1 | 1.1% |
| δ-cadinene | 204 | C15H24 | 51.3 | 1.2% |
| Humulene epoxide | 220 | C15H24O | 55.5 | 1.1% |
| Cembrene | 272 | C20H32 | 73.4 | 2.4% |
| Compounds <1% | - | - | - | 11.3% |
| Xanthohumol | Co-Humulone | N-Humulone | Ad-Humulone | Co-Lupulone | N-Lupulone | Ad-Lupulone | |
|---|---|---|---|---|---|---|---|
| Crude Hydro-Ethanolic Extracts (CHE) | |||||||
| Cones | 19.475 ± 1.738 | 64.452 ± 6.000 | 146.784 ± 13.715 | 41.584 ± 3.824 | 46.806 ± 4.173 | 32.053 ± 2.824 | 13.524 ± 1.195 |
| Leaves | 1.084 ± 0.126 | 0.674 ± 0.142 | 1.741 ± 0.211 | 0.556 ± 0.071 | 1.851 ± 0.240 | 4.290 ± 0.527 | 0.676 ± 0.091 |
| Stems | 0.106 ± 0.009 | <LOQ | 0.066 ± 0.017 | <LOD | <LOQ | <LOQ | <LOQ |
| Rhizomes | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| DCM sub-extracts (DSE) | |||||||
| Cones | 19.017 ± 0.546 | 71.232 ± 2.956 | 162.368 ± 6.608 | 45.805 ± 1.772 | 35.247 ± 1.212 | 24.189 ± 1.182 | 10.191 ± 0.441 |
| Leaves | 1.029 ± 0.051 | 0.542 ± 0.030 | 1.379 ± 0.066 | 0.401 ± 0.017 | 0.461 ± 0.021 | 2.335 ± 0.150 | 0.161 ± 0.011 |
| Stems | 0.685 ± 0.021 | 0.166 ± 0.007 | 0.590 ± 0.005 | 0.101 ± 0.003 | <LOD | 0.268 ± 0.011 | <LOQ |
| Rhizomes | 0.062 ± 0.004 | 0.077 ± 0.006 | 0.307 ± 0.018 | 0.374 ± 0.043 | <LOD | <LOQ | <LOQ |
| Mycelial Growth | Spore Germination | ||||
|---|---|---|---|---|---|
| IC50 | Confidence Interval (95%) | IC50 | Confidence Interval (95%) | ||
| (mg.L−1) | 2.5% | 97.5% | (mg.L−1) | 2.5% | 97.5% |
| 1 295 | 1 159 | 2 181 | 5 355 | 1 714 | 9 775 |
| Branched Mycelium | Lysed Mycelium | ||
|---|---|---|---|
| Untreated | - | - | |
| Cones | CHE | + | + |
| DSE | + | + | |
| Leaves | CHE | + | + |
| DSE | 0 | 0 | |
| Stems | CHE | - | + |
| DSE | - | + | |
| Rhizomes | CHE | + | + |
| DSE | - | + | |
| Copper sulfate | + | + | |
| Purified compounds | Xanthohumol | + | + |
| α-acids | + | + | |
| αβ-acids | + | + | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacquin, J.; Moureu, S.; Deweer, C.; Hakem, A.; Paguet, A.-S.; Bonneau, N.; Bordage, S.; Dermont, C.; Sahpaz, S.; Muchembled, J.; et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy 2022, 12, 2826. https://doi.org/10.3390/agronomy12112826
Jacquin J, Moureu S, Deweer C, Hakem A, Paguet A-S, Bonneau N, Bordage S, Dermont C, Sahpaz S, Muchembled J, et al. Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy. 2022; 12(11):2826. https://doi.org/10.3390/agronomy12112826
Chicago/Turabian StyleJacquin, Justine, Sophie Moureu, Caroline Deweer, Asma Hakem, Anne-Sophie Paguet, Natacha Bonneau, Simon Bordage, Charles Dermont, Sevser Sahpaz, Jérôme Muchembled, and et al. 2022. "Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans" Agronomy 12, no. 11: 2826. https://doi.org/10.3390/agronomy12112826
APA StyleJacquin, J., Moureu, S., Deweer, C., Hakem, A., Paguet, A.-S., Bonneau, N., Bordage, S., Dermont, C., Sahpaz, S., Muchembled, J., & Rivière, C. (2022). Hop (Humulus lupulus L.) Specialized Metabolites: Extraction, Purification, Characterization in Different Plant Parts and In Vitro Evaluation of Anti-Oomycete Activities against Phytophthora infestans. Agronomy, 12(11), 2826. https://doi.org/10.3390/agronomy12112826