Screening Soybean Genotypes for High-Temperature Tolerance by Maximin-Minimax Method Based on Yield Potential and Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Temperature Treatments
2.2. Plant Material and Growing Conditions
2.3. Growth Analysis
2.4. Seed Yield Attributes
2.5. Reproductive Efficiency
2.6. Gas Exchange and Chlorophyll Fluorescence Measurements
2.7. Pollen Germination
2.8. Maximin-Minimax Approach
2.9. Cumulative Stress Response Index (CSRI)
2.10. Statistical Analysis
3. Results
3.1. Weather Conditions
3.2. Effect of Temperature on Growth Parameters
3.2.1. Leaf Area
3.2.2. Above-Ground Biomass
3.2.3. Below-Ground Biomass
3.3. Effect of Temperature Photosynthetic Rate
3.4. Effect of Temperature on Fv/Fm
3.5. Effect of Temperature on Pollen Germination
3.6. Effect of Temperature on Reproductive Efficiency
3.7. Effect of Temperature on Seed Yield and Its Attributes
3.7.1. Seed Yield
3.7.2. Total Biomass
3.7.3. Harvest Index
3.7.4. Number of Pods/Plant
3.7.5. Seeds/Pod
3.7.6. 100 Seed Weight
3.7.7. 0-,1-,2-,3-, and 4-Seeded Pods
3.8. Maximin-Minimax Approach
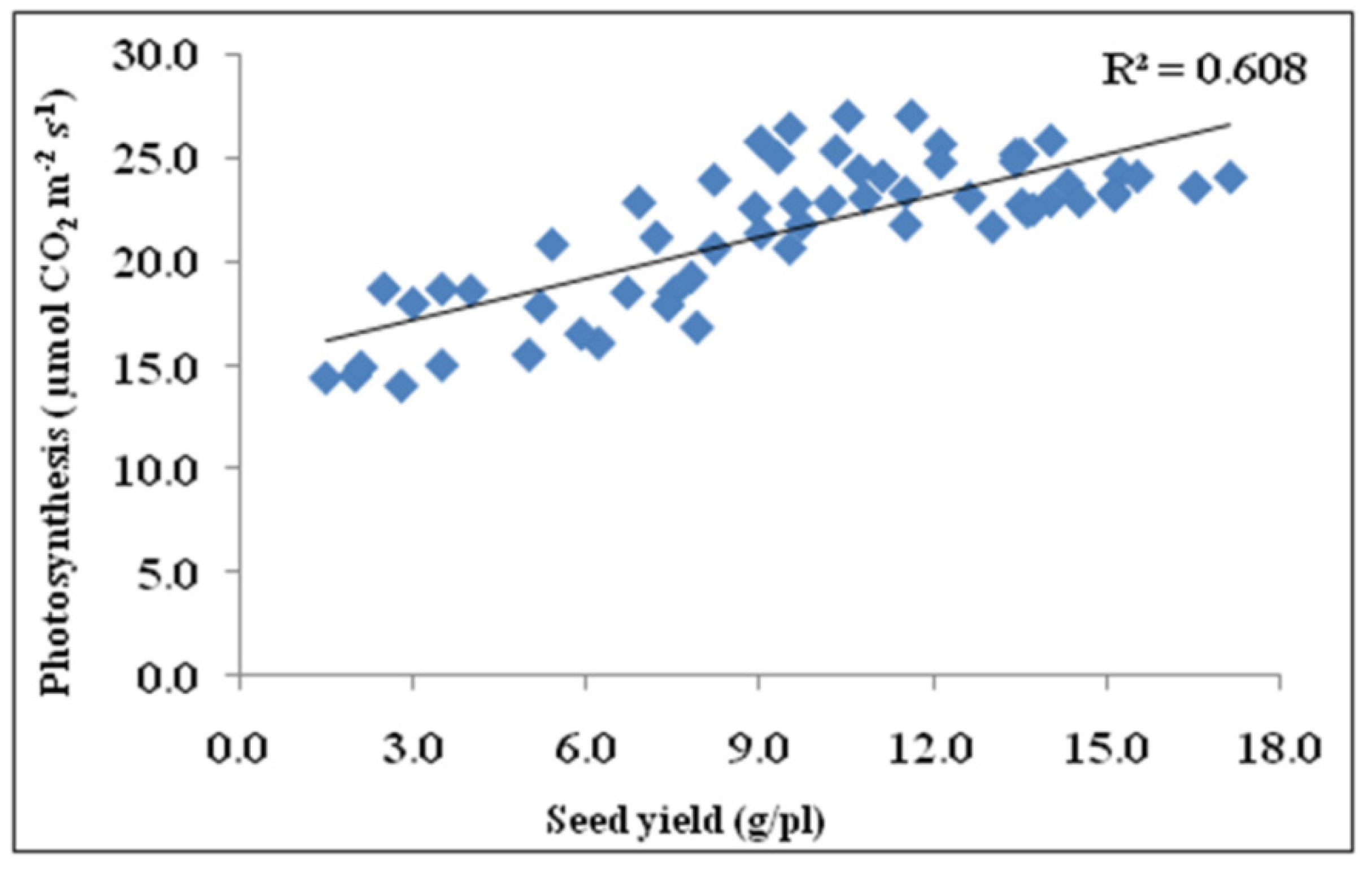
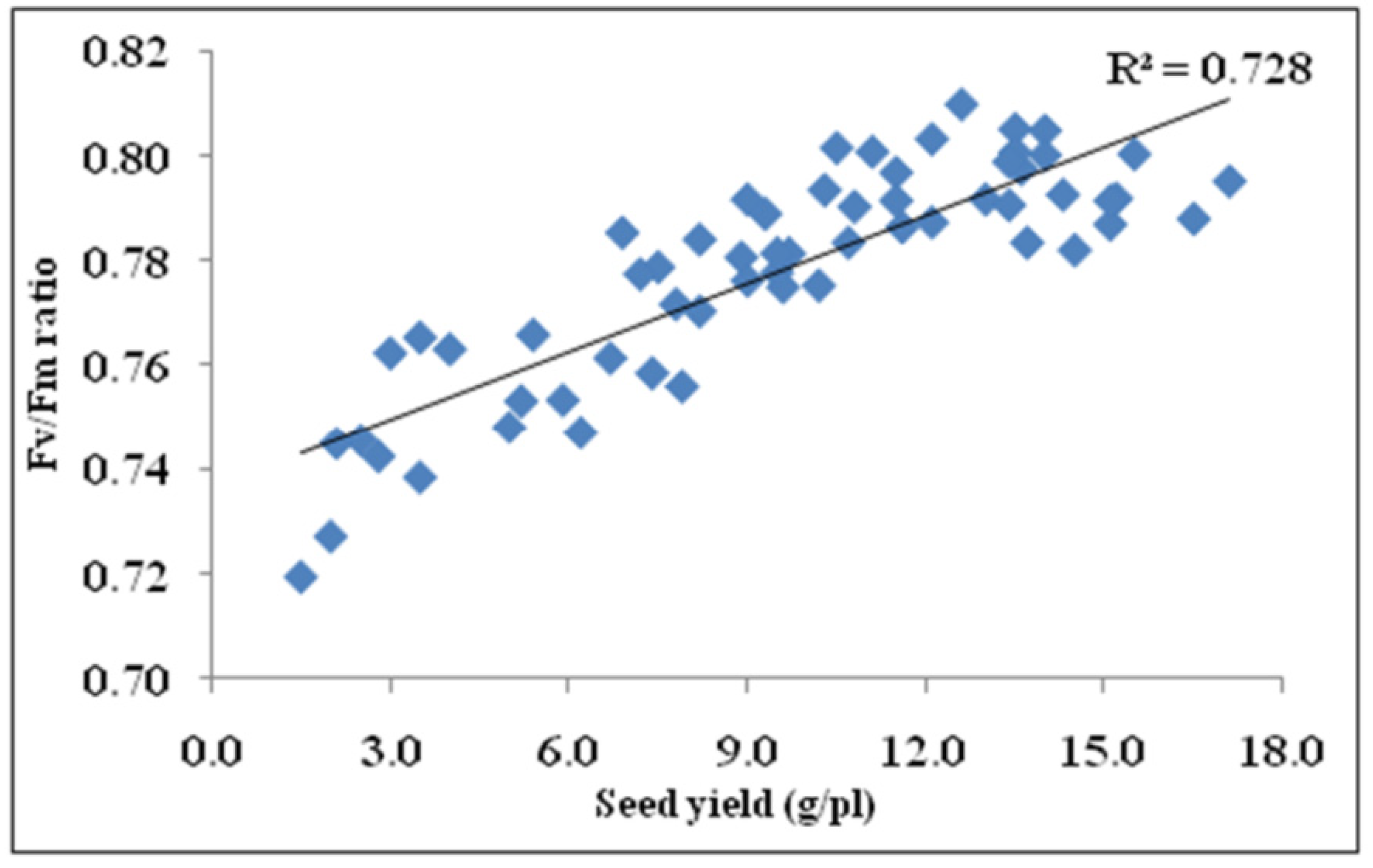
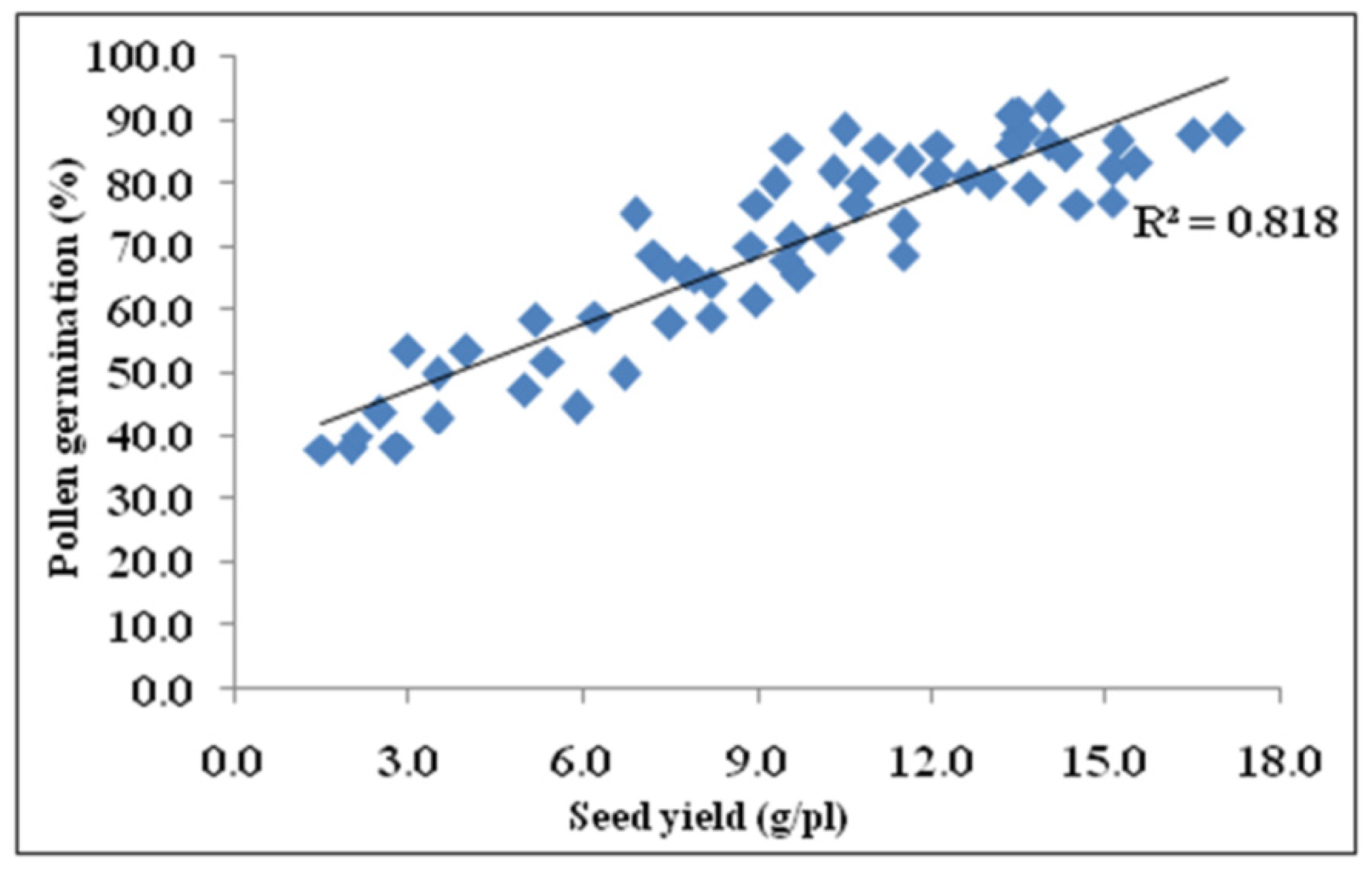
3.9. Correlation of Seed Yield with Photosynthesis, Fv/Fm, and Pollen Germination
3.10. Cumulative Stress Response Index (CSRI)
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.S.; Fahey, D.W.; Skowron, A.; Allen, M.R.; Burkhardt, U.; Chen, Q.; Doherty, S.J.; Freeman, S.; Forster, J.; Fuglestvedt, P.M.; et al. The contribution of global aviation to anthropogenic climate forcing for 2000 to 2018. Atmos. Environ. 2021, 244, 117834. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Intergovernmental Panel on Climate Change [IPCC]. Summary for policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P., Pirani, R., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Qin, J.; Zhang, J.; Liu, D.; Yin, C.; Wang, F.; Chen, P.; Chen, H.; Ma, J.; Zhang, B.; Xu, J. iTRAQ-based analysis of developmental dynamics in the soybean leaf proteome reveals pathways associated with leaf photosynthetic rate. Mol. Genet. Genom. 2016, 291, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Screening soybean genotypes for high temperature tolerance by in vitro pollen germination, pollen tube length, reproductive efficiency, and seed yield. Indian J. Plant Physiol. 2018, 23, 77–90. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Alamri, S.; Siddiqui, M.H.; Rastogi, A. Inoculation with arbuscular mycorrhizal fungi alleviates the adverse effects of high temperature in soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef]
- Liu, X.B.; Jin, J.; Wang, G.H.; Herbert, S.J. Soybean yield physiology and development of high yielding practices in Northeast China. Field Crops Res. 2008, 105, 157–171. [Google Scholar] [CrossRef]
- Tacarindua, C.R.P.; Shiraiwa, T.; Homma, K.; Kumagai, E.; Sameshima, R. The response of soybean seed growth characteristics to increased temperature under near-field conditions in a temperature gradient chamber. Field Crop Res. 2012, 131, 26–31. [Google Scholar] [CrossRef]
- Tacarindua, C.R.P.; Shiraiwa, T.; Homma, K.; Kumagai, E.; Sameshima, R. The effects of increased temperature on crop growth and yield of soybean grown in a temperature gradient chamber. Field Crop Res. 2013, 131, 74–81. [Google Scholar] [CrossRef]
- Ortiz, A.C.; De Smet, I.; Sozzani, R.; Locke, A.M. Field-grown soybean shows genotypic variation in physiological and seed composition responses to heat stress during seed development. Environ. Exp. Bot. 2021, 195, 104768. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hodges, H.F. Crop ecosystem responses to global climate change: Cotton. In Climate Change and Global Productivity; Reddy, K.R., Hodges, H.F., Eds.; CAB International: Wallingford, CT, USA, 2000; pp. 161–187. [Google Scholar]
- Ashraf, M.; Hafeez, M. Thermotolerance of pearl millet and maize at early growth stages: Growth and nutrient relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress—Opportunities, challenges and future directions. Field Crops Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Gaye, A.J.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; Raper, S.C.B.; et al. (Eds.) Global Climate Projections; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 749–844. [Google Scholar]
- Bheemanahalli, R.; Sunoj, V.S.J.; Saripalli, G.; Prasad, P.V.V.; Balyan, H.S.; Gupta, P.K.; Grant, N.; Gill, K.S.; Krishna Jagadish, S.V. Quantifying the heat stress on pollen germination, seed set, and grain filling in spring wheat. Crop Sci. 2019, 59, 684–696. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Sathishraj, R.; Tack, J.; Nalley, L.L.; Muthurajan, R.; Jagadish, K.S. Temperature thresholds for spikelet sterility and associated warming impacts for sub-tropical rice. Agric. For. Meteorol. 2016, 221, 122–130. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Allen, L.H., Jr.; Gallo Meagher, M.; Davis, J.M. Elevated temperature and carbon dioxide effects on soybean seed germination and transcript abundance. Crop Sci. 2003, 43, 1548–1557. [Google Scholar] [CrossRef]
- Wheeler, T.; von Braun, J. Climate Change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; Siebers, M.; Gray, S.B.; Drag, D.W.; Rosenthal, D.; Kimball, M.; Ort, B.A.; Bernacchi, C.J.R.D. Global warming can negate the expected CO2 stimulation in photosynthesis and productivity for soybean grown in the Midwestern United States. Plant Physiol. 2013, 162, 410–423. [Google Scholar] [CrossRef]
- Siebers, M.H.; Yendrek, C.R.; Drag, D.; Locke, A.M.; Acosta, L.R.; Leakey, A.D.B.; Ort, D.R. Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Glob. Chang. Biol. 2015, 21, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Thomey, M.L.; Slattery, R.A.; Kohler, I.H.; Bernacchi, C.J.; Ort, D.R. Yield response of field-grown soybean exposed to heat waves under current and elevated [CO2]. Glob. Chang. Biol. 2019, 25, 4352–4368. [Google Scholar] [CrossRef] [PubMed]
- Alsajri, F.A.; Singh, B.; Wijewardana, C.; Irby, T.; Gao, W.; Reddy, K.R. Evaluating soybean cultivars for low- and high-temperature tolerance during the seedling growth stage. Agronomy 2019, 9, 13. [Google Scholar] [CrossRef]
- Kumangai, E.; Sameshima, R. Genotypic differences in soybean yield responses to increasing temperature in a cool climate are related to maturity group. Agric. For. Meteorol. 2014, 198–199, 265–272. [Google Scholar] [CrossRef]
- Herritt, M.T.; Fritschi, F.B. Characterization of photosynthetic phenotypes and chloroplast ultrastructural changes of soybean (Glycine max) in responses to elevated air temperature. Front. Plant Sci. 2020, 11, 153. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Boote, K.J.; Allen, L.H. Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum (Sorghum bicolor (L.) Moench) are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric. For. Meteorol. 2006, 139, 237–251. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef]
- Salem, M.A.; Kakani, V.G.; Koti, S.; Reddy, K.R. Pollen based screening of soybean genotypes for high temperatures. Crop Sci. 2007, 47, 219–231. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.S.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Pipolo, E.A.; Sinclair, T.S.; Camara, G.M.S. Effects of temperature on oil and protein concentration in soybean seeds cultured in vitro. Ann. Appl Biol. 2004, 144, 71–76. [Google Scholar] [CrossRef]
- Chebrolu, K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, K.R. Developing functional relationships between temperature and soybean yield and seed quality. Agron. J. 2020, 112, 194–204. [Google Scholar] [CrossRef]
- Nakagawa, A.C.S.; Ario, N.; Tomita, Y.; Tanka, S.; Murayama, N.; Mizuta, C.; IwayaInoue, M.; Ishibashi, Y. High temperature during soybean seed development differentially alters lipid and protein metabolism. Plant Prod. Sci. 2020, 23, 504–516. [Google Scholar] [CrossRef]
- Koti, S.; Reddy, K.R.; Kakani, V.G.; Zhao, D.; Gao, W. Effects of carbon dioxide, temperature and ultraviolet-B radiation and their interactions on soybean (Glycine max L.) growth and development. Environ. Exp. Bot. 2007, 60, 1–10. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Schapaugh, W.T. High day or nighttime temperature alters leaf assimilation, reproductive success and phosphatidic acid of pollen grain in soybean (Glycine max (L.) Merr.). Crop Sci. 2013, 53, 1594–1604. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Boyle, D.L.; Schapaugh, W.T. Soybean pollen anatomy, viability and pod set under high temperature stress. J. Agron. Crop Sci. 2013, 199, 171–177. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Schapaugh, W.; Fritschi, F.; Nguyen, H.; Prasad, P.V.V. Reproductive success of soybean (Glycine max L. Merril) cultivars and exotic lines under high daytime temperature. Plant Cell Environ. 2019, 42, 321–336. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Metaanalysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef]
- Odulaja, A.; Nokoe, S. A maximin-minimax approach for classifying crop varieties into resistant groups based on yield potential and loss. Int. J. Pest Managem. 1993, 39, 64–67. [Google Scholar] [CrossRef]
- Koti, S.; Reddy, K.R.; Kakani, V.G.; Zhao, D.; Reddy, V.R. Soybean (Glycine max) pollen germination characteristics flower and pollen morphology in response to enhanced ultraviolet-B radiation. Ann. Bot. 2005, 94, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Boote, K.J.; Allen, L.H.; Prasad, P.V.V.; Baker, J.T.; Gesch, R.W.; Snyder, A.M.; Pan, D.; Thomas, J.M.G. Elevated temperature and carbon dioxide impacts on pollination, reproductive growth and yield of several globally important Crops. J. Agric. Meteorol. 2005, 60, 469–474. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Combined effect of temperature and water stress on physiological and biochemical processes in soybean (Glycine max). Physiol Mol. Biol. Plants. 2019, 25, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S. Impact of elevated temperatures on growth and yield of chickpea (Cicer arietinum L.). Field Crops Res. 2014, 164, 90–97. [Google Scholar] [CrossRef]
- Pereira-Flores, M.E.; Justino, F.; Ruiz-Vera, U.M.; Stordal, F.; Melo, A.A.M.; Rodrigues, R.D.Á. Response of soybean yield components and allocation of dry matter to increased temperature and CO2 concentration. Aust. J. Crop Sci. 2016, 10, 808–818. [Google Scholar] [CrossRef]
- Camejo, D.; Rodriguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcon, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Boote, K.J. Improving soybean cultivars for adaptation to climate change and climate variability. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, E.A., Eds.; John Wiley and Sons, Ltd.: West Sussex, UK, 2011; pp. 370–395. [Google Scholar]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A.; et al. Net carbondioxide losses of northern ecosystems in response to autumn warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef]
- Egli, D.B. Soybean reproductive sink size and short-term reductions in photosynthesis during flowering and pod set. Crop Sci. 2010, 50, 1971–1977. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Saibo, N.J.M.; Lourenço, T.; Oliveira, M.M. Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann. Bot. 2009, 103, 609–623. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V. Ethylene production under high temperature stress causes premature leaf senescence in soybean. Funct. Plant Biol. 2010, 37, 1071–1084. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, L.; Yu, M.; Zhong, M. Warming decreases photosynthates and yield of soybean [Glycine max (L.) Merrill] in the North China Plain. Crop J. 2016, 4, 139–146. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Huan, F.; Lizhe, A.; Ling, T.; Zong Dong, H.; Xunling, W. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 Taxa in vitro. Environ. Exp. Bot. 2000, 43, 45–53. [Google Scholar]
- Kakani, V.G.; Prasad, P.V.V.; Craufurd, P.Q.; Wheeler, T.R. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotype to temperature. Plant Cell Environ. 2002, 25, 1651–1661. [Google Scholar] [CrossRef]
- Bhatia, V.S.; Jumrani, K.; Pandey, G.P. Developing drought tolerance in soybean using physiological approaches. Soybean Res. 2014, 12, 1–19. [Google Scholar]
- Bhatia, V.S.; Jumrani, K.; Pandey, G.P. Evaluation of the usefulness of senescing agent potassium iodide as a screening tool for tolerance to terminal drought in soybean. Plant Knowl. J. 2014, 3, 23–30. [Google Scholar]
- Bhatia, V.S.; Jumrani, K. A maximin-minimax approach for classifying soybean genotypes for drought tolerance based on yield potential and loss. Plant Breed. 2016, 135, 691–700. [Google Scholar] [CrossRef]

| Treatments | Leaf Area (cm2) | Above-Ground Biomass (g/Plant) | Below-Ground Biomass (g/Plant) |
|---|---|---|---|
| Day/Night Temperatures (°C) | |||
| Ambient | 2206 a | 23.4 a | 4.94 a |
| 30/22 °C | 2196 a | 23.0 b | 3.63 b |
| 34/24 °C | 1960 b | 20.5 c | 2.72 c |
| 38/26 °C | 1613 c | 16.3 d | 2.13 d |
| 42/28 °C | 1302 d | 14.5 e | 1.62 e |
| LSD (T) | 66.9 | 0.34 | 0.147 |
| Genotypes | |||
| JS 97-52 | 2566 a | 24.2 b | 4.86 a |
| EC 602288 | 2301 b | 26.3 a | 4.60 a |
| JS 95-60 | 1047 f | 10.0 i | 1.25 g |
| JS 93-05 | 1104 f | 12.8 h | 2.43 e |
| EC 456548 | 2099 c | 20.4 e | 2.91 cd |
| Hardee | 2375 b | 22.4 c | 3.01 c |
| NRC 37 | 2316 b | 22.4 c | 2.65 de |
| JS 335 | 2001 c | 22.1 c | 3.51 b |
| JS 71-05 | 1773 d | 21.3 d | 2.88 cd |
| EC 538828 | 1320 e | 17.4 f | 1.91 f |
| NRC 7 | 1619 d | 20.7 de | 2.66 de |
| Punjab-1 | 1742 d | 14.8 g | 3.42 b |
| Mean | 1855.1 | 19.6 | 3.01 |
| LSD (G) | 78.3 | 0.65 | 0.274 |
| LSD (T × G) | NS | 1.44 | 0.612 |
| ANOVA | |||
| T | <0.0001 | <0.0001 | <0.0001 |
| G | <0.0001 | <0.0001 | <0.0001 |
| T × G | 0.1409 | <0.0001 | <0.0001 |
| Treatments | Photosynthetic Rate | Fv/Fm | Pollen Germination (%) | Reproductive Efficiency (%) |
|---|---|---|---|---|
| Day/Night Temperatures (°C) | ||||
| Ambient | 24.9 a | 0.797 a | 87.3 a | 42 a |
| 30/22 °C | 24.2 b | 0.795 a | 82.0 b | 40 b |
| 34/24 °C | 22.9 c | 0.785 b | 72.2 c | 37 c |
| 38/26 °C | 19.9 d | 0.770 c | 58.7 d | 32 d |
| 42/28 °C | 16.0 e | 0.745 d | 48.0 e | 28 e |
| LSD (T) | 0.33 | 0.0037 | 1.28 | 1.2 |
| Genotypes | ||||
| JS 97-52 | 22.2 bc | 0.785 b | 71.9 d | 24 e |
| EC 602288 | 21.2 efg | 0.778 c | 70.5 e | 25 e |
| JS 95-60 | 22.0 bcd | 0.766 e | 60.1 j | 39 c |
| JS 93-05 | 21.6 cde | 0.770 e | 64.6 i | 40 c |
| EC 456548 | 21.2 efg | 0.777 c | 75.0 c | 39 c |
| Hardee | 20.6 gh | 0.793 a | 68.5 gf | 28 d |
| NRC 37 | 20.3 h | 0.775 cd | 64.4 i | 21 f |
| JS 335 | 21.7 bcde | 0.770 e | 65.7 hi | 39 c |
| JS 71-05 | 22.4 b | 0.786 b | 68.9 f | 41 c |
| EC 538828 | 20.9 fgh | 0.786 b | 78.4 b | 54 b |
| NRC 7 | 23.3 a | 0.785 b | 81.3 a | 58 a |
| Punjab-1 | 21.4 def | 0.772 de | 66.7 gh | 21 f |
| Mean | 21.6 | 0.779 | 69.7 | 36 |
| LSD (G) | 0.66 | 0.0036 | 1.31 | 2.0 |
| LSD (T × G) | 1.47 | 0.0081 | 2.93 | 4.5 |
| ANOVA | ||||
| T | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| G | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T × G | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Genotypes | Ambient | 30/22 °C | 34/24 °C | 38/26 °C | 42/28 °C | Mean |
|---|---|---|---|---|---|---|
| JS 97-52 | 17.1 | 15.5 | 15.1 | 9.0 | 6.7 | 12.7 a |
| EC 602288 | 16.5 | 15.1 | 14.5 | 8.2 | 5.0 | 11.9 b |
| JS 95-60 | 10.5 | 9.0 | 8.2 | 2.5 | 1.5 | 6.3 f |
| EC 538828 | 13.5 | 13.6 | 13.0 | 9.5 | 7.9 | 11.5 b |
| JS 71-05 | 13.5 | 12.1 | 11.5 | 7.2 | 5.2 | 9.9 c |
| JS 93-05 | 11.6 | 10.3 | 8.9 | 3.5 | 2.0 | 7.3 e |
| EC 456548 | 15.2 | 14.3 | 13.7 | 7.8 | 6.2 | 11.4 b |
| Hardee | 14.0 | 12.6 | 11.5 | 7.5 | 5.9 | 10.3 c |
| NRC 37 | 11.1 | 10.8 | 9.7 | 4.0 | 2.8 | 7.7 e |
| JS 335 | 12.1 | 10.7 | 10.2 | 5.4 | 3.5 | 8.4 d |
| NRC 7 | 14.0 | 13.4 | 13.4 | 9.6 | 7.4 | 11.6 b |
| Punjab 1 | 9.5 | 9.3 | 6.9 | 3.0 | 2.1 | 6.2 f |
| Mean | 13.2 a | 12.2 b | 11.4 c | 6.4 d | 4.7 e | 9.6 |
| LSD | ||||||
| Temperature (T) | 0.33 | |||||
| Genotype (G) | 0.58 | |||||
| T × G | 1.29 | |||||
| ANOVA | ||||||
| T | <0.0001 | |||||
| G | <0.0001 | |||||
| T × G | <0.0001 |
| Treatments | TBM (g/Plant) | HI (%) | Pods/Plant | 100 Seed Weight (g) | Seeds/Pods |
|---|---|---|---|---|---|
| Day/Night Temperatures (°C) | |||||
| Ambient | 30.3 a | 43.8 a | 61 a | 12.5 a | 1.94 a |
| 30/22 °C | 28.7 b | 43.0 a | 59 b | 12.3 a | 1.90 a |
| 34/24 °C | 26.8 c | 42.7 a | 56 c | 12.0 a | 1.91 a |
| 38/26 °C | 20.2 d | 32.2 b | 40 d | 10.1 b | 1.70 b |
| 42/28 °C | 17.7 e | 26.4 c | 35 e | 8.8 c | 1.61 c |
| LSD (T) | 0.60 | 1.09 | 1.4 | 0.64 | 0.075 |
| Genotypes | |||||
| JS 97-52 | 32.0 a | 38.6 cd | 80 a | 7.9 g | 1.97 cd |
| EC 602288 | 32.3 a | 35.3 e | 79 a | 8.3 fg | 1.72 ef |
| JS 95-60 | 15.7 g | 37.0 de | 20 h | 12.1 c | 2.29 a |
| JS 93-05 | 17.7 f | 37.8 cde | 32 f | 9.5 e | 2.11 b |
| EC 456548 | 26.6 c | 42.6 b | 47 d | 14.9 b | 1.59 fg |
| Hardee | 26.6 c | 37.9 cde | 62 b | 9.6 e | 1.68 ef |
| NRC 37 | 25.1 d | 29.4 g | 58 c | 8.0 g | 1.53 g |
| JS 335 | 26.4 c | 30.6 fg | 48 d | 9.1 ef | 1.84 de |
| JS 71-05 | 26.3 c | 36.6 de | 49 d | 11.5 d | 1.72 ef |
| EC 538828 | 21.5 e | 53.3 a | 24 g | 23.7 a | 2.03 bc |
| NRC 7 | 28.4 b | 40.2 c | 63 b | 11.9 cd | 1.52 g |
| Punjab-1 | 18.3 f | 31.9 f | 43 e | 7.4 g | 1.75 ef |
| Mean | 24.7 | 37.6 | 50 | 11.2 | 1.81 |
| LSD (G) | 0.83 | 2.57 | 2.2 | 1.00 | 0.139 |
| LSD (T × G) | 1.85 | 5.75 | 5.0 | NS | NS |
| ANOVA | |||||
| T | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| G | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T × G | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Treatments | 0 Seeded Pods (%) | 1 Seeded Pods (%) | 2 Seeded Pods (%) | 3 Seeded Pods (%) | 4 Seeded Pods (%) |
|---|---|---|---|---|---|
| Day/Night Temperatures (°C) | |||||
| Ambient | 1.3 e | 6.4 e | 63.8 a | 26.8 a | 1.8 a |
| 30/22 °C | 2.1 d | 9.8 d | 64.1 a | 22.4 b | 1.7 a |
| 34/24 °C | 2.8 c | 12.7 c | 63.1 b | 20.1 c | 1.2 b |
| 38/26 °C | 5.6 b | 22.8 b | 57.5 c | 13.7 d | 0.4 c |
| 42/28 °C | 7.4 a | 26.6 a | 55.0 d | 10.8 e | 0.2 d |
| LSD (T) | 0.17 | 0.29 | 0.38 | 0.53 | 0.09 |
| Genotypes | |||||
| JS 97-52 | 3.0 e | 15.8 fg | 69.3 d | 12.0 g | 0.0 c |
| EC 602288 | 2.9 e | 12.7 i | 70.6 c | 13.8 f | 0.0 c |
| JS 95-60 | 7.2 a | 18.5 c | 31.8 i | 37.5 b | 4.9 b |
| JS 93-05 | 5.0 b | 11.6 j | 32.2 i | 43.5 a | 7.9 a |
| EC 456548 | 4.0 d | 19.1 b | 58.8 g | 18.0 e | 0.0 c |
| Hardee | 2.5 f | 17.2 d | 72.2 b | 8.1 i | 0.0 c |
| NRC 37 | 4.4 c | 20.7 a | 68.2 e | 6.7 j | 0.0 c |
| JS 335 | 5.1 b | 16.7 e | 58.3 g | 19.9 d | 0.0 c |
| JS 71-05 | 3.1 e | 13.2 h | 54.1 h | 29.7 c | 0.0 c |
| EC 538828 | 1.8 g | 11.0 k | 77.7 a | 9.6 h | 0.0 c |
| NRC 7 | 3.1 e | 16.1 f | 63.0 f | 17.8 e | 0.0 c |
| Punjab-1 | 3.9 d | 15.4 g | 72.2 b | 8.5 i | 0.0 c |
| Mean | 3.8 | 15.7 | 60.7 | 18.8 | 1.0 |
| LSD (G) | 0.17 | 0.49 | 0.66 | 0.62 | 0.14 |
| LSD (T × G) | 0.39 | 1.09 | 1.48 | 1.39 | 0.31 |
| ANOVA | |||||
| T | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| G | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| T × G | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Genotypes | 30/22 °C | 34/24 °C | 38/26 °C | 42/28 °C | TSRI |
|---|---|---|---|---|---|
| EC 538828 | +1.0 | −25 | −117 | −191 | −333 |
| NRC 7 | −15 | −21 | −138 | −194 | −367 |
| EC 456548 | −18 | −62 | −223 | −273 | −575 |
| JS 97-52 | −41 | −58 | −212 | −269 | −580 |
| JS 71-05 | −38 | −100 | −199 | −250 | −587 |
| Hardee | −39 | −85 | −219 | −264 | −607 |
| EC 602288 | −43 | −68 | −232 | −302 | −644 |
| JS 335 | −62 | −79 | −265 | −305 | −711 |
| NRC 37 | −16 | −79 | −300 | −328 | −724 |
| Punjab 1 | −17 | −102 | −322 | −329 | −770 |
| JS 93-05 | −39 | −115 | −317 | −378 | −848 |
| JS 95-60 | −79 | −151 | −393 | −445 | −1068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumrani, K.; Bhatia, V.S.; Kataria, S.; Landi, M. Screening Soybean Genotypes for High-Temperature Tolerance by Maximin-Minimax Method Based on Yield Potential and Loss. Agronomy 2022, 12, 2854. https://doi.org/10.3390/agronomy12112854
Jumrani K, Bhatia VS, Kataria S, Landi M. Screening Soybean Genotypes for High-Temperature Tolerance by Maximin-Minimax Method Based on Yield Potential and Loss. Agronomy. 2022; 12(11):2854. https://doi.org/10.3390/agronomy12112854
Chicago/Turabian StyleJumrani, Kanchan, Virender Singh Bhatia, Sunita Kataria, and Marco Landi. 2022. "Screening Soybean Genotypes for High-Temperature Tolerance by Maximin-Minimax Method Based on Yield Potential and Loss" Agronomy 12, no. 11: 2854. https://doi.org/10.3390/agronomy12112854
APA StyleJumrani, K., Bhatia, V. S., Kataria, S., & Landi, M. (2022). Screening Soybean Genotypes for High-Temperature Tolerance by Maximin-Minimax Method Based on Yield Potential and Loss. Agronomy, 12(11), 2854. https://doi.org/10.3390/agronomy12112854







