Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flowering Chinese Cabbage Harvest and Storage
2.2. Analysis of Macro- and Microelements in Plants
2.3. Colour Measurements
2.4. Chlorophyll and Carotenoid Content Measurements
2.5. TPC and TFC Measurements
2.6. Antioxidant Activity Measurements
2.7. Statistical Analysis
3. Results
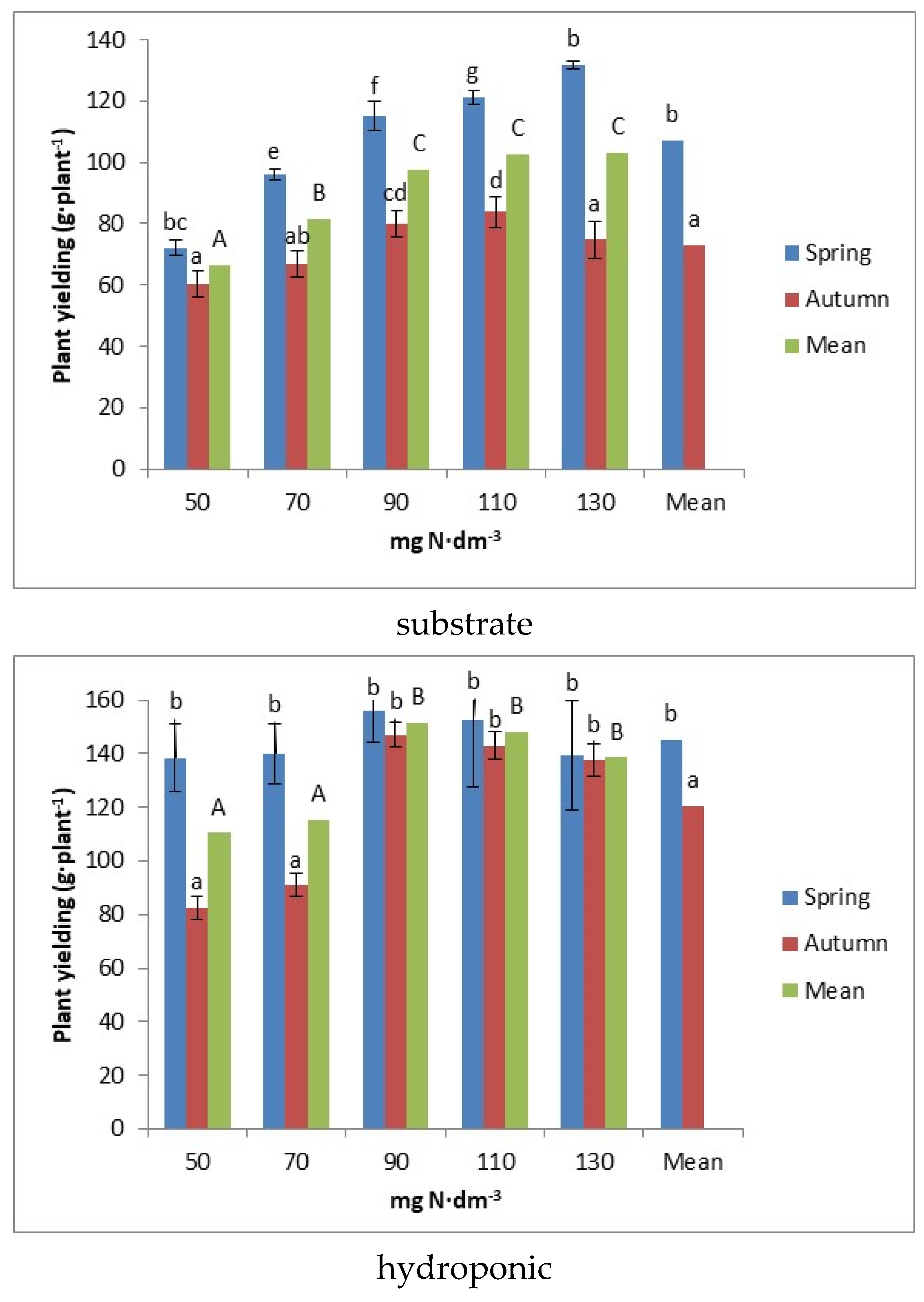
3.1. Plant Yields
3.2. Macroelements
3.3. Microelements and Sodium
3.4. Colour Parameters
3.5. Chorophyll and Carotenoid Content
3.6. Phenolic Content and the Antioxidant Activity
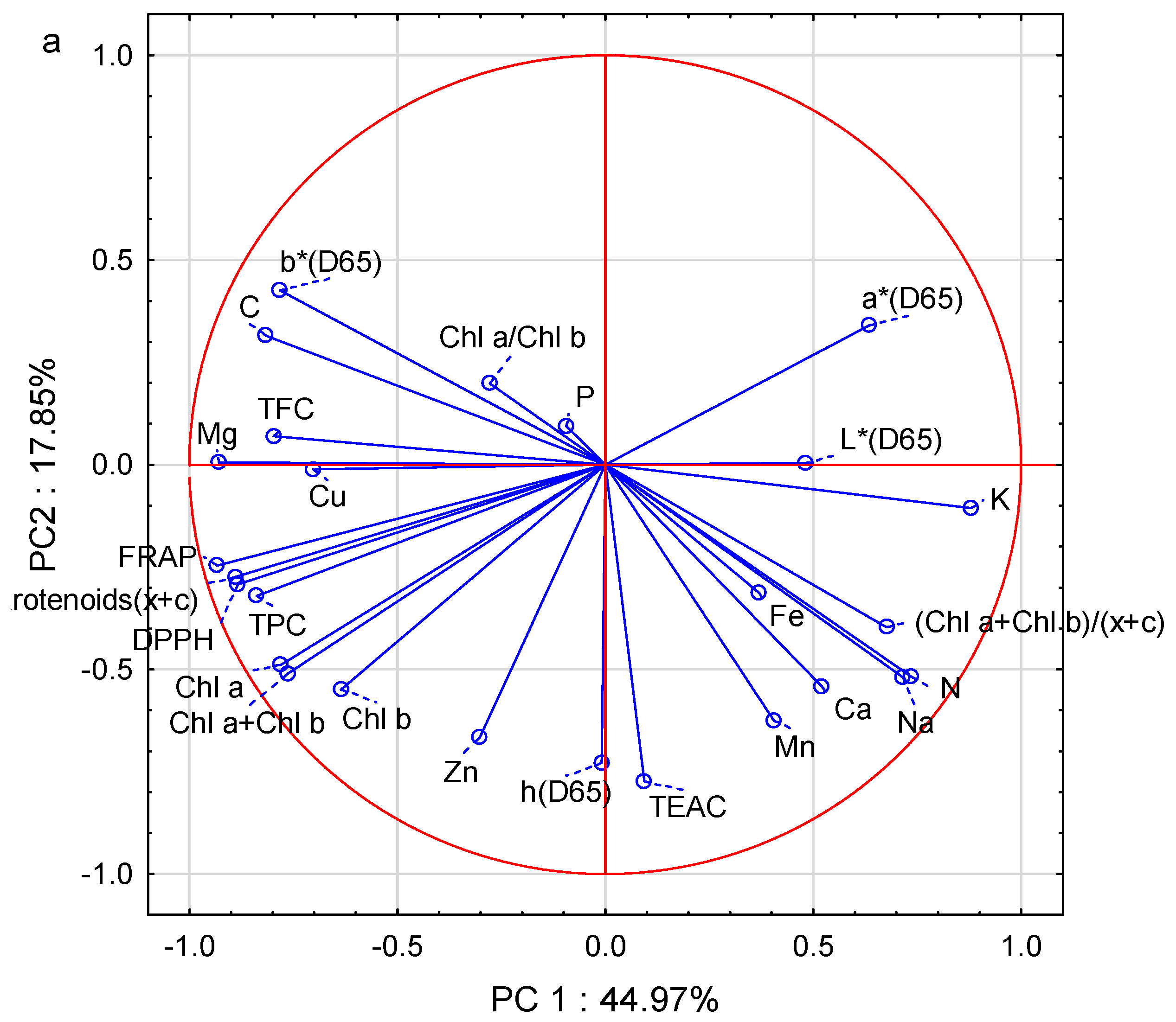
3.7. Principle Component Analysis (PCA)
4. Discussion
4.1. Plant Yields, Chlorophyll Content, and Colour
4.2. Plant Nutrient Status
4.3. Antioxidant Activity and Total Phenolic Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Zhang, H.; Huang, H.; Qiao, Y.; Zheng, Y. Research Progress on Flowering Chinese Cabbage Breeding in Guangdong Province. China Veg. 2011, 20, 9–14. [Google Scholar]
- Yuan, Y.; Zhou, X.; Li, G.; Zheng, Y.; Jiang, D.; Ren, H.; Lei, J.; Zhang, H. Comprehensive Evaluation and Comparison of Nutritional Values of Amino Acids of Chinese Flowering Cabbage and Related Subspecies Vegetables. Food Ferment. Ind. 2019, 45, 102–107. [Google Scholar] [CrossRef]
- Mo, H.Z.; Zhu, Y.Y.; Zhang, M. Selenium Enrichment Pattern in Flowering Chinese Cabbage, Cabbage and Asparagus. Agro Food Ind. Hi-Tech 2006, 17, 39–42. [Google Scholar]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological Traits, Antioxidant Capacity, and Nitrogen Metabolism in Ramie under Nitrogen Fertilizer. Agron J. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Din, I.; Khan, H.; Khan, N.A.; Khil, A. Inoculation of Nitrogen Fixing Bacteria in Conjugation with Integrated Nitrogen Sources Induce Changes in Phenology, Growth, Nitrogen Assimilation and Productivity of Wheat Crop. J. Saudi Soc. Agric. Sci. 2021, 20, 459–466. [Google Scholar]
- Zhang, Y.-Y.; Tian, J.-P.; Jun, C.; Hong, Y.-H.; Luan, Y.-S. Effects of Different NH4+/NO3- Ratios on the Photosynthetic and Physiology Responses of Blueberry (Vaccinium Spp.) Seedlings Growth. J. Plant Nutr. 2021, 44, 854–864. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; El Hadj, S.B.; Braham, M.; Lemeur, R.; Van Labeke, M.-C. Effects of Nitrogen Deficiency on Leaf Photosynthesis, Carbohydrate Status and Biomass Production in Two Olive Cultivars ‘Meski’and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Luo, Z.-B. Phosphorus and Nitrogen Physiology of Two Contrasting Poplar Genotypes When Exposed to Phosphorus and/or Nitrogen Starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive Nitrogen Application Dampens Antioxidant Capacity and Grain Filling in Wheat as Revealed by Metabolic and Physiological Analyses. Sci. Rep. 2017, 7, 43363. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Nie, J.; Liao, Y.; Huang, S.; Tang, J. Effects of Different Nitrogen Fertilizer Rates on Yield and Quality of Brassica Compestris L. Var. Purpurea Bailey. Hunan Agric. Sci. 2011, 239, 70–76. [Google Scholar]
- IUNG. Analytical Methods in Agricultural Chemistry Stations; IUNG: Puławy, Poland, 1972; pp. 25–83. [Google Scholar]
- Liu, W.; Muzolf-Panek, M.; Kleiber, T. The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy 2022, 12, 737. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS. Curr. Protoc. Food Anal. Chem. 2001, 1, F4. 3.1–F4. 3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol Vitic 1965, 16, 144–158. [Google Scholar]
- Re, R.; Nicoletta, P.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved Abts Radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 76, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen Supply Influences Photosynthesis Establishment along the Sugarcane Leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-w.; Lei, Y.-l.; Huang, X.-m.; Su, W.; Chen, R.-y.; Hao, Y.-w. Crosstalk of Cold and Gibberellin Effects on Bolting and Flowering in Flowering Chinese Cabbage. J. Integr. Agric. 2019, 18, 992–1000. [Google Scholar] [CrossRef]
- Chen, L.-S.; Cheng, L. Carbon Assimilation and Carbohydrate Metabolism of Concord’ Grape (Vitis Labrusca L.) Leaves in Response to Nitrogen Supply. J. Am. Soc. Hortic. Sci. 2003, 128, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Hikosaka, K.; Osone, Y. A Paradox of Leaf-Trait Convergence: Why Is Leaf Nitrogen Concentration Higher in Species with Higher Photosynthetic Capacity? J. Plant Res. 2009, 122, 245–251. [Google Scholar] [CrossRef]
- Walcroft, A.S.; Whitehead, D.; Silvester, W.B.; Kelliher, F.M. The Response of Photosynthetic Model Parameters to Temperature and Nitrogen Concentration in Pinus Radiata D. Don. Plant Cell Environ. 1997, 20, 1338–1348. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef] [Green Version]
- Krezel, J.; Koota, E. The Effect of Nitrogen Fertilization on Yield and Biological Value of Chinese Cabbage Grown from Seed Growing for Autumn Harvest. Folia Univ. Agric. Stetin. Agric. 2004, 95, 197–200. [Google Scholar]
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil Environmental Quality in Greenhouse Vegetable Production Systems in Eastern China: Current Status and Management Strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.K.; Guo, W.Z.; Xu, Z.G.; Wang, L.H.; Ma, L. Yield, Nitrogen Use Efficiency and Economic Benefits of Biochar Additions to Chinese Flowering Cabbage in Northwest China. Nutr. Cycl. Agroecosyst 2019, 113, 337–348. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, M.; Qiao, H.; Li, F.; Zhang, S.; Zhang, S.; Zhang, H.; Sun, R. Characterization of Interspecific Hybrids between Flowering Chinese Cabbage and Broccoli. Sci. Hortic. 2018, 240, 552–557. [Google Scholar] [CrossRef]
- Song, S.; Yi, L.; Zhu, Y.; Liu, H.; Sun, G.; Chen, R. Effects of Ammonium and Nitrate Ratios on Plant Growth, Nitrate Concentration and Nutrient Uptake in Flowering Chinese Cabbage. Bangladesh J. Bot. 2017, 46, 1259–1267. [Google Scholar]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3– Ratio Triggers Plant Growth and Nutrient Uptake of Flowering Chinese Cabbage by Optimizing the PH Value of Nutrient Solution. Front. Plant Sci. 2021, 12, 724. [Google Scholar] [CrossRef]
- Evans, J.R.; Terashima, I. Photosynthetic Characteristics of Spinach Leaves Grown with Different Nitrogen Treatments. Plant Cell Physiol. 1988, 29, 157–165. [Google Scholar]
- Evans, J.R. Photosynthesis and Nitrogen Relationships in Leaves of C3 Plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic Nitrogen-Use Efficiency of Species That Differ Inherently in Specific Leaf Area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; Schapendonk, A.H.C.M.; van Oijen, M.; Pot, C.S.; Rabbinge, R. Radiation and Nitrogen Use at the Leaf and Canopy Level by Wheat and Oilseed Rape during the Critical Period for Grain Number Definition. Funct. Plant Biol. 2000, 27, 899–910. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A. Distribution of N, Rubisco and Photosynthesis in Pinus Pinaster and Acclimation to Light. Plant Cell Environ. 2001, 24, 597–609. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Miyazawa, K.; Hikosaka, K.; Nagashima, H.; Hirose, T. Leaf Nitrogen Distribution in Relation to Leaf Age and Photon Flux Density in Dominant and Subordinate Plants in Dense Stands of a Dicotyledonous Herb. Oecologia 1998, 113, 314–324. [Google Scholar] [CrossRef]
- Rosati, A.; Day, K.R.; DeJong, T.M. Distribution of Leaf Mass per Unit Area and Leaf Nitrogen Concentration Determine Partitioning of Leaf Nitrogen within Tree Canopies. Tree Physiol. 2000, 20, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, X.; Sinoquet, H.; Vandame, M. Spatial Distribution of Leaf Dry Weight per Area and Leaf Nitrogen Concentration in Relation to Local Radiation Regime within an Isolated Tree Crown. Tree Physiol. 1999, 19, 181–188. [Google Scholar] [CrossRef]
- Kamnqa, U.; Etsassala, N.G.E.R.; Akinpelu, E.A.; Nchu, F. Effects of Varying Nitrogen Fertilization on Growth, Yield and Flowering of Capsicum Annuum (California Wonder). In Proceedings of the 18th SOUTH AFRICA Int’l Conference on Agricultural, Chemical, Biological & Environmental Sciences (ACBES-20), Johannesburg, South Africa, 16–17 November 2020; pp. 255–259. [Google Scholar] [CrossRef]
- Bojović, B.; Marković, A. Correlation Between Nitrogen and Chlorophyll Content in Wheat (Triticum aestivum L.). Kragujev. J. Sci. 2009, 31, 69–74. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of Nitrogen Fertilization on Synthesis of Primary and Secondary Metabolites in Three Varieties of Kacip Fatimah (Labisia Pumila Blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Hamilton, E.W.; Giovannini, M.S.; Moses, S.A.; Coleman, J.S.; McNaughton, S.J. Biomass and Mineral Element Responses of a Serengeti Short-Grass Species to Nitrogen Supply and Defoliation: Compensation Requires a Critical. Oecologia 1998, 116, 407–418. [Google Scholar] [CrossRef]
- Fang, Y.; Xun, F.; Bai, W.; Zhang, W.; Li, L. Long-Term Nitrogen Addition Leads to Loss of Species Richness Due to Litter Accumulation and Soil Acidification in a Temperate Steppe. PLoS ONE 2012, 7, e47369. [Google Scholar] [CrossRef]
- Tian, Q.; Liu, N.; Bai, W.; Li, L.; Chen, J.; Reich, P.B.; Yu, Q.; Guo, D.; Smith, M.D.; Knapp, A.K.; et al. A Novel Soil Manganese Mechanism Drives Plant Species Loss with Increased Nitrogen Deposition in a Temperate Steppe. Ecology 2016, 97, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R. Zinc Application and Its Availability to Plants. Ph.D. Thesis, Murdoch University, Perth, Australia, 2005. [Google Scholar]
- Sinkhorn, E.R.; Perakis, S.S.; Compton, J.E.; Cromack, K.; Bullen, T.D. Non-Linear Nitrogen Cycling and Ecosystem Calcium Depletion Along a Temperate Forest Soil Nitrogen Gradient. AGU Fall Meet. Abstr. 2007, 2007, B31A-0061. [Google Scholar]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Interactive Effects of Nitrogen and Potassium on Photosynthesis and Photosynthetic Nitrogen Allocation of Rice Leaves. BMC Plant Biol. 2019, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Smolen, S.; Sady, W. The effect of nitrogen fertilizer form on the content of sixteen elements in red cabbage. Acta Scien. Pol. Hort. Cult. 2008, 7, 35–44. [Google Scholar]
- Rutkowska, B.; Szulc, W.; Labetowicz, J. Influence of Soil Fertilization on Concentration of Microelements in Soil Solution of Sandy Soil. J. Elem. 2009, 14, 349–355. [Google Scholar] [CrossRef]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N Fertilization on the Content of Phenolic Compounds in Jerusalem Artichoke (Helianthus tuberosus L.) Tubers and Their Antioxidant Capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Tavarini, S.; Sgherri, C.; Ranieri, A.M.; Angelini, L.G. Effect of Nitrogen Fertilization and Harvest Time on Steviol Glycosides, Flavonoid Composition, and Antioxidant Properties in Stevia Rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Cui, R.; Su, L.; Sun, X.; Borras-Hidalgo, O.; Li, K.; Wei, J.; Yue, Q.; Zhao, L. Effects of Nitrogen Application on Phytochemical Component Levels and Anticancer and Antioxidant Activities of Allium Fistulosum. PeerJ 2021, 9, e11706. [Google Scholar] [CrossRef]
- Sugiharto, B.; Sugiyama, T. Effects of Nitrate and Ammonium on Gene Expression of Phosphoenolpyruvate Carboxylase and Nitrogen Metabolism in Maize Leaf Tissue during Recovery from Nitrogen Stress. Plant Physiol. 1992, 98, 1403–1408. [Google Scholar] [CrossRef] [Green Version]
- Margna, U.; Margna, E.; VainjÄrv, T. Influence of Nitrogen Nutrition on the Utilization of L-Phenylalanine for Building Flavonoids in Buckwheat Seedling Tissues. J. Plant Physiol. 1989, 134, 697–702. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waśkiewicz, A.; Muzolf-Panek, M.; Goliński, P. Phenolic Content Changes in Plants Under Salt Stress. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 283–314. ISBN 978-1-4614-4747-4. [Google Scholar]
- Zhao, X.; Nechols, J.R.; Williams, K.A.; Wang, W.; Carey, E.E. Comparison of Phenolic Acids in Organically and Conventionally Grown Pac Choi (Brassica Rapa L. Chinensis). J. Sci. Food Agric. 2009, 89, 940–946. [Google Scholar] [CrossRef]
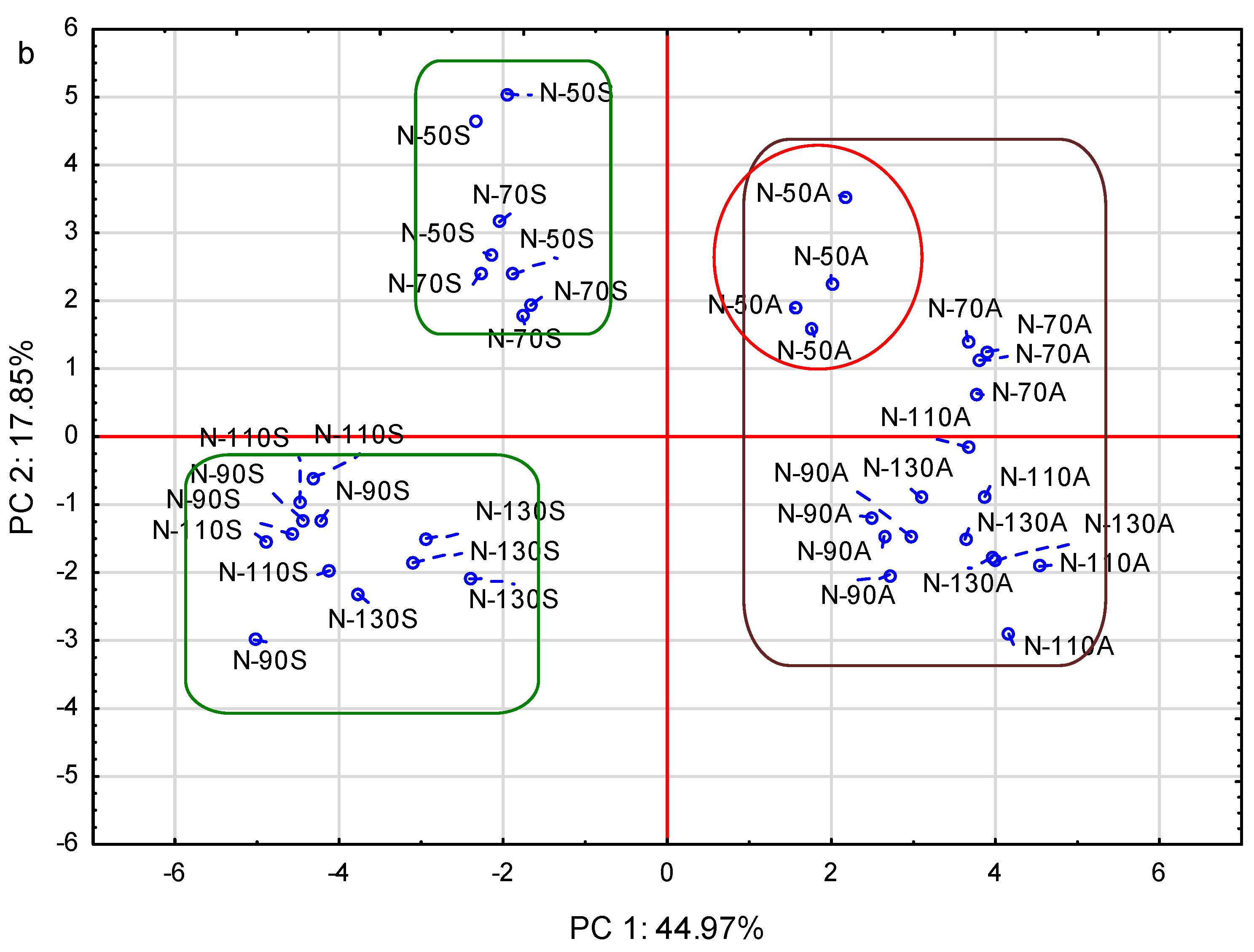
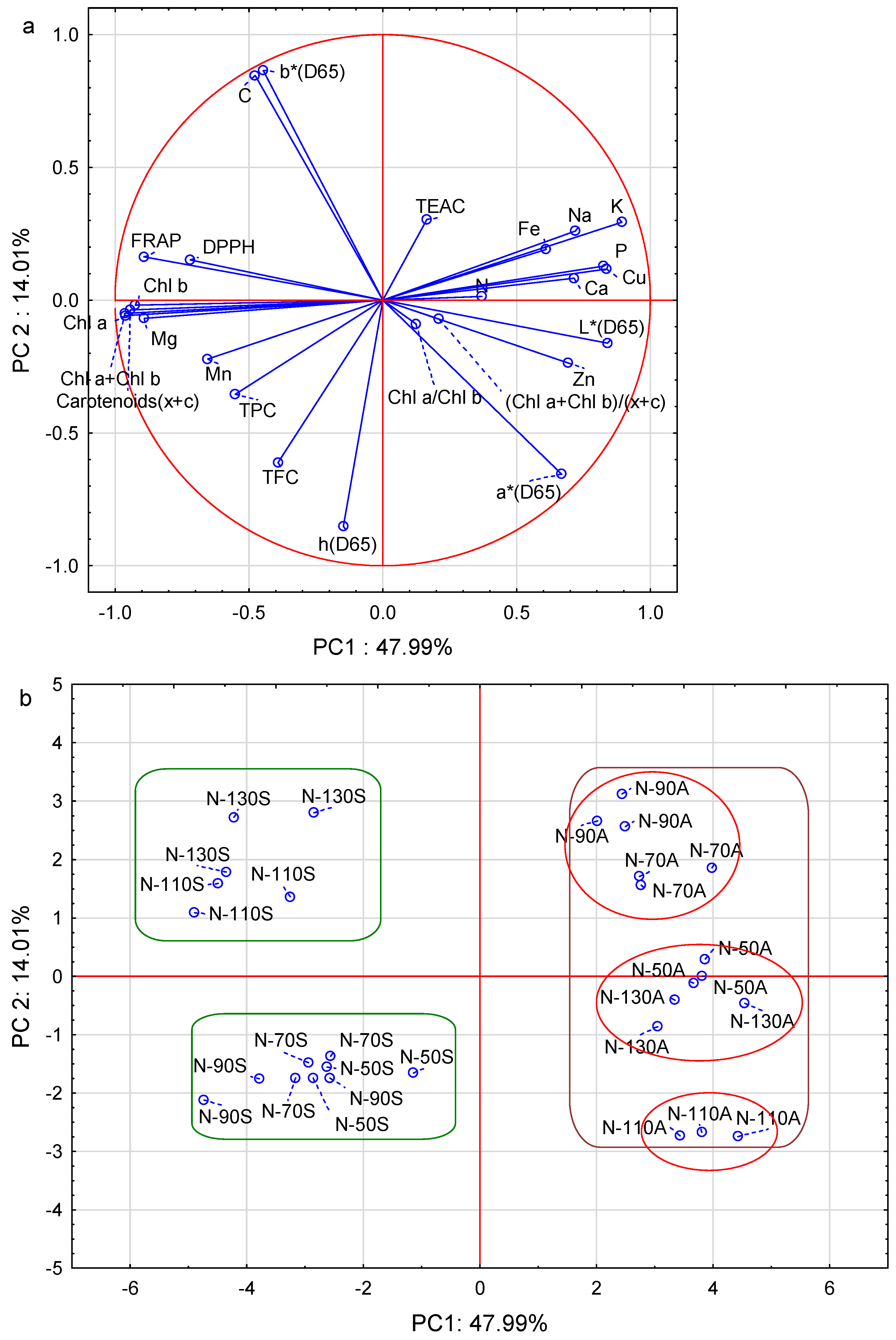
| N Level | N | P | K | Ca | Mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring * | Autumn * | Mean ** | Spring | Autumn | Mean | Spring | Autumn | Mean | Spring | Autumn | Mean | Spring | Autumn | Mean | |
| Substrate | |||||||||||||||
| N-50 | 1.92 a | 4.15 d | 3.03 a | 0.46 a | 0.59 c | 0.53 b | 4.62 a | 7.70 d | 6.16 a | 2.13 a | 2.40 ab | 2.27 ab | 2.13 bc | 0.54 a | 1.33 ab |
| N-70 | 2.14 a | 4.11 d | 3.12 a | 0.50 ab | 0.47 ab | 0.48 ab | 4.96 ab | 7.40 cd | 6.17 a | 1.92 a | 2.28 ab | 2.10 a | 1.92 b | 0.49 a | 1.21 a |
| N-90 | 2.84 b | 4.32 de | 3.58 b | 0.46 a | 0.47 ab | 0.47 a | 4.62 a | 6.93 c | 5.78 a | 2.01 a | 2.64 b | 2.32 ab | 2.01 b | 0.52 a | 1.26 ab |
| N-110 | 3.19 bc | 4.43 de | 3.81 bc | 0.50 ab | 0.44 a | 0.47 a | 5.01 ab | 7.05 cd | 6.03 a | 2.39 ab | 3.28 c | 2.83 c | 2.39 c | 0.49 a | 1.44 b |
| N-130 | 3.50 c | 4.71 e | 4.10 c | 0.54 bc | 0.47 ab | 0.51 ab | 5.38 b | 7.17 cd | 6.28 a | 2.40 ab | 2.66 b | 2.53 bc | 2.40 c | 0.51 a | 1.45 b |
| Mean ** | 2.71 a | 4.34 b | 0.49 a | 0.49 a | 4.92 a | 7.25 b | 2.17 a | 2.65 b | 2.17 b | 0.51 a | |||||
| ±SD | 0.67 | 0.38 | 0.05 | 0.07 | 0.47 | 0.48 | 0.32 | 0.44 | 0.32 | 0.04 | |||||
| Hydroponic | |||||||||||||||
| N-50 | 4.39 ab | 4.53 ab | 4.46 a | 0.50 a | 0.59 cd | 0.55 a | 4.96 a | 8.21 c | 6.59 a | 2.01 a | 2.55 ab | 2.28 a | 2.01 b | 1.14 a | 1.57 a |
| N-70 | 4.34 ab | 4.67 ab | 4.50 a | 0.52 ab | 0.64 cd | 0.58 a | 5.20 a | 8.03 c | 6.62 a | 2.19 ab | 2.49 ab | 2.34 a | 2.19 b | 1.10 a | 1.64 a |
| N-90 | 4.48 ab | 4.36 ab | 4.42 a | 0.52 a | 0.58 bc | 0.55 a | 5.17 a | 8.06 c | 6.62 a | 2.02 a | 2.53 ab | 2.28 a | 2.02 b | 1.11 a | 1.56 a |
| N-110 | 4.18 a | 4.53 ab | 4.35 a | 0.52 ab | 0.61 cd | 0.57 a | 5.23 a | 7.06 b | 6.14 a | 2.01 a | 2.68 b | 2.35 a | 2.01 b | 1.05 a | 1.53 a |
| N-130 | 4.62 ab | 4.95 b | 4.78 a | 0.53 ab | 0.64 d | 0.59 a | 5.26 a | 7.36 bc | 6.31 a | 2.20 ab | 2.49 ab | 2.35 a | 2.20 b | 1.05 a | 1.63 a |
| Mean | 4.40 a | 4.61 a | 0.52 a | 0.61 b | 5.16 a | 7.74 b | 2.09 a | 2.55 b | 2.09 b | 1.09 a | |||||
| ±SD | 0.43 | 0.22 | 0.03 | 0.04 | 0.38 | 0.72 | 0.26 | 0.24 | 0.26 | 0.07 | |||||
| N Level | Fe | Mn | Zn | Cu | Na | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring * | Autumn * | Mean ** | Spring | Autumn | Mean | Spring | Autumn | Mean | Spring | Autumn | Mean | Spring | Autumn | Mean | |
| Substrate | |||||||||||||||
| N-50 | 126.1 ab | 111.4 a | 118.8 a | 28.1 a | 39.0 bcd | 33.6 a | 42.8 a | 47.9 abc | 45.4 a | 7.55 cd | 5.20 a | 6.37 a | 0.09 a | 0.19 b | 0.14 a |
| N-70 | 117.4 ab | 154.1 b | 135.8 a | 32.8 ab | 38.4 bcd | 35.6 ab | 47.3 abc | 46.4 a | 46.8 a | 8.41de | 5.07 a | 6.74 ab | 0.11 a | 0.22 b | 0.16 ab |
| N-90 | 126.1 ab | 119.5 ab | 122.8 a | 38.8 bcd | 40.6 bcd | 39.7 bc | 59.5 d | 46.7 ab | 53.1 b | 7.03 bcd | 6.07 abc | 6.55 ab | 0.12 a | 0.28 c | 0.20 b |
| N-110 | 115.3 ab | 147.6 ab | 131.5 a | 36.4 abc | 43.8 cd | 40.1 bc | 55.6 cd | 51.0 abcd | 53.3 b | 9.24 e | 5.77 ab | 7.51 b | 0.18 b | 0.30 c | 0.24 c |
| N-130 | 152.5 b | 209.4 c | 181.0 b | 40.3 bcd | 46.7 d | 43.5 c | 57.2 d | 55.3 bcd | 56.2 b | 7.84 de | 6.31 abc | 7.07 ab | 0.20 b | 0.31 c | 0.25 c |
| Mean ** | 127.5 a | 148.4 b | 35.3 a | 41.7 b | 52.5 a | 49.5 a | 8.01 b | 5.68 a | 0.14 a | 0.26 b | |||||
| ±SD | 22.6 | 41.6 | 7.81 | 5.87 | 6.22 | 6.14 | 1.51 | 0.83 | 0.05 | 0.06 | |||||
| Hydroponic | |||||||||||||||
| N-50 | 86.8 a | 108.4 bc | 97.6 a | 73.95 e | 44.45 a | 59.20 ab | 39.00 c | 31.67 a | 35.33 a | 4.03 ab | 5.08 cd | 4.56 a | 0.18 a | 0.25 d | 0.22 a |
| N-70 | 92.1 ab | 106.4 abc | 99.3 a | 65.08 cde | 51.75 ab | 58.42 a | 32.29 ab | 45.17 d | 38.73 a | 3.92 ab | 5.36 de | 4.64 a | 0.19 a | 0.25 d | 0.22 a |
| N-90 | 99.6 abc | 118.2 c | 108.9 a | 66.29 cde | 57.63 bc | 61.96 abc | 37.02 bc | 47.78 d | 42.40 b | 3.78 a | 5.89 e | 4.84 a | 0.19 ab | 0.25 d | 0.22 a |
| N-110 | 93.2 ab | 117.1 c | 105.1 a | 66.62 cde | 63.28 cd | 64.95 bc | 30.72 a | 59.97 e | 45.35 bc | 3.71 a | 5.97 e | 4.84 a | ‘0.19 a | 0.23 bcd | 0.21 a |
| N-130 | 107.8 bc | 113.9 c | 110.8 a | 70.99 de | 60.28 c | 65.63 c | 32.15 ab | 59.83 e | 45.99 c | 4.57 bc | 6.65 f | 5.61 b | 0.20 abc | 0.24 cd | 0.22 a |
| Mean | 95.9 a | 112.8 b | 68.59 b | 55.48 a | 34.23 a | 48.89 b | 4.00 a | 5.79 b | 0.19 a | 0.24 b | |||||
| ±SD | 12.07 | 16.38 | 5.51 | 9.49 | 4.35 | 9.90 | 0.44 | 0.62 | 0.02 | 0.02 | |||||
| L* (D65) | a* (D65) Green | b* (D65) Yellow | h* (°) (D65) | C* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Dose | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn |
| Substrate | ||||||||||
| N-50 | 47.50 g | 43.82 b | −8.66 c | −7.32 j | 22.36 j | 20.21 f | 111.16 c | 109.91 a | 23.98 j | 21.50 f |
| N-70 | 45.16 c | 48.27 h | −7.99 g | −7.40 i | 20.43 g | 19.14 c | 111.36 d | 111.14 b | 21.94 g | 20.52 c |
| N-90 | 45.5 e | 48.32 i | −9.17 b | −8.60 d | 21.32 h | 18.47 a | 113.29 h | 114.98 j | 23.21 h | 20.37 a |
| N-110 | 45.63 f | 48.97 j | −9.19 a | −8.31 e | 21.38 i | 19.20 d | 113.26 g | 113.41 i | 23.27 i | 20.93 d |
| N-130 | 43.65 a | 45.24 d | −8.03 f | −7.81 h | 19.80 e | 18.89 b | 112.08 e | 112.47 f | 21.36 e | 20.44 b |
| Hydroponic | ||||||||||
| N-50 | 47.42 f | 49.01 j | −8.36 d | −7.64 g | 19.47 e | 18.46 b | 113.23 gh | 112.48 d | 21.18 e | 19.98 b |
| N-70 | 44.75 c | 48.82 i | −8.35 d | −8.18 e | 19.47 e | 21.11 f | 113.20 g | 111.18 a | 21.18 e | 22.64 f |
| N-90 | 43.84 a | 45.68 e | −8.34 d | −8.97 c | 19.40 d | 22.28 g | 113.26 h | 111.91 b | 21.12 d | 24.02 g |
| N-110 | 45.28 d | 48.66 h | −9.49 a | −7.54 h | 22.67 i | 16.73 a | 112.71 e | 114.24 i | 24.57 i | 18.35 a |
| N-130 | 44.35 b | 48.46 g | −9.25 b | −8.08 f | 22.52 h | 19.13 c | 112.32 c | 112.91 f | 24.35 h | 20.77 c |
| Chl a (mg/g DM) | Chl b (mg/g DM) | Carotenoid (x + c) (mg/g DM) | Chl a + Chl b (mg/g DM) | Chl a/Chl b | (Chl a + Chl b)/(x + c) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Level | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn |
| Substrate | ||||||||||||
| N-50 | 5.14 a | 5.50 a | 1.43 a | 1.44 a | 1.40 b | 1.32 ab | 6.57 a | 6.94 a | 3.77 a | 3.99 a | 4.65 a | 5.28 ab |
| N-70 | 5.47 a | 4.64 a | 1.59 ab | 1.52 ab | 1.34 ab | 0.98 a | 7.06 a | 6.16 a | 3.44 a | 3.05 a | 5.29 ab | 6.31 b |
| N-90 | 8.13 b | 5.26 a | 2.16 b | 1.64 ab | 2.01 c | 1.09 ab | 10.29 b | 6.90 a | 3.76 a | 3.21 a | 5.19 ab | 6.34 b |
| N-110 | 7.62 b | 5.05 a | 2.00 bc | 1.48 ab | 2.03 c | 1.07 ab | 9.62 b | 6.53 a | 3.81 a | 3.49 a | 4.74 a | 6.18 b |
| N-130 | 8.33 b | 4.88 a | 2.29 c | 1.32 a | 1.95 c | 1.06 ab | 10.62 b | 6.20 a | 3.64 a | 3.79 a | 5.47 ab | 5.95 b |
| Hydroponic | ||||||||||||
| N-50 | 7.46 b | 4.68 a | 1.98 b | 1.38 a | 1.71 b | 1.00 a | 9.44 b | 6.06 a | 3.77 a | 3.41 a | 5.55 abc | 6.06 abc |
| N-70 | 7.72 cb | 5.03 a | 2.10 b | 1.27 a | 1.78 bc | 1.21 a | 9.82 bc | 6.31 a | 3.68 a | 4.09 a | 5.53 abc | 5.24 a |
| N-90 | 8.75 c | 4.87 a | 2.36 b | 1.42 a | 1.99 bc | 1.02 a | 11.11 c | 6.29 a | 3.70 a | 3.46 a | 5.61 abc | 6.14 bc |
| N-110 | 8.31 cb | 4.84 a | 2.28 b | 1.36 a | 1.87 bc | 0.98 a | 10.59 bc | 6.20 a | 3.68 a | 3.71 a | 5.67 abc | 6.30 c |
| N-130 | 8.90 c | 5.44 a | 2.42 b | 1.32 a | 2.02 c | 1.26 a | 11.31 c | 6.77 a | 3.68 a | 4.24 a | 5.61 abc | 5.42 ab |
| TEAC (μmol/g) | DPPH (μmol TE/g) | FRAP (mmol TE/g) | TPC (mg GAE/g) | TFC (mg QE/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N Dose | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn | Spring | Autumn |
| Substrate | ||||||||||
| N-50 | 396.54 a | 392.85 a | 120.19 bc | 108.78 b | 33.23 d | 24.59 b | 6.28 c | 4.22 a | 1.89 d | 1.45 bc |
| N-70 | 417.17 b | 414.83 b | 148.00 d | 107.41 b | 32.83 d | 24.42 b | 6.44 c | 4.21 a | 3.90 g | 1.16 ab |
| N-90 | 501.87 ef | 465.38 d | 188.21 f | 125.56 c | 44.75 e | 27.58 c | 8.96 e | 6.37 c | 2.73 ef | 1.47 c |
| N-110 | 484.75 e | 511.69 fg | 199.31 f | 89.41 a | 46.11 e | 21.26 a | 7.37 d | 3.61 a | 2.86 f | 1.13 a |
| N-130 | 437.90 c | 524.21 g | 164.73 e | 106.98 b | 45.45 e | 23.64 b | 8.81 e | 5.16 b | 2.48 e | 1.29 abc |
| Hydroponic | ||||||||||
| N-50 | 410.87 ab | 447.63 bcde | 101.48 a | 121.05 c | 37.16 de | 21.56 a | 6.01 cde | 5.31 abcd | 1.40 bcd | 0.97 a |
| N-70 | 363.92 a | 419.34 bc | 148.11 d | 103.95 a | 34.75 cd | 28.28 abc | 6.32 de | 4.88 ab | 1.62 de | 1.25 abc |
| N-90 | 434.20 bcd | 481.87 de | 140.79 d | 111.37 abc | 38.04 de | 30.12 bc | 8.55 f | 5.53 bcd | 1.81 e | 1.11 ab |
| N-110 | 467.72 cde | 497.63 e | 151.29 d | 119.44 bc | 39.31 de | 25.52 ab | 8.18 f | 6.73 e | 1.33 bc | 1.31 bc |
| N-130 | 480.25 de | 423.21 bc | 170.05 e | 105.87 ab | 43.03 e | 25.97 ab | 4.95 abc | 4.38 a | 1.01 a | 1.41 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Muzolf-Panek, M.; Kleiber, T. Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy 2022, 12, 2869. https://doi.org/10.3390/agronomy12112869
Liu W, Muzolf-Panek M, Kleiber T. Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy. 2022; 12(11):2869. https://doi.org/10.3390/agronomy12112869
Chicago/Turabian StyleLiu, Wenping, Małgorzata Muzolf-Panek, and Tomasz Kleiber. 2022. "Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage" Agronomy 12, no. 11: 2869. https://doi.org/10.3390/agronomy12112869
APA StyleLiu, W., Muzolf-Panek, M., & Kleiber, T. (2022). Effect of Nitrogen Nutrition and Planting Date on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy, 12(11), 2869. https://doi.org/10.3390/agronomy12112869





