Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. PSTVd Strains
2.3. Plant Inoculation and Tolerance Assessment
2.4. Detection of PSTVd in Potato and Tomato Plants by RT-PCR and RT-qPCR
2.5. Detection of viral infection in potato plants by ELISA (enzyme-linked immunosorbent assay) and RT-qPCR
2.6. Statistical Analysis
3. Results
3.1. Assessment of Symptoms on Tomato cv. Rutgers after Inoculation by Four PSTVd Strains
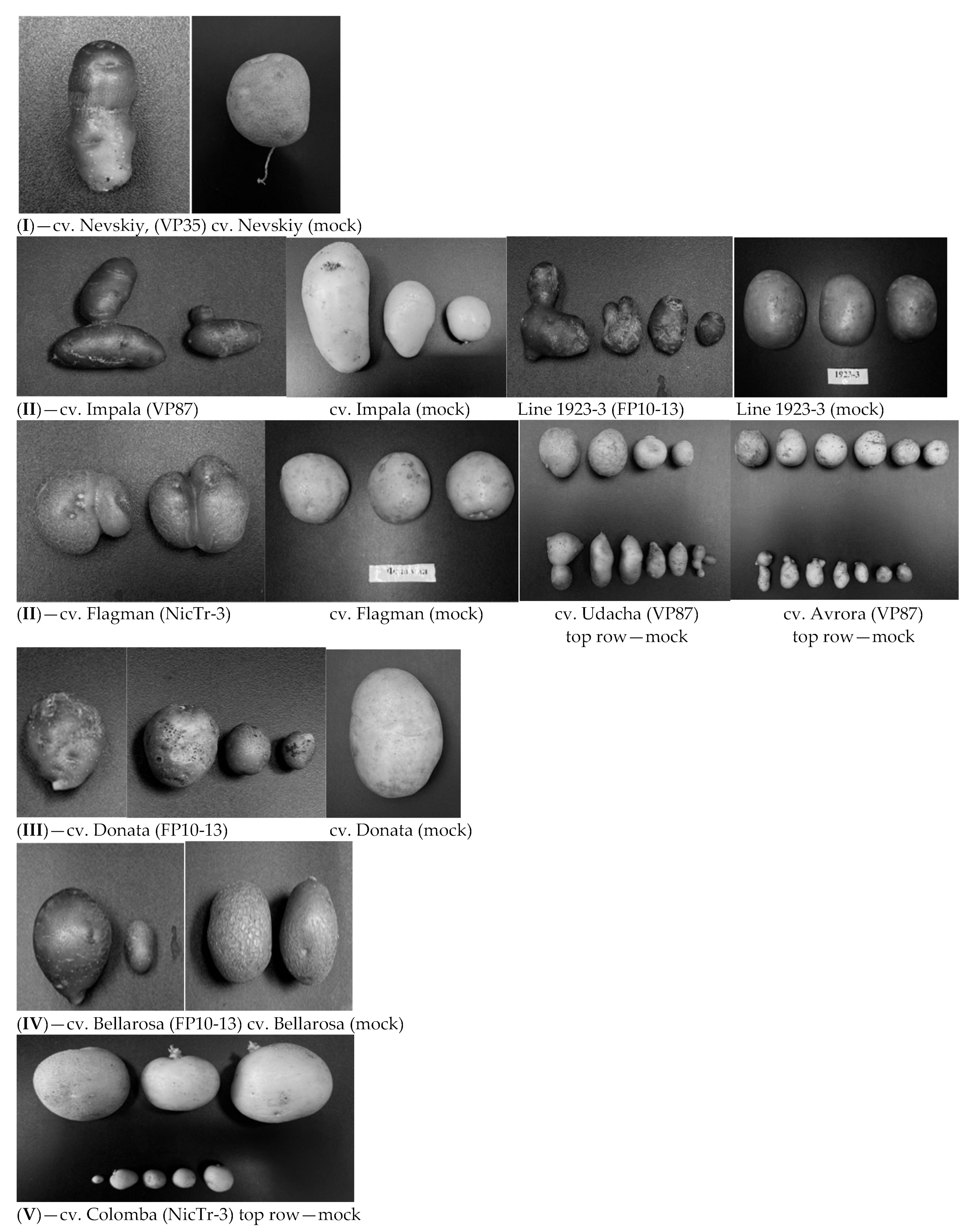
3.2. Disease Symptoms
3.3. Symptoms on Second Generation Plants Derived from PSTVd-Infected but Normal in Shape Tubers
3.4. Symptoms of Viroid/Viral Mixed Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hadidi, A.; Flores, R.; Palukaitis, P.; Randles, J. Viroids and Satellites; Academic Press: Oxford, UK, 2017; p. 716. [Google Scholar]
- Puchta, H.; Herold, T.; Verhoeven, K.; Roenhorst, A.; Ramm, K.; Schmidt-Puchta, W.; Sänger, H.L. A new strain of potato spindle tuber viroid (PSTVd-N) exhibits major sequence differences as compared to all other PSTVd strains sequenced so far. Plant Mol. Biol. 1990, 15, 509–511. [Google Scholar] [CrossRef]
- Owens, R.A.; Verhoeven, J.T.J. Potato spindle tuber viroid. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 149–158. [Google Scholar]
- Singh, R.P. Experimental host range of the potato spindle tuber ‘virus’. Am. Potato J. 1973, 50, 111–123. [Google Scholar] [CrossRef]
- Owens, R.A.; Tech, K.B.; Shao, J.Y.; Sano, T.; Baker, C.J. Global analysis of tomato gene expression during Potato spindle tuber viroid infection reveals a complex array of changes affecting hormone signaling. Mol. Plant Microbe Interact. 2012, 25, 582–598. [Google Scholar] [CrossRef]
- Krasnova, M.V. Potato Spindle Tuber Viroid in the North-West Region of the Russian Federation. Ph.D. Thesis, Petrozavodsk State University, St. Petersburg, Russia, 2000; p. 18. [Google Scholar]
- Mackie, A.E.; Barbetti, M.J.; Rodoni, B.; McKirdy, S.J.; Jones, R.A.C. Effects of a Potato Spindle Tuber Viroid Tomato Strain on the Symptoms, Biomass, and Yields of Classical Indicator and Currently Grown Potato and Tomato Cultivars. Plant Dis. 2019, 103, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, Y.A. Spindle Tuber Virus (Gothic)—One of the Main Virus Diseases in the Volga Region. Ph.D. Thesis, Agricultural Institute, Pushkin, Russia, 1971; p. 43. [Google Scholar]
- Romanova, S.A.; Ledneva, V.A. Viruses, viroids and mycoplasma diseases affecting sanitized potato in the Far East. In Protection of Plants under Reforming of Agroindustrial Complex; All-Russian Research Institute of Plant Protection: St. Petersburg, Russia, 1995; pp. 80–81. [Google Scholar]
- Mozhaeva, K.A.; Vasilieva, T.Y.; Kastalieva, T.B. Distribution of potato spindle tuber viroid with virus-free seed potatoes. In Actual Problems of Modern Potato Breeding; Belarus Institute for Potato Growing: Minsk, Belarus, 1997; p. 101. [Google Scholar]
- Matsushita, Y.; Yanagisawa, H.; Khiutti, A.; Mironenko, N.; Ohto, Y.; Afanasenko, O. Genetic diversity and pathogenicity of potato spindle tuber viroid and chrysanthemum stunt viroid isolates in Russia. Eur. J. Plant Pathol. 2021, 161, 529–542. [Google Scholar] [CrossRef]
- Malko, A.; Frantsuzov, P.; Nikitin, M.; Statsuyk, N.; Dzhavakhiya, V.; Golikov, A. Potato Pathogens in Russia’s Regions: An Instrumental Survey with the Use of Real-Time PCR/RT-PCR in Matrix Format. Pathogens 2019, 8, 18. [Google Scholar] [CrossRef]
- Singh, R.P. Clones of Solanum berthauitii resistant to potato spindle tuber viroid. Phytopathology 1985, 75, 1432–1434. [Google Scholar] [CrossRef]
- Naoi, T.; Hataya, T. Tolerance Even to Lethal Strain of Potato Spindle Tuber Viroid Found in Wild Tomato Species Can Be Introduced by Crossing. Plants 2021, 10, 575. [Google Scholar] [CrossRef]
- Matsushita, Y.; Penmetcha, K.K.R. In Vitro-Transcribed Chrysanthemum stunt viroid RNA Is Infectious to Chrysanthemum and Other Plants. Phytopathology 2009, 99, 58–66. [Google Scholar] [CrossRef]
- Behjatnia, A.; Dry, I.; Krake, L.; Condé, B.D.; Connelly, M.I.; Randles, J.; Rezaian, M.A. New potato spindle tuber viroid and tomato leaf curl geminivirus strains from a wild Solanum sp. Phytopathology 1996, 86, 880–886. [Google Scholar] [CrossRef]
- Gross, H.J.; Domdey, H.; Lossow, C.; Jank, P.; Raba, M.; Alberty, H.; Sänger, H.L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 1978, 273, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Sano, T.; Hase, S.; Matsushita, Y. Influence of the terminal left domain on horizontal and vertical transmissions of tomato planta macho viroid and potato spindle tuber viroid through pollen. Virology 2019, 526, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Hernández, C.; de Alba, A.E.M.; Daròs, J.A.; Di Serio, F. Viroids and viroid–host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral diseases in potato. In The Potato Crop. Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 389–430. [Google Scholar] [CrossRef]
- Constable, F.; Chambers, G.; Penrose, L.; Daly, A.; Mackie, J.; Davis, K.; Rodoni, B.; Gibbs, M. Viroid-infected tomato and capsicum seed shipments to Australia. Viruses 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Kastalieva, T.B.; Mozhaeva, K.A.; Pisetskaya, N.F.; Romanova, S.A.; Trofimets, L.N. Spindle tuber viroid and potato health improvement. Bull. Russ. Acad. Agric. Sci. 1992, 3, 22–24. [Google Scholar]
- Owens, R.A.; Hammond, R.W. Viroid Pathogenicity: One process, many faces. Viruses 2009, 1, 298–316. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Bolduc, F.; Bru, P.; Perreault, J.-P. Insights Into Potato Spindle Tuber Viroid Quasi-Species From Infection to Disease. Front. Microbiol. 2020, 11, 1235. [Google Scholar] [CrossRef]
- Kitabayashi, S.; Tsushima, D.; Adkar-Purushothama, C.R.; Sano, T. Identification and Molecular Mechanisms of Key Nucleotides Causing Attenuation in Pathogenicity of Dahlia Isolate of Potato Spindle Tuber Viroid. Int. J. Mol. Sci. 2020, 21, 7352. [Google Scholar] [CrossRef]
- Schnölzer, M.; Haas, B.; Ramm, K.; Hofmann, H.; Sanger, H.L. Correlation between structure and pathogenicity of Potato spindle tuber viroid (PSTV). EMBO J. 1985, 4, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Gruner, R.; Fels, A.; Qu, F.; Zimmat, R.; Steger, G.; Riesner, D. Interdependence of pathogenicity and replicability with potato spindle tuber viroid. Virology 1995, 209, 60–69. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, B. Inhibition of cell growth and shoot development by a specific nucleotide sequence in a noncoding viroid RNA. Plant Cell 2003, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Mozhayeva, K.A. Productivity of potatoes infected with potato spindle tuber viroids. Proc. TSKHA 2011, 6, 60–68. [Google Scholar]
- Sofy, A.R.; Mahfouze, S.A.; El-Enany, M.A.M. Isozyme markers for response of wild potato species to Potato spindle tuber viroid egyptian isolate. World Appl. Sci. J. 2013, 27, 1010–1022. [Google Scholar] [CrossRef]
- Domingo-Calap, M.L.; Moreno, A.B.; Pendon, J.A.D.; Moreno, A.; Fereres, A.; Lopez-Moya, J.J. Assessing the Impact on Virus Transmission and Insect Vector Behavior of a Viral Mixed Infection in Melon. Phytopathology 2020, 110, 174–186. [Google Scholar] [CrossRef] [PubMed]













| No | Cultivar | The First Inoculation by Strains: | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VP87 | VP35 | FP10-13 | NicTr-3 | ||||||
| RT-PCR | Form | RT-PCR | Form | RT-PCR | Form | RT-PCR | Form | ||
| 1 | Avrora | −/+ | D | No data | N | No data | |||
| 2 | Arizona | +/+* | N | + | N | +/+* | N | + | S |
| 3 | Ariel | + | N | + | N/S | +/+* | N | + | N |
| 4 | Armada | + | N | No data | No data | + | M | ||
| 5 | Bellarosa | + | N | + | N | +/−*/+* | N\P | + | N |
| 6 | Berkut | + | N | + | N/P | + | N | No data | |
| 7 | Bernina | + | N | +/+* | N | +/+* | N | +/+* | N/P |
| 8 | Bizon | + | N | + | M | No data | No data | ||
| 9 | Briz | + | N/D | +/+* | N | +/+* | N | No data | |
| 10 | Charoit | + | N | + | N | +/+* | N | +/+* | N/P |
| 11 | Colomba | + | N | + | N/D | +/+* | N | +/+* | N/D |
| 12 | Donata | +/+* | N | + | N | +/+* | N/P | + | N |
| 13 | Elizaveta | −/+ | N | + | N | No data | No data | ||
| 14 | Favorit | −/+ | N/P/M | + | N/P | + | N/P | +/+ | N |
| 15 | Flagman | +/+* | N | +/+* | M/D | No data | +/+* | N/D | |
| 16 | Gala | + | N/M | No data | +/+* | N | +/+* | P | |
| 17 | Impala | + | D | + | N/D | + | N/D | +/+* | N |
| 18 | Krepysh | +/+* | N/P/M | + | N | + | N/P | No data | |
| 19 | Labadia | + | N | +/+* | N | +/+* | N | +/+* | N |
| 20 | Madeira | + | N | + | N | +/+* | N | + | N/S |
| 21 | Manifest | + | N | + | N | No data | + | N | |
| 22 | Meteor | No data | No data | + | N\D | No data | |||
| 23 | Mirazh | N | + | N/S | + | N | + | N | |
| 24 | Nandina | + | N/P | +/+* | N | +/+* | N | +/+* | N |
| 25 | Navigator | + | D | + | S | +/+* | N | +/+* | N/S |
| 26 | Nevskiy | +/+* | N | +/+* | N/S | No data | +/+* | N | |
| 27 | Phioletovyi | +/−/+* | N/P | No data | + | N/D | +/+* | N/P | |
| 28 | Queen Anna | + | N | +/+* | N | +/+* | N/P | + | N/S/M |
| 29 | Riviera | N | + | N | + | N | No data | ||
| 30 | Sanibel | + | N/P | + | S | +/+* | N/P | No data | |
| 31 | Sapsan | - | N | N | + | M | + | N | |
| 32 | Sineglazka2016 | No data | + | N | + | N | No data | ||
| 33 | Siurpriz | + | N | + | N | + | N | +/+* | N |
| 34 | Sorentina | +/+* | N | No data | + | N | +/+* | N | |
| 35 | Sprinter | +/+* | N | + | N | +/+ | N/D | + | N |
| 36 | Udacha | + | D | + | N | + | N | +/+* | N |
| 37 | Vineta | + | N | +/+* | N/P | +/+* | N | +/−* | N/D |
| 38 | 1923-3 | + | N | + | N/S | + | N/D | + | D |
| 39 | 4434-1 | + | N | + | N/D | + | N/D | + | N |
| Total cultivars with viroid symptoms | 11 | 14 | 13 | 13 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afanasenko, O.S.; Lashina, N.M.; Mironenko, N.V.; Kyrova, E.I.; Rogozina, E.V.; Zubko, N.G.; Khiutti, A.V. Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection. Agronomy 2022, 12, 2916. https://doi.org/10.3390/agronomy12122916
Afanasenko OS, Lashina NM, Mironenko NV, Kyrova EI, Rogozina EV, Zubko NG, Khiutti AV. Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection. Agronomy. 2022; 12(12):2916. https://doi.org/10.3390/agronomy12122916
Chicago/Turabian StyleAfanasenko, Olga S., Nina M. Lashina, Nina V. Mironenko, Elena I. Kyrova, Elena V. Rogozina, Natalia G. Zubko, and Aleksander V. Khiutti. 2022. "Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection" Agronomy 12, no. 12: 2916. https://doi.org/10.3390/agronomy12122916
APA StyleAfanasenko, O. S., Lashina, N. M., Mironenko, N. V., Kyrova, E. I., Rogozina, E. V., Zubko, N. G., & Khiutti, A. V. (2022). Evaluation of Responses of Potato Cultivars to Potato Spindle Tuber Viroid and to Mixed Viroid/Viral Infection. Agronomy, 12(12), 2916. https://doi.org/10.3390/agronomy12122916







