Highly Dispersed Blast-Furnace Sludge as a New Micronutrient Fertilizer: Promising Results on Rapeseed
Abstract
:1. Introduction
2. Materials and Methods
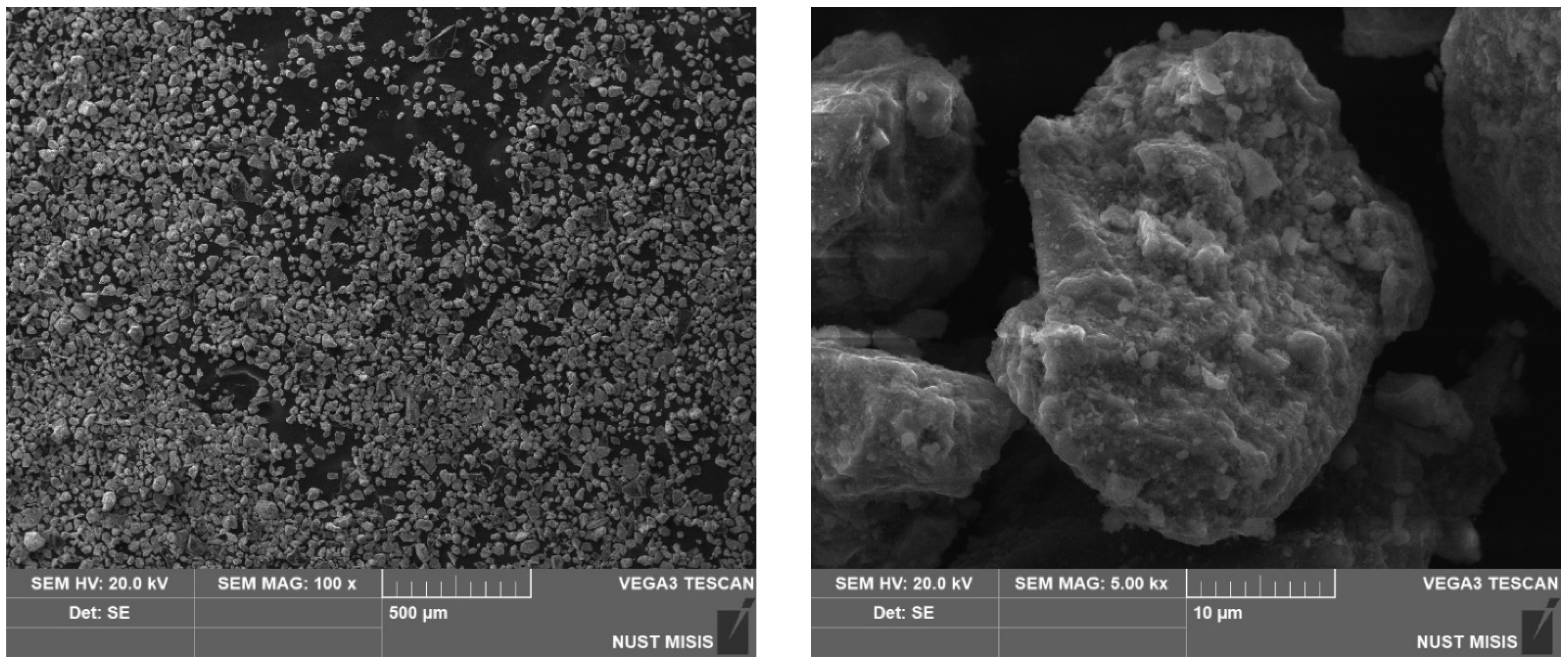
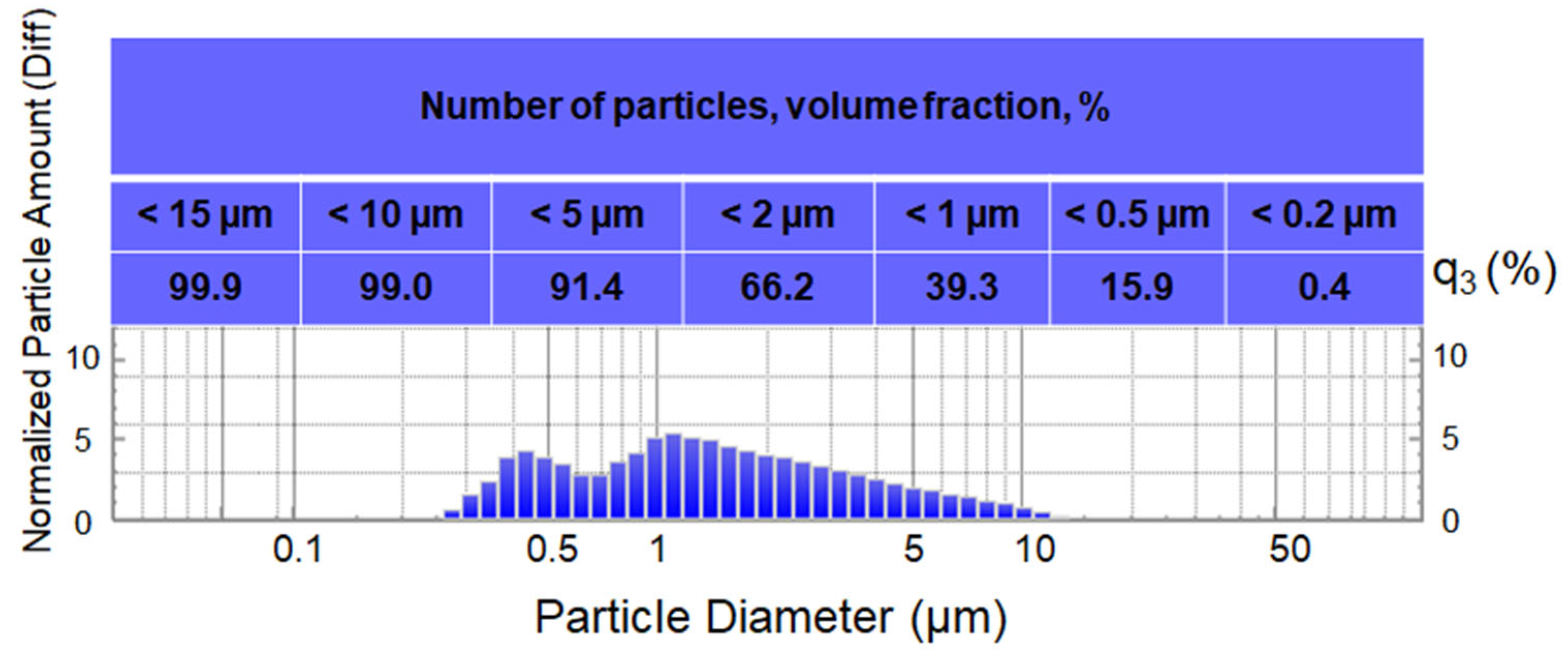
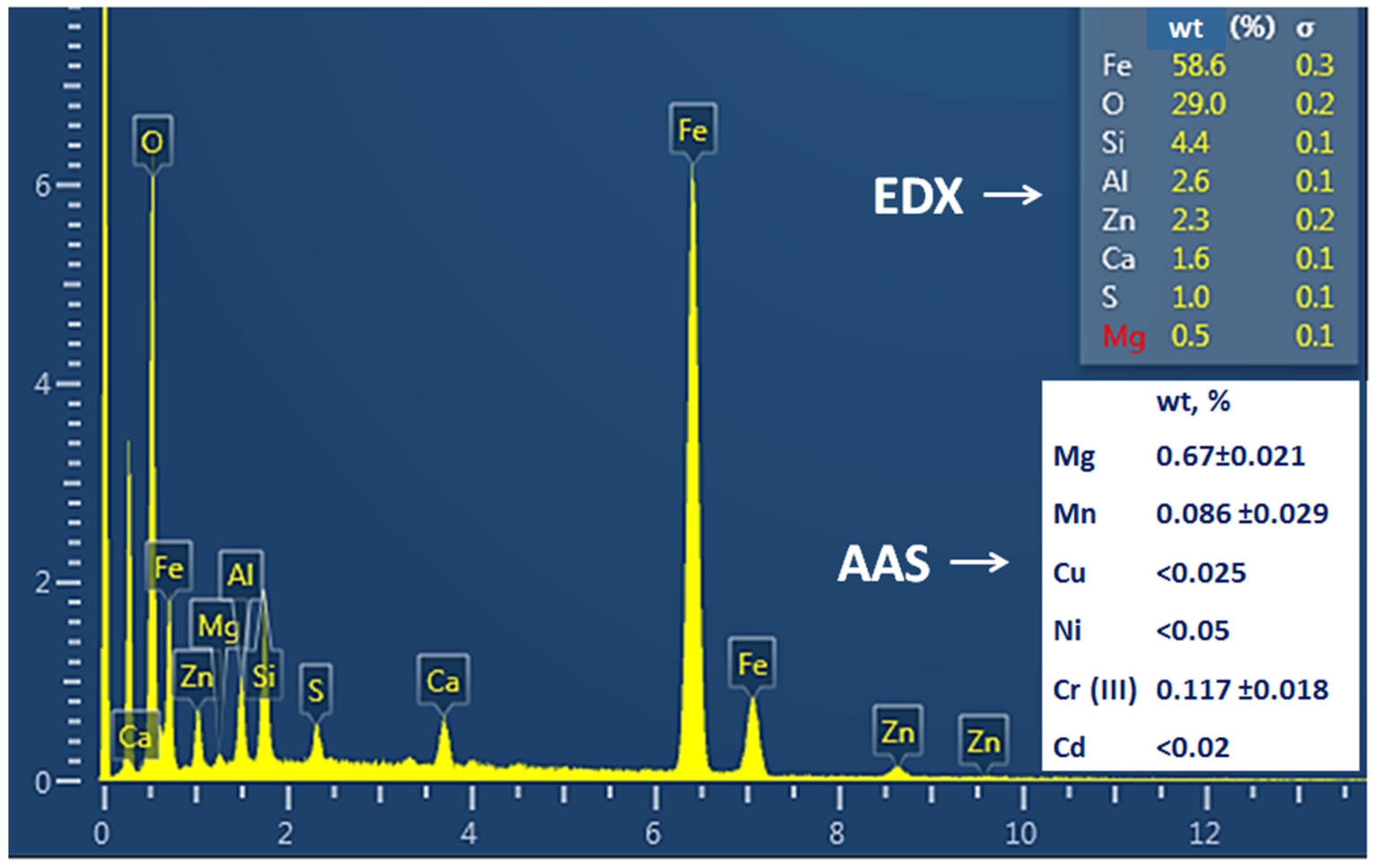
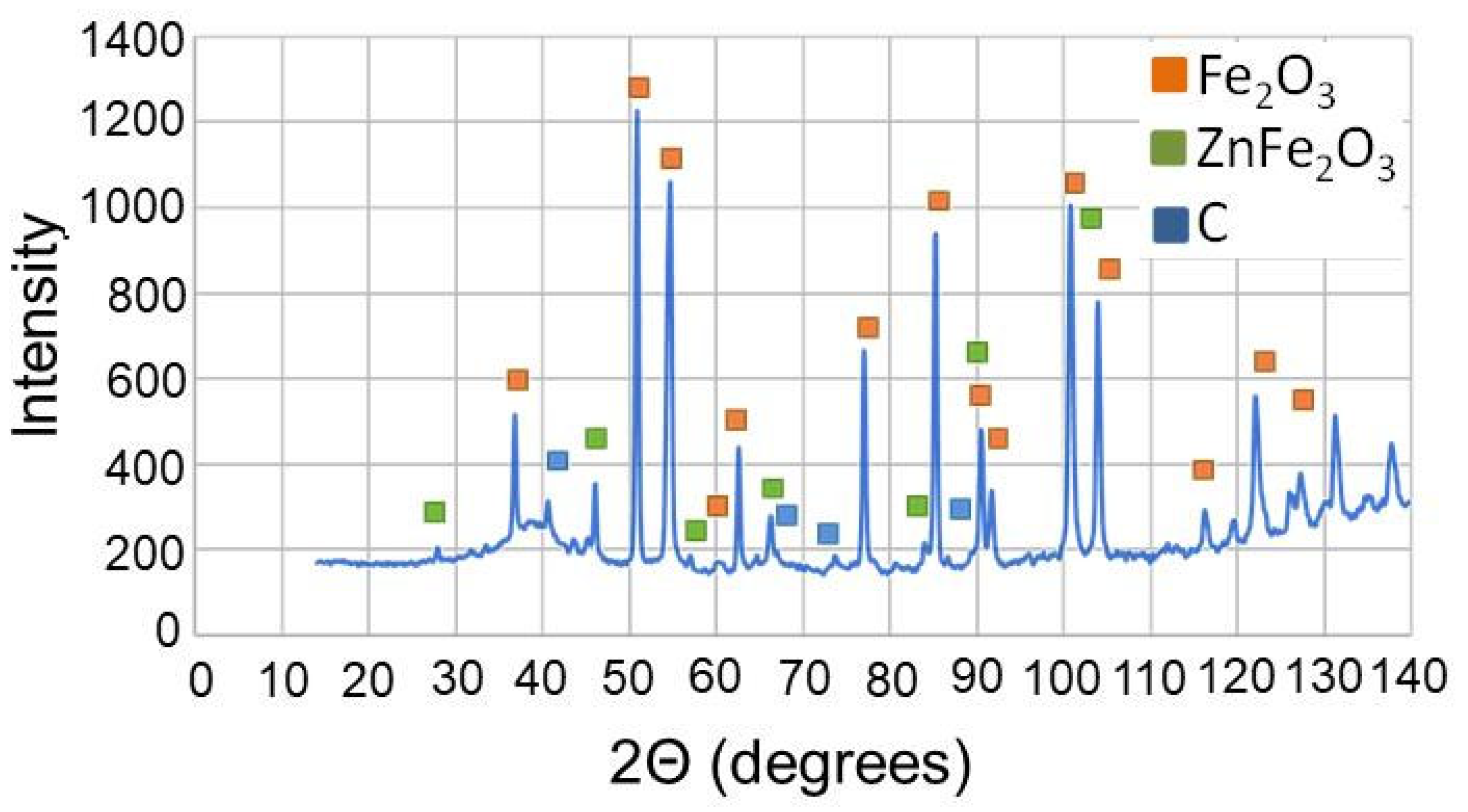
2.1. Blast-Furnace Sludge
2.2. Laboratory Experiment
2.3. Greenhouse Experiment
2.4. Field Experiment
2.5. Data Analysis
3. Results
3.1. Sludge Analysis
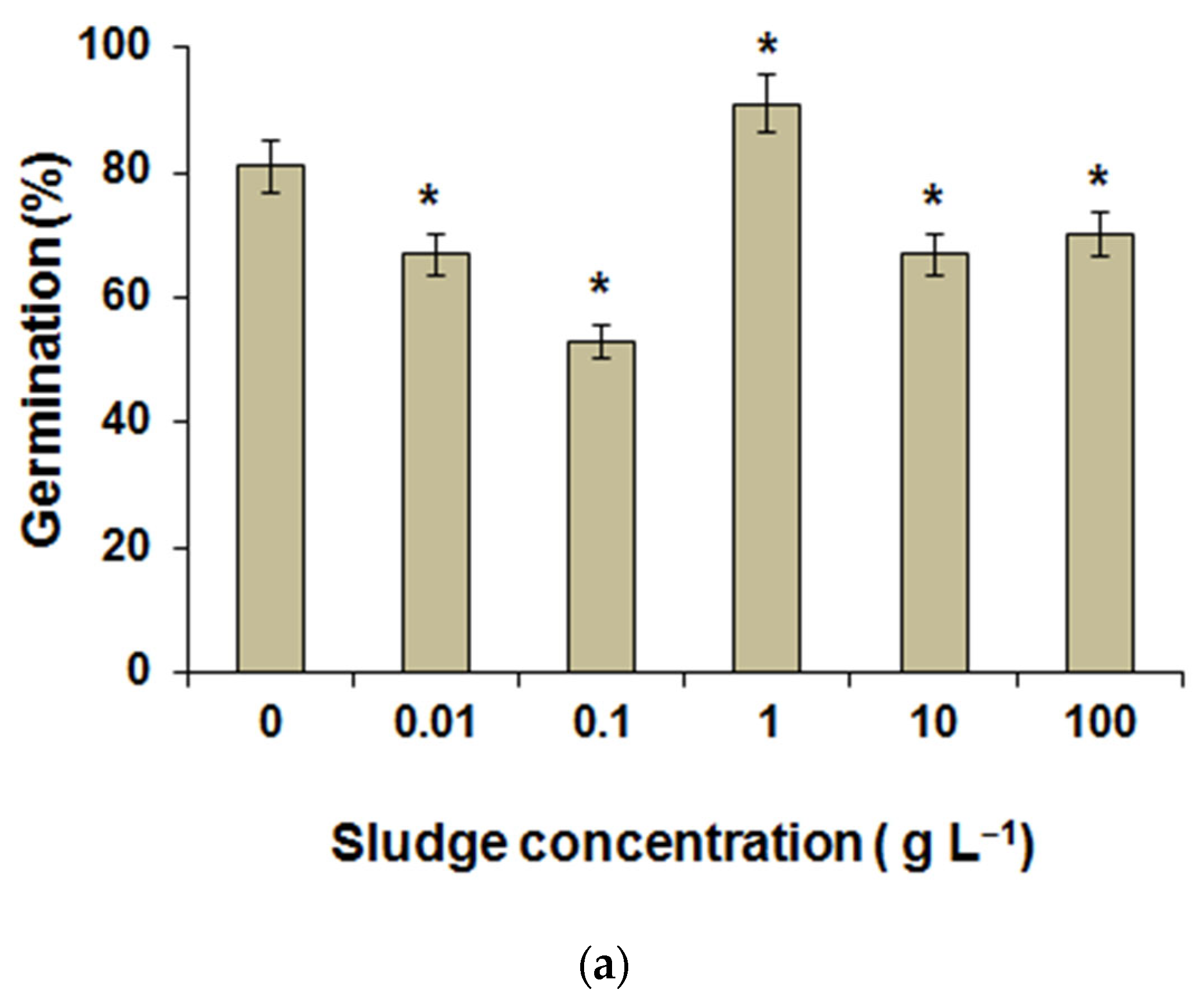
3.2. Laboratory Research Results
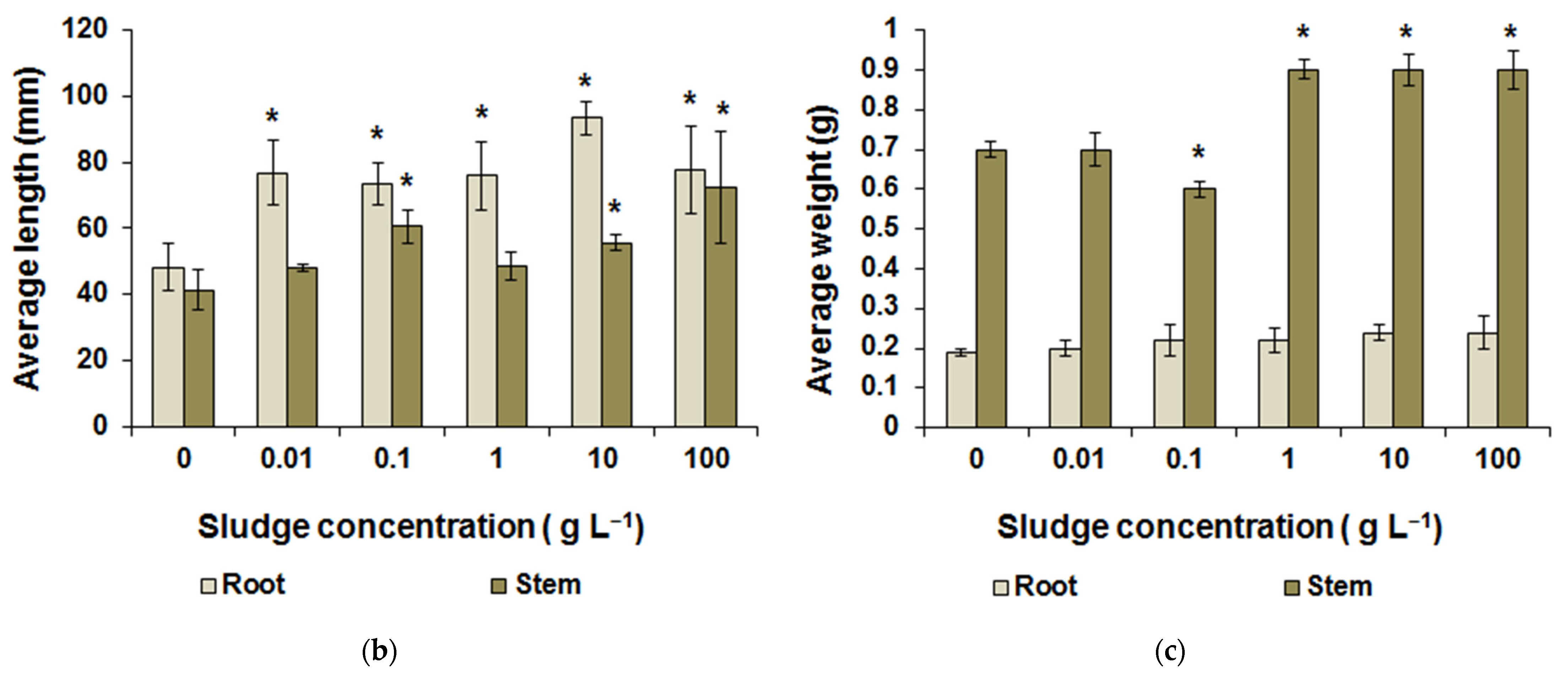
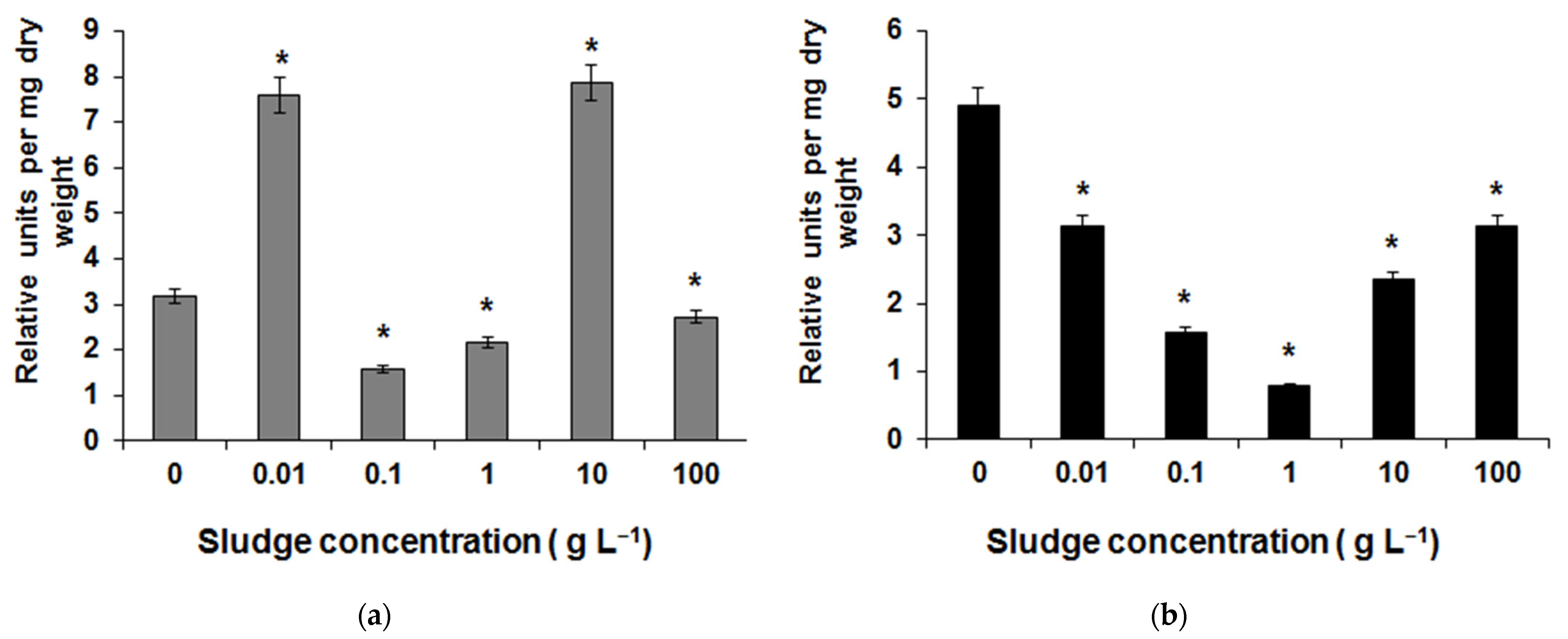
3.3. Greenhouse Research Results
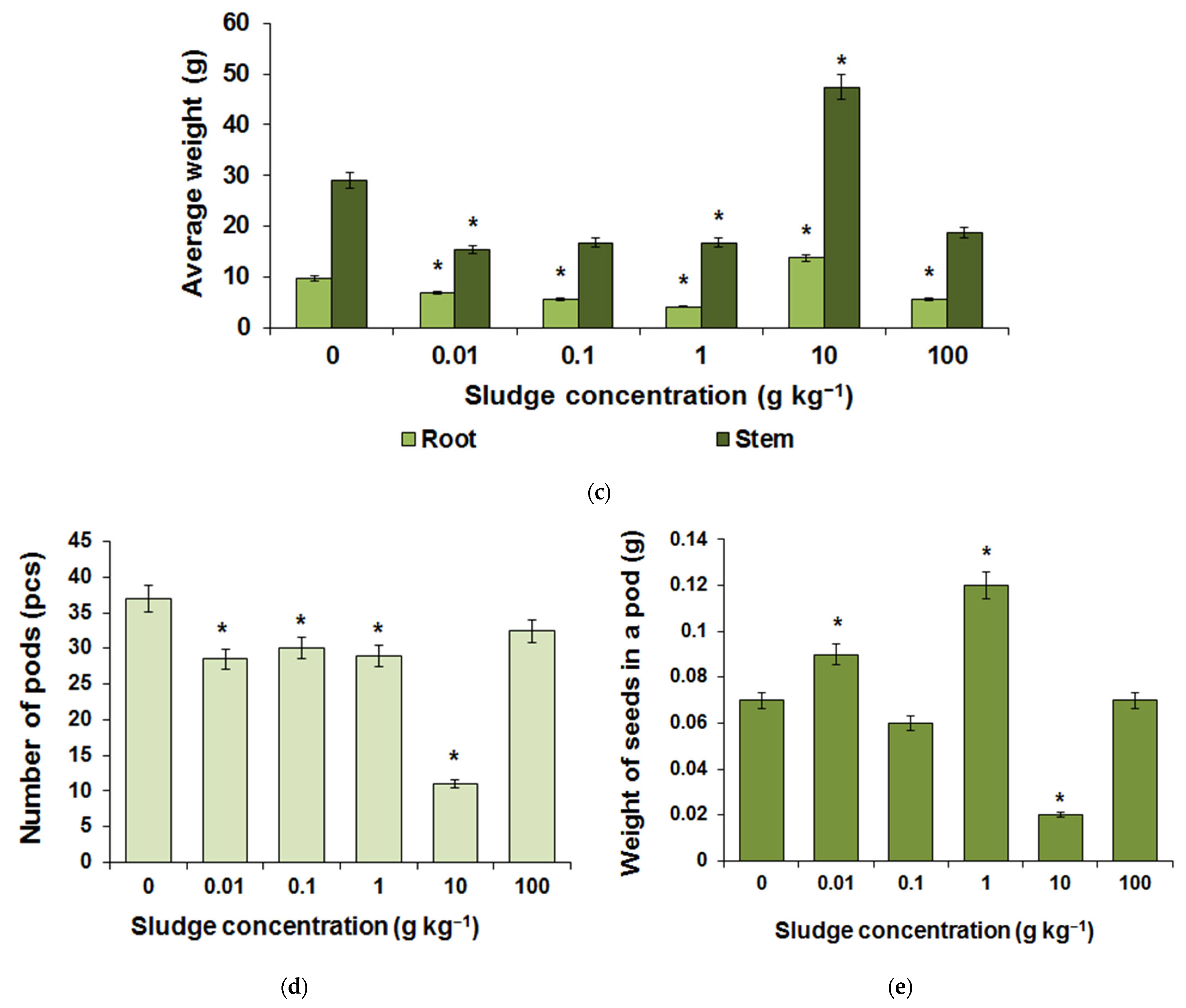
3.4. Field Research Results
3.5. Accumulation of Sludge Components in Plant Organs
3.6. Heavy Metal Concentration in Soils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Delaune, T.; Silva, J.V.; Descheemaeker, K.; van de Ven, G.; Schut, A.G.T.; van Wijk, M.; Hammond, J.; Hochman, Z.; Taulya, G.; et al. The future of farming: Who will produce our food? Food Secur. 2021, 13, 1073–1099. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Wiśniewska, A.; Saeid, A.; Chojnacka, K. Trace Elements in Agricultural and Industrial Wastes. In Recent Advances in Trace Elements; Wiley-Blackwell: Oxford, UK, 2018; pp. 121–142. [Google Scholar]
- Anderson, W.B.; Parkpian, P. Plant availability of an iron waste product utilized as an agricultural fertilizer on calcareous soil. J. Plant Nutr. 1984, 7, 223–233. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [Green Version]
- Lis, T.; Nowacki, K.; Żelichowska, M.; Kania, H. Innovation in metallurgical waste management. Metal. Sisak Zagreb 2015, 54, 283–285. [Google Scholar]
- Volynkina, E.P. Development of the concept of waste management and development of a methodology for its implementation at a metallurgical enterprise. Dr. Sc. Thesis. 2007. (Moscow). Available online: https://www.dissercat.com/content/razvitie-kontseptsii-upravleniya-otkhodami-i-razrabotka-metodologii-ee-realizatsii-na-metall (accessed on 26 October 2022).
- Zakharova, O.; Gusev, A.; Skripnikova, E.; Skripnikova, M.; Krutyakov, Y.; Kudrinsky, A.; Mikhailov, I.; Senatova, S.; Chuprunov, C.; Kuznetsov, D. Study of ecologo-biological reactions of common flax to finely dispersed metallurgical wastes. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012018. [Google Scholar] [CrossRef]
- Kiventerä, J.; Leiviskä, T.; Keski-Ruismäki, K.; Tanskanen, J. Characteristics and settling behaviour of particles from blast furnace flue gas washing. J. Environ. Manag. 2016, 172, 162–170. [Google Scholar] [CrossRef]
- Singh, D.J.; Kalamdhad, A. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Phytotoxicity of heavy metals. In Fertilizers and Environment: Proceedings of the International Symposium “Fertilizers and Environment”, Held in Salamanca, Spain, 26–29, September, 1994; Rodriguez-Barrueco, C., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 423–430. [Google Scholar]
- Fuentes, A.; Lloréns, M.; Sáez, J.; Aguilar, M.I.; Pérez-Marín, A.B.; Ortuño, J.F.; Meseguer, V.F. Ecotoxicity, phytotoxicity and extractability of heavy metals from different stabilised sewage sludges. Environ. Pollut. 2006, 143, 355–360. [Google Scholar] [CrossRef]
- Trinkel, V.; Mallow, O.; Aschenbrenner, P.; Rechberger, H.; Fellner, J. Characterization of Blast Furnace Sludge with Respect to Heavy Metal Distribution. Ind. Eng. Chem. Res. 2016, 55, 5590–5597. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Paananen, T. Effect of Blast Furnace Sludge (BFS) Characteristics on Suitable Recycling Process Determining. J. Miner. Mater. Charact. Eng. 2017, 05, 185–197. [Google Scholar] [CrossRef]
- Andersson, A. Recycling of Blast Furnace Sludge within the Integrated Steel Plant: Potential for Complete Recycling and Influence on Operation. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2019. [Google Scholar]
- Hamann, C.; Spanka, M.; Stolle, D.; Auer, G.; Weingart, E.; Al-Sabbagh, D.; Ostermann, M.; Adam, C. Recycling of blast-furnace sludge by thermochemical treatment with spent iron(II) chloride solution from steel pickling. J. Hazard. Mater. 2021, 402, 123511. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J.; Rodríguez-Celma, J. Iron homeostasis in plants—A brief overview. Metallomics 2017, 9, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Rudani, K.; Prajapati, K. The importance of zinc in plant growth—A review. Int. Res. J. Nat. Appl. Sci. 2018, 5, 2349–4077. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Mohammadi Torkashvand, A.; Sedaghathoor, S. Converter slag as a liming agent in the amelioration of acidic soils. Int. J. Agric. Biol. 2007, 9, 715–720. [Google Scholar]
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of Steel-Slag-Based Silicate Fertilizer on Soil Acidity and Silicon Availability and Metals-Immobilization in a Paddy Soil. PLoS ONE 2016, 11, e0168163. [Google Scholar] [CrossRef] [Green Version]
- Ning, D.; Liang, Y.; Song, A.; Duan, A.; Liu, Z. In situ stabilization of heavy metals in multiple-metal contaminated paddy soil using different steel slag-based silicon fertilizer. Environ. Sci. Pollut. Res. Int. 2016, 23, 23638–23647. [Google Scholar] [CrossRef]
- Radić, S.; Crnojević, H.; Sandev, D.; Jelić, S.; Sedlar, Z.; Glavaš, K.; Pevalek-Kozlina, B. Effect of Electric Arc Furnace Slag on Growth and Physiology of Maize (Zea mays L.). Acta Biol. Hung. 2013, 64, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.S.; Fanijo, E.O. A Review of the Influence of Steel Furnace Slag Type on the Properties of Cementitious Composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping With Slag to Address Soil, Environment, and Food Security. Front. Microbiol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Belanov, I.; Naumova, N.; Semina, I.; Savenkov, O. Metallurgical production slags -promising material for technological waste reclamation. Izvestiya Vysshikh Uchebnykh Zavedenij. Chernaya Metallurgiya 2019, 61, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hao, S.; Wufeng, J.; Ren, Q.; Zhang, Y. Study of Comprehensive Utilization on the Steel Slag. Appl. Mech. Mater. 2014, 488–489, 137–140. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Potentiality of sewage sludge-based organo-mineral fertilizer production in Poland considering nutrient value, heavy metal content and phytotoxicity for rapeseed crops. J. Environ. Manag. 2019, 248, 109283. [Google Scholar] [CrossRef]
- Cocârţă, D.; Subtirelu, V.; Badea, A. Effect of sewage sludge application on wheat crop productivity and heavy metal accumulation in soil and wheat grain. Environ. Eng. Manag. J. 2017, 16, 1093–1100. [Google Scholar] [CrossRef]
- Tran, T.; Tan, L. Heavy Metal Accumulation in Soil and Water in Pilot Scale Rice Field Treated with Sewage Sludge. ChemEngineering 2021, 5, 77. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, L.; Huang, Y.; Luo, Y.; Christie, P. Total concentrations of heavy metals and occurrence of antibiotics in sewage sludges from cities throughout China. J. Soils Sediments 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Adamcová, D.; Vaverková, M.D.; Břoušková, E. The toxicity of two types of sewage sludge from wastewater treatment plant for plants in czech republic. J. Ecol. Eng. 2016, 17, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Rastetter, N.; Gerhardt, A. Toxic potential of different types of sewage sludge as fertiliser in agriculture: Ecotoxicological effects on aquatic, sediment and soil indicator species. J. Soils Sediments 2017, 17, 106–121. [Google Scholar] [CrossRef]
- Iglesias, M.; Marguí, E.; Camps, F.; Hidalgo, M. Extractability and crop transfer of potentially toxic elements from mediterranean agricultural soils following long-term sewage sludge applications as a fertilizer replacement to barley and maize crops. Waste Manag. 2018, 75, 312–318. [Google Scholar] [CrossRef]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef]
- Latare, A.M.; Kumar, O.; Singh, S.K.; Gupta, A. Direct and residual effect of sewage sludge on yield, heavy metals content and soil fertility under rice–wheat system. Ecol. Eng. 2014, 69, 17–24. [Google Scholar] [CrossRef]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In The Brassica Napus Genome, Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Antunes, G.A.; dos Santos, H.S.; da Silva, Y.P.; Silva, M.M.; Piatnicki, C.M.S.; Samios, D. Determination of Iron, Copper, Zinc, Aluminum, and Chromium in Biodiesel by Flame Atomic Absorption Spectrometry Using a Microemulsion Preparation Method. Energy Fuels 2017, 31, 2944–2950. [Google Scholar] [CrossRef]
- Abdulwahid, W.; Dawood, J.; Mohammed, J.; Jamur, J.; Lecturer, A.; Lecturer, D. Flame Atomic Absorption Spectrophotometry Analysis of Heavy Metals in Some Food Additives Available in Baghdad Markets, Iraq. Indian J. Forensic Med. Toxicol. 2020, 14, 451. [Google Scholar]
- Metto, M.; Adamu, F.; Kassie, B. Determination of heavy metals in soil used for potato cultivation by atomic absorption spectroscopy in awi Zone, Amhara Region, Ethiopia. MOJ Ecol. Environ. Sci. 2021, 6, 28–33. [Google Scholar] [CrossRef]
- Alici, E.; Arabaci, G. Determination of SOD, POD, PPO and cat enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plant. 2009, 135, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, S.; Chen, K.; Li, G. Spectrophotometric determination of chlorophylls in different solvents related to the leaf traits of the main tree species in Northeast China. IOP Conf. Ser. Earth Environ. Sci. 2021, 836, 012008. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- SanPiN (Sanitary and Epidemiologiсal Norms) 1.2.3685-2; Hygienic Standards and Requirements for Ensuring the Safety and (or) Harmlessness of Environmental Factors for Humans (In Russia). Russian Government: Moscow, Russia, 2021.
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- van Oijen, T.; van Leeuwe, M.A.; Gieskes, W.W.C.; de Baar, H.J.W. Effects of iron limitation on photosynthesis and carbohydrate metabolism in the Antarctic diatom Chaetoceros brevis (Bacillariophyceae). Eur. J. Phycol. 2004, 39, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Parkpian, P.; Anderson, W.B. Iron availability from a steel industry by-product. J. Plant Nutr. 1986, 9, 1027–1038. [Google Scholar] [CrossRef]
- Anderson, W.B.; Parkpian, P. Effect of soil applied iron by-product on micronutrient concentrations in sorghum cultivars. J. Plant Nutr. 1988, 11, 1333–1343. [Google Scholar] [CrossRef]
- Abbaspour, A.; Kalbasi, M.; Shariatmadari, H. Effect of Steel Converter Sludge as Iron Fertilizer and Soil Amendment in Some Calcareous Soils. J. Plant Nutr. 2004, 27, 377–394. [Google Scholar] [CrossRef]
- Briat, J.-F.; Curie, C.; Gaymard, F. Iron utilization and metabolism in plants. Current opinion in plant biology 2007, 10, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Thomine, S.; Buckhout, T.J. Editorial: Iron Nutrition and Interactions in Plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhailova, S.I.; Zotikova, A.P.; Zueva, T.I.; Surnina, E.N.; Astafurova, T.P.; Morgalev, Y.N. Influence of highly dispersed sludge from ferrous metallurgy on the early growth of leguminous crops. Mod. Probl. Sci. Educ. 2014, 2, 566. (In Russian) [Google Scholar]
- Kuznetsov, D.V.; Kondakov, S.E.; Churilov, G.I.; Polishchuk, A.A.; Kolesnikov, E.A.; Chuprunov, K.O.; Lyovina, V.V.; Leibo, D.V. Phytostimulating effects of metallurgical sludge on sunflower (Helianthus) plants. Mod. Probl. Sci. Educ. 2013, 5, 25–35. (In Russian) [Google Scholar]
- Kuznetsov, D.V.; Kondakov, S.E.; Churilov, G.I.; Kutskir, M.V.; Kolesnikov, E.A. Biological effects of the impact of highly dispersed industrial waste on cereals. Internet J. Sci. 2013, 5, 15. (In Russian) [Google Scholar]
- Gusev, A.; Akimova, O.; Zakharova, O.; Godymchuk, A.; Krutyakov, Y.; Klimov, A.; Denisov, A.; Kuznetsov, D. Morphometric Parameters and Biochemical Status of Oilseed Rape Exposed to Fine-Dispersed Metallurgical Sludge, PHMB-Stabilized Silver Nanoparticles and Multi-Wall Carbon Nanotubes. Adv. Mater. Res. 2014, 880, 212–218. [Google Scholar] [CrossRef]
- Suchkova, S.A.; Borovikova, G.V.; Verkhoturova, G.S.; Postovalova, V.M.; Morgalev, Y.N. Influence of highly dispersed sludge waste from metallurgy on the growth and development of Lycopersicon Esculentum: Scientific review. Biol. Sci. 2014, 1, 121. (In Russian) [Google Scholar]
- Karimi, E.; Teixeira, I.F.; Gomez, A.; de Resende, E.; Gissane, C.; Leitch, J.; Jollet, V.; Aigner, I.; Berruti, F.; Briens, C.; et al. Synergistic co-processing of an acidic hardwood derived pyrolysis bio-oil with alkaline Red Mud bauxite mining waste as a sacrificial upgrading catalyst. Appl. Catal. B Environ. 2014, 145, 187–196. [Google Scholar] [CrossRef]
- Radu, L. Plant growth suitable nutritive red mud composite materials from the romanian dry landfilled red mud. I. Red mud chemical and agrochemical characterization. Rev. Roum. Chim. 2014, 65, 1008–1014. [Google Scholar]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in India—A review. Int. J. Miner. Process. 2013, 118, 43–55. [Google Scholar] [CrossRef]
- Ayres, R.; Holmberg, J.; Sandén, B. Materials and the Global Environment: Waste Mining in the 21st Century. MRS Bull. 2001, 26, 477–480. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S. Waste Materials Used in Concrete Manufacturing; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Aini, S.; Iskandar, I.; Darmawan, D. The Utilization of Red Mud as a Plant Growing Medium with The Addition of Ultisol Soil Material and Compost. Sains Tanah J. Soil Sci. Agroclimatol. 2017, 14, 51. [Google Scholar] [CrossRef]
- Ujaczki, É.; Feigl, V.; Farkas, É.; Vaszita, E.; Gruiz, K.; Molnár, M. Red mud as acidic sandy soil ameliorant: A microcosm incubation study. J. Chem. Technol. Biotechnol. 2016, 91, 1596–1606. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in Understanding Environmental Risks of Red Mud After the Ajka Spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Mišík, M.; Burke, I.T.; Reismüller, M.; Pichler, C.; Rainer, B.; Mišíková, K.; Mayes, W.M.; Knasmueller, S. Red mud a byproduct of aluminum production contains soluble vanadium that causes genotoxic and cytotoxic effects in higher plants. Sci. Total Environ. 2014, 493, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Pinto, F.M.; Pereira, R.A.; Souza, T.M.; Saczk, A.A.; Magriotis, Z.M. Treatment, reuse, leaching characteristics and genotoxicity evaluation of electroplating sludge. J. Environ. Manag. 2021, 280, 111706. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense Against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.F.; Miyake, Y.; Takahashi, E. Chapter 2 Silicon as a beneficial element for crop plants. In Studies in Plant Science, Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 17–39. [Google Scholar]
- Gong, H.J.; Randall, D.P.; Flowers, T.J. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 2006, 29, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Flam-Shepherd, R.; Huynh, W.; Coskun, D.; Hamam, A.; Britto, D.; Kronzucker, H. Membrane fluxes, bypass flows, and sodium stress in rice: The influence of silicon. J. Exp. Bot. 2018, 69, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef] [Green Version]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 177. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N. Effects of Iron Oxide Nanoparticles (Fe3O4) on Growth, Photosynthesis, Antioxidant Activity and Distribution of Mineral Elements in Wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Deng, C.; Tang, Q.; Yang, Z.; Dai, Z.; Cheng, C.; Xu, Y.; Chen, X.; Zhang, X.; Su, J. Effects of iron oxide nanoparticles on phenotype and metabolite changes in hemp clones (Cannabis sativa L.). Front. Environ. Sci. Eng. 2022, 16, 134. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, J.; Gan, Q.; Wang, Y.; Xing, B. Comparative impacts of iron oxide nanoparticles and ferric ions on the growth of Citrus maxima. Environ. Pollut. 2017, 221, 199–208. [Google Scholar] [CrossRef]


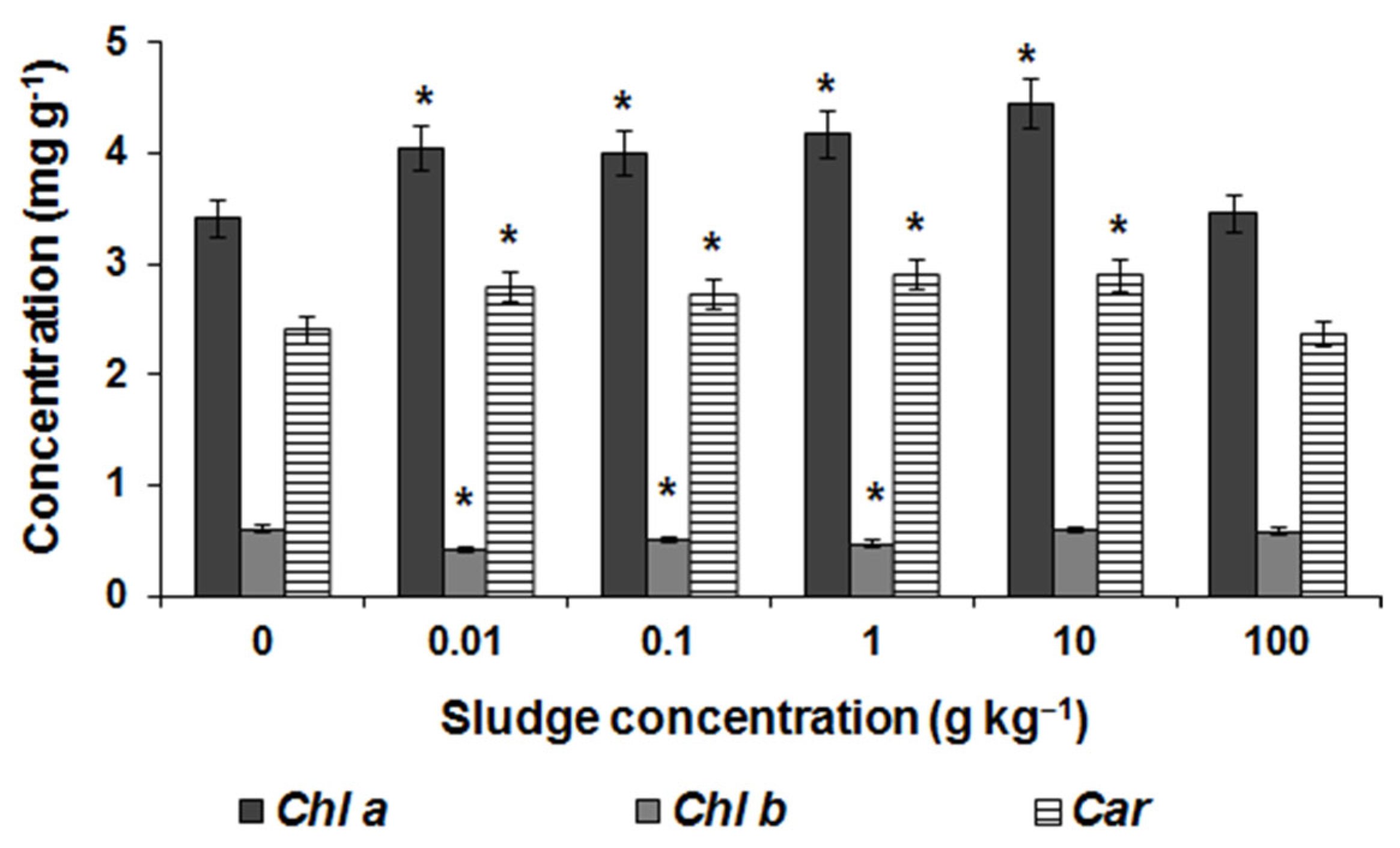
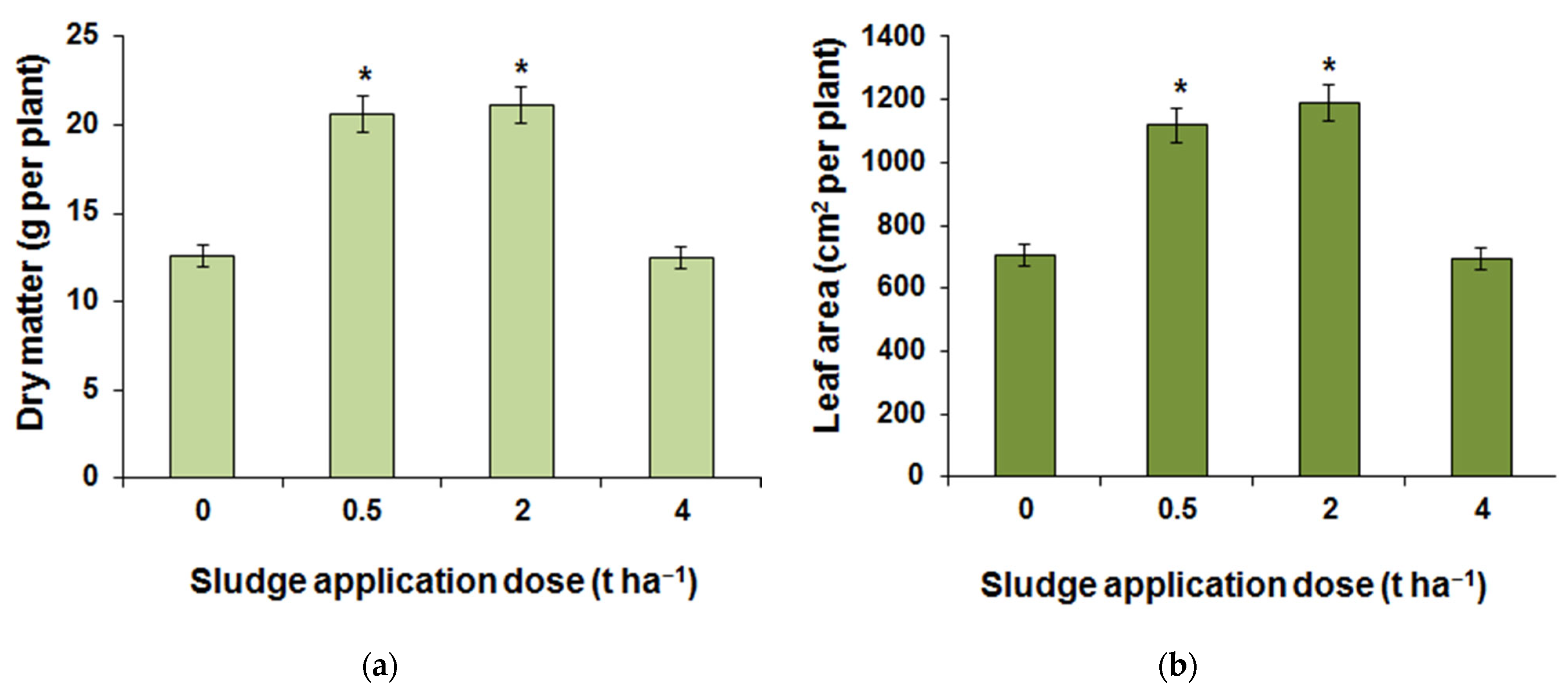

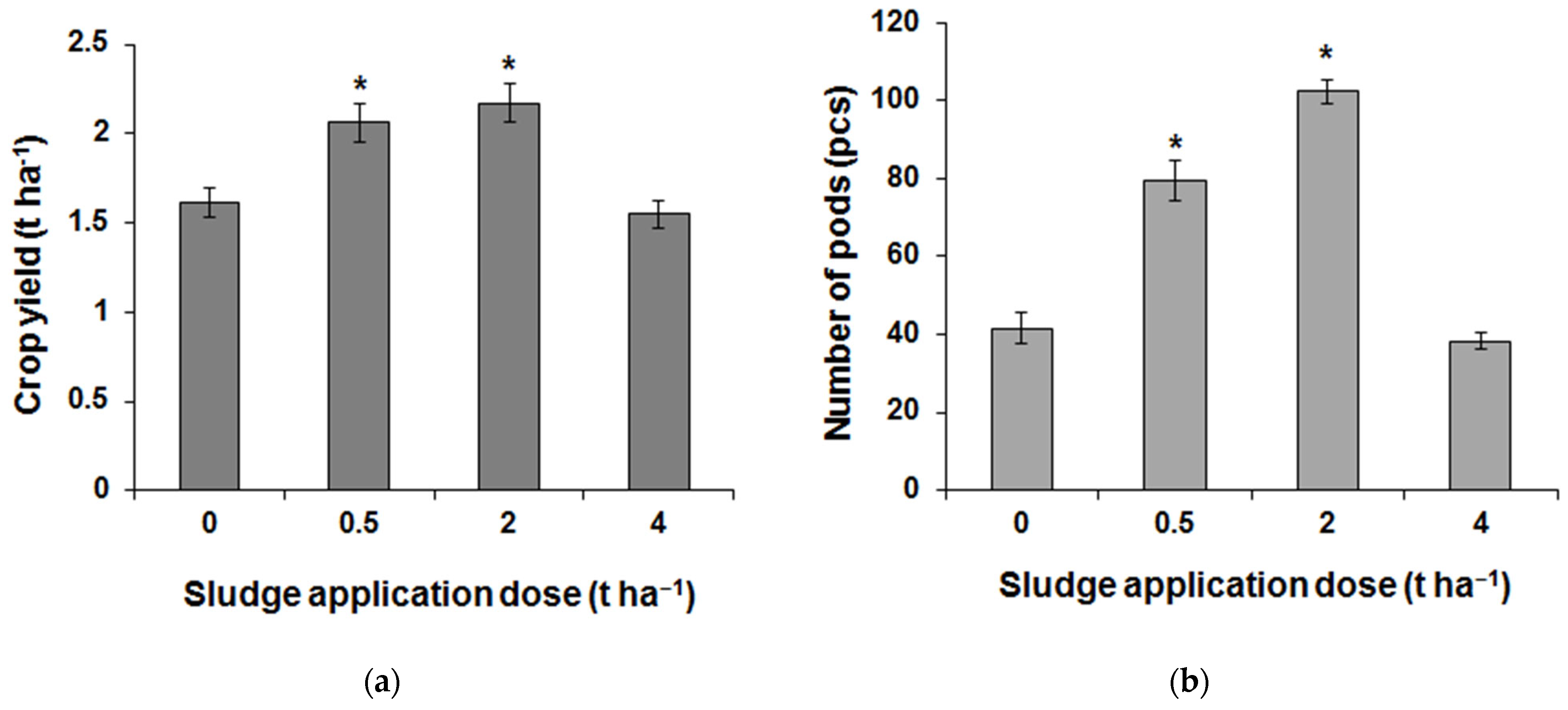
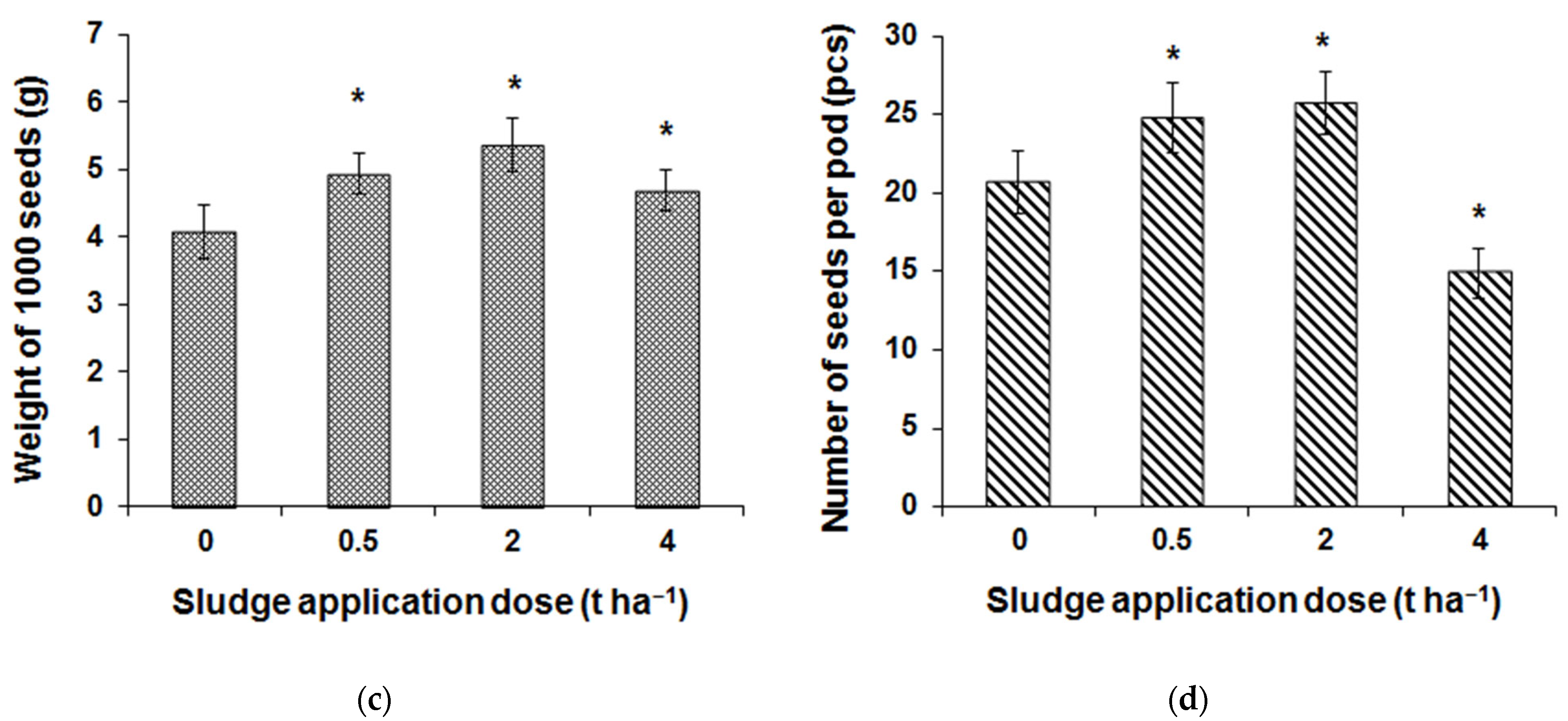
| Element | 0 t ha−1 | 0.5 t ha−1 | 2 t ha−1 | 4 t ha−1 |
|---|---|---|---|---|
| Root | ||||
| Zn | 29.9 ± 1.3 | 25.0 ± 3.8 | 21.9 ± 6.4 | 25.0 ± 5.3 |
| Fe | 124.4 ± 10.2 | 173.4 ± 13.3 * | 171.0 ± 16.1 * | 213.2 ± 15.7 * |
| Stem | ||||
| Zn | 6.4 ± 1.6 | 8.4 ± 1.1 | 7.3 ± 1.5 | 8.6 ± 1.7 |
| Fe | 96.6 ± 6.3 | 102.2 ± 9.1 | 148.5 ± 11.3 * | 201.8 ± 8.9 * |
| Seeds | ||||
| Zn | 15.8 ± 2.3 | 15.6 ± 1.7 | 18.8 ± 2.1 | 16.8 ± 1.6 |
| Fe | 45.4 ± 4.9 | 46.2 ± 4.3 | 51.0 ± 6.2 | 63.2 ± 5.1 * |
| Element | 0 t ha−1 | 0.5 t ha−1 | 2 t ha−1 | 4 t ha−1 | Maximum Allowable Concentration [53] |
|---|---|---|---|---|---|
| Pb | 7.9 ± 0.88 | 8.0 ± 0.87 | 8.9 ± 0.46 | 8.4 ± 0.72 | 130 |
| Ni | 9.2 ± 1.1 | 10.2 ± 0.92 | 9.3 ± 0.67 | 9.8 ± 0.89 | 80 |
| Mn | 251.6 ± 10.1 | 245.8 ± 9.2 | 271.8 ± 15.2 | 266.4 ± 12.7 | 1500 |
| Cu | 7.2 ± 0.45 | 7.8 ± 0.67 | 7.9 ± 0.71 | 6.9 ± 0.81 | 132 |
| Cd | 0.48 ± 0.23 | 0.46 ± 0.12 | 0.50 ± 0.18 | 0.49 ± 0.21 | 2 |
| Zn | 30.4 ± 5.1 | 40.9 ± 4.3 * | 45.8 ± 2.8 * | 47.4 ± 3.6 * | 220 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, O.V.; Baranchikov, P.A.; Grodetskaya, T.A.; Kuznetsov, D.V.; Gusev, A.A. Highly Dispersed Blast-Furnace Sludge as a New Micronutrient Fertilizer: Promising Results on Rapeseed. Agronomy 2022, 12, 2929. https://doi.org/10.3390/agronomy12122929
Zakharova OV, Baranchikov PA, Grodetskaya TA, Kuznetsov DV, Gusev AA. Highly Dispersed Blast-Furnace Sludge as a New Micronutrient Fertilizer: Promising Results on Rapeseed. Agronomy. 2022; 12(12):2929. https://doi.org/10.3390/agronomy12122929
Chicago/Turabian StyleZakharova, Olga V., Peter A. Baranchikov, Tatiana A. Grodetskaya, Denis V. Kuznetsov, and Alexander A. Gusev. 2022. "Highly Dispersed Blast-Furnace Sludge as a New Micronutrient Fertilizer: Promising Results on Rapeseed" Agronomy 12, no. 12: 2929. https://doi.org/10.3390/agronomy12122929






