Temporal Assessment of Biofumigation Using Mustard and Oilseed Rape Tissues on Verticillium dahliae, Soil Microbiome and Yield of Eggplant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Real-Time PCR Assay to Estimate Soil Abundance of Verticillium dahliae
2.3. Illumina MiSeq Sequencing for Analysis of Soil Microorganism Abundance
2.4. Investigating Morbidity and Yield of Eggplant
2.5. Glucosinolate Concentration Analysis
2.6. Soil Enzyme Activities
2.7. Statistical Analysis
3. Results
3.1. Variation of Soil Verticillium dahliae Abundance and Yield Improvement Affected by Biofumigation
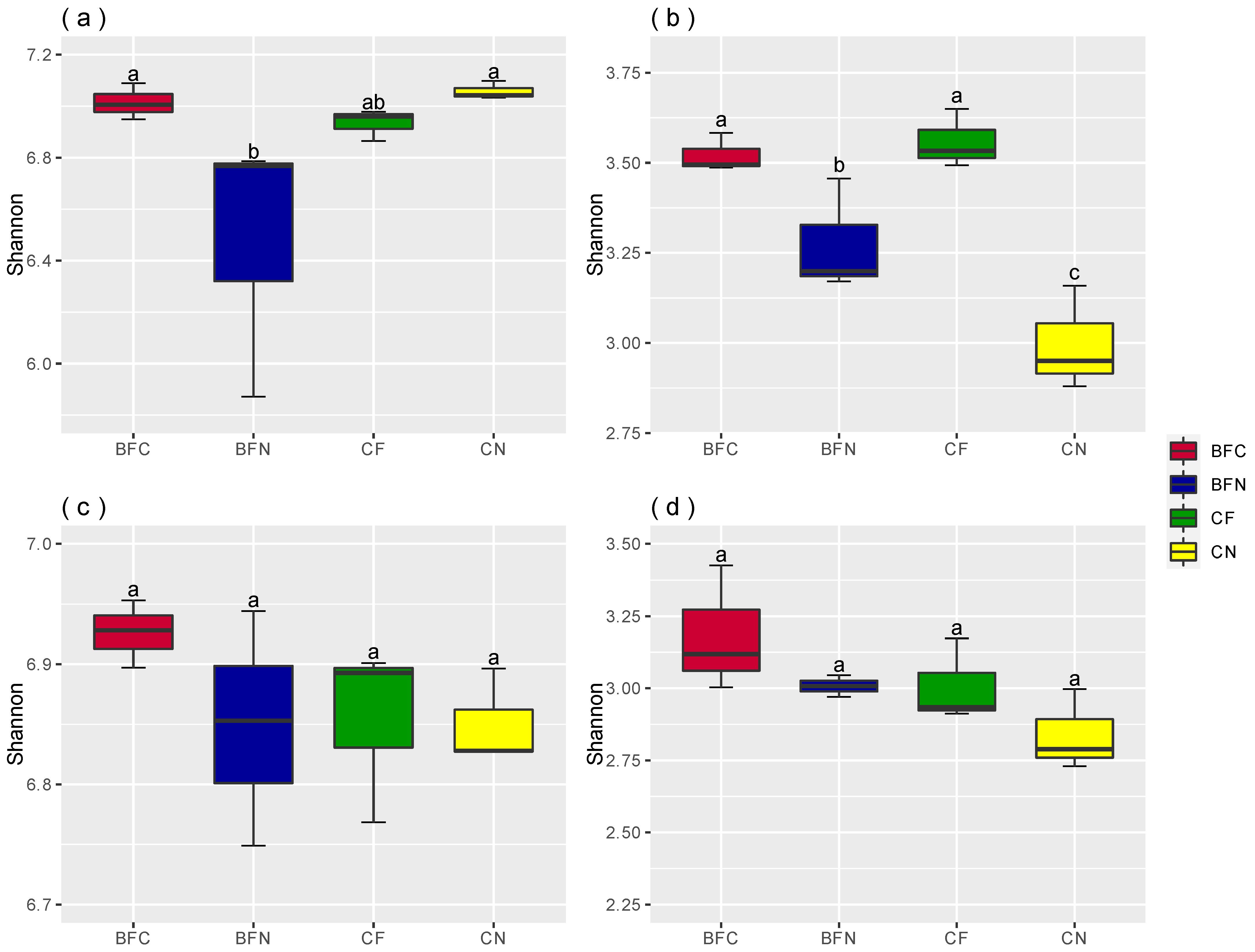
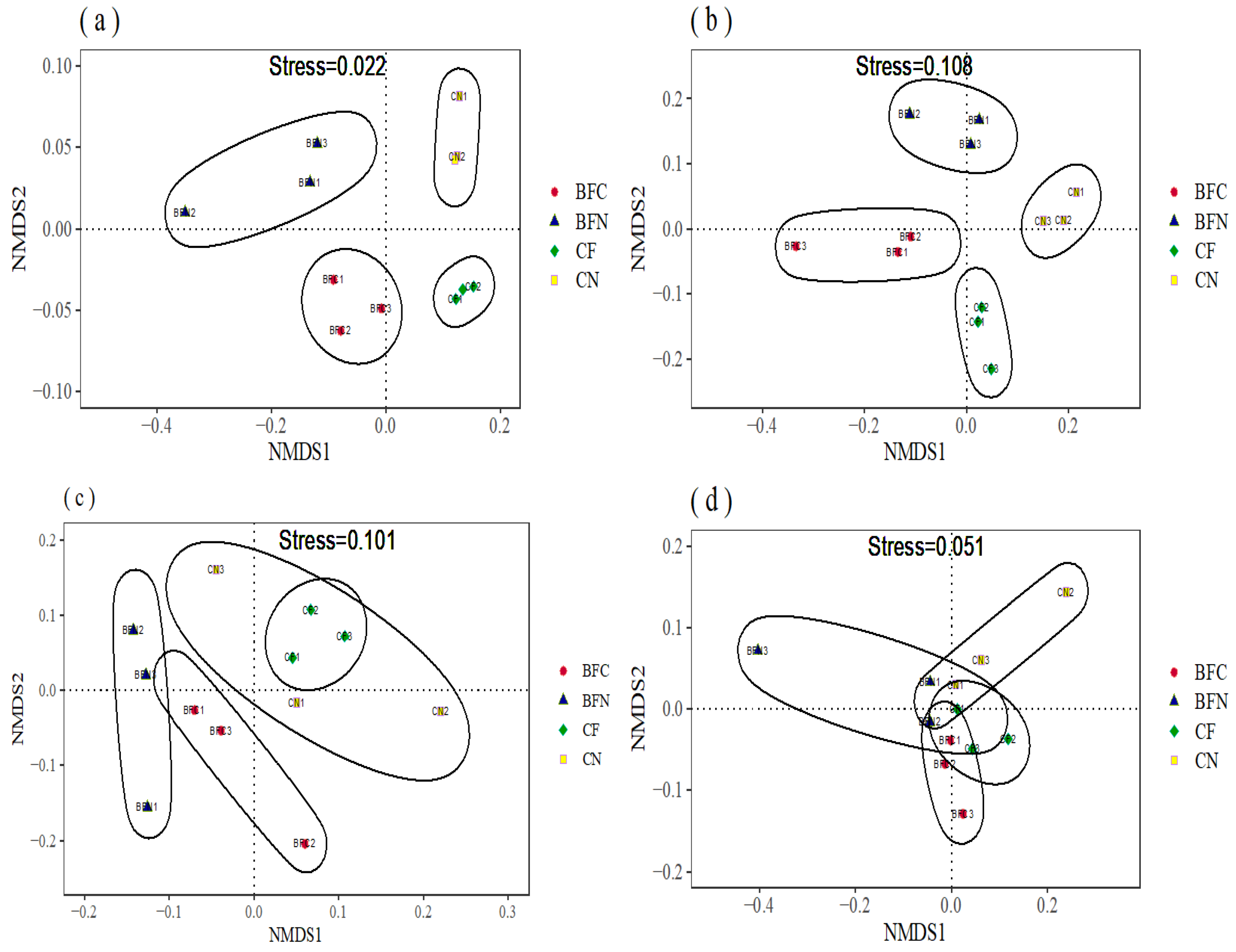
3.2. Soil Microbial Diversity Affected by Biofumigation
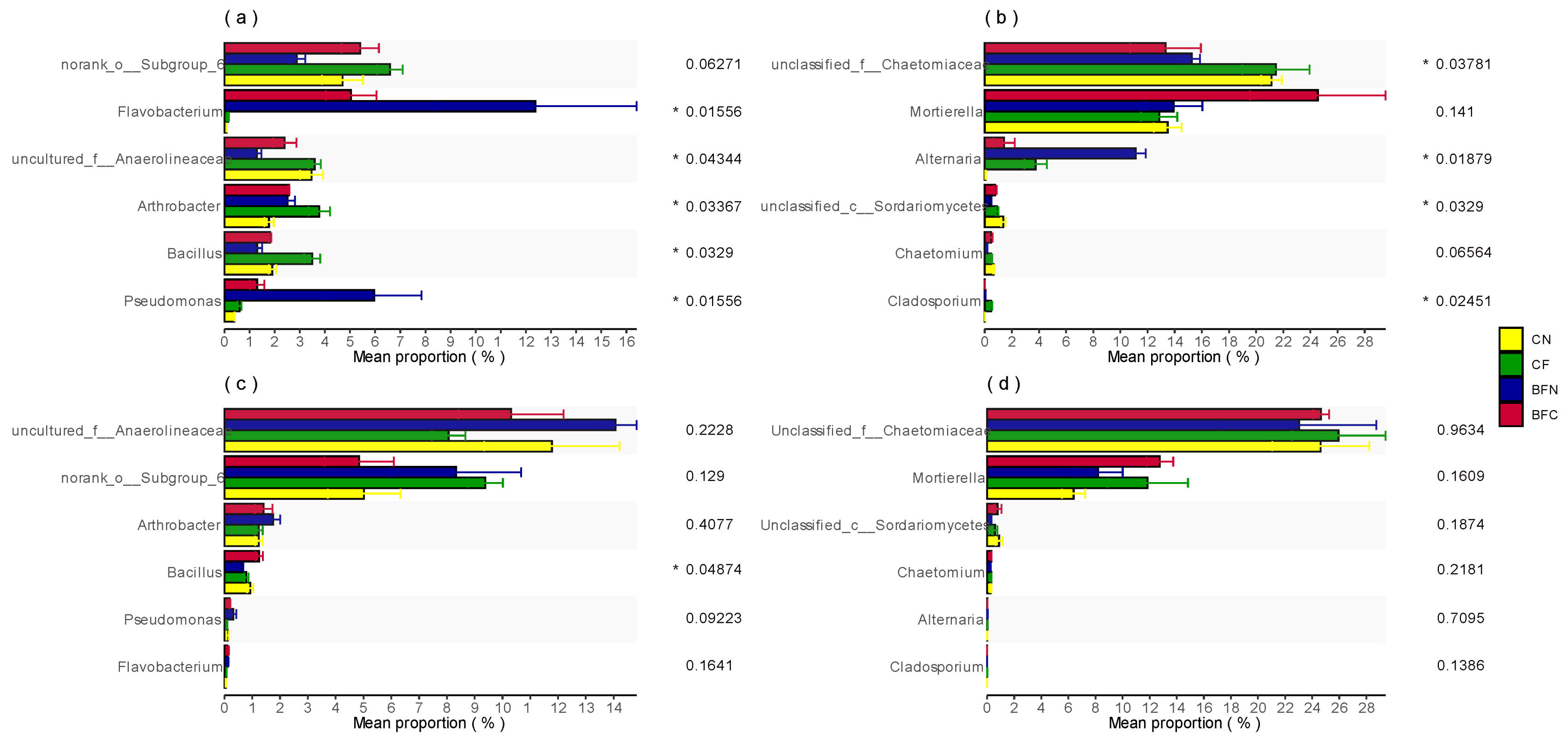
3.3. Soil Beneficial Microbial Composition Affected by Biofumigation
3.4. Glucosinolate Profiles in Tissues of the Biofumigation Materials
3.5. Soil Enzymatic Activities Affected by Biofumigation
4. Discussion
4.1. Biofumigation Could Control Verticillium dahliae
4.2. Biofumigation Could Change Soil Microbial Diversity and Composition
4.3. Biofumigation Change Soil Enzymatic Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, D.; Solberg, S.O.; Prohens, J.; Chou, Y.-Y.; Rakha, M.; Wu, T.-H. World Vegetable Center Eggplant Collection: Origin, Composition, Seed Dissemination and Utilization in Breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Statistical Database. 2020. Available online: http://www.fao.org/faostat/en/ (accessed on 10 January 2022).
- Ogundeji, A.O.; Meng, L.; Cheng, Z.; Hou, J.; Yin, T.; Zhang, S.; Liu, X.; Liu, X.; Li, S. Integrated crop practices management stimulates soil microbiome for Verticillium wilt suppression. Eur. J. Agron. 2022, 140, 126594. [Google Scholar] [CrossRef]
- Scholz, S.S.; Schmidt-Heck, W.; Guthke, R.; Furch, A.C.; Reichelt, M.; Gershenzon, J.; Oelmüller, R. Verticillium dahliae-Arabidopsis Interaction Causes Changes in Gene Expression Profiles and Jasmonate Levels on Different Time Scales. Front. Microbiol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Ateş, A. Investigation of resistance to Verticillium wilt disease (Verticillium dahliae Kleb.) in eggplant genotypes. Plant Prot. Bull. 2020, 60, 5–11. [Google Scholar]
- Guo, H.; Zhao, X.; Rosskopf, E.N.; Gioia, F.D.; Hong, J.C.; McNear, D.H. Impacts of anaerobic soil disinfestation and chemical fumigation on soil microbial communities in field tomato production system. Appl. Soil Ecol. 2018, 126, 165–173. [Google Scholar] [CrossRef]
- Wei, F.; Passey, T.; Xu, X. Effects of individual and combined use of bio-fumigation-derived products on the viability of Verticillium dahliae microsclerotia in soil. Crop Prot. 2016, 79, 170–176. [Google Scholar] [CrossRef]
- Luca, L.; Giovanna, C.; Onofrio, L.; Elisabetta, D. Effects of glucosinolates and their enzymatic hydrolysis products via myrosinase on the root-knot nematode Meloidogyne incognita (Kofoid et White) Chitw. J. Agric. Food Chem. 2004, 52, 6703–6707. [Google Scholar]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Clematis, F.; Gullino, M.L.; Lazzeri, L.; Malaguti, L. Effect of Green Brassica Manure and Brassica Defatted Seed Meals in Combination with Grafting and Soil Solarization against Verticillium Wilt of Eggplant and Fusarium Wilt of Lettuce and Basil. Acta Hortic. 2010, 883, 295–302. [Google Scholar] [CrossRef]
- Srivastava, J.N.; Ghatak, A. Biofumigation:A Control Method for the Soil-Borne Diseases. Int. J. Plant Prot. 2017, 10, 453–460. [Google Scholar]
- Ugolini, L.; Martini, C.; Lazzeri, L.; D’Avino, L.; Mari, M. Control of postharvest grey mould (Botrytis cinerea Per.: Fr.) on strawberries by glucosinolate-derived allyl-isothiocyanate treatments. Postharvest Biol. Technol. 2014, 90, 34–39. [Google Scholar] [CrossRef]
- Pazolini, K.; Santos, I.D.; Giaretta, R.D.; Marcondes, M.M.; Reiner, D.A.; Citadin, I. The use of brassica extracts and thermotherapy for the postharvest control of brown rot in peach. Sci. Hortic. 2016, 209, 41–46. [Google Scholar] [CrossRef]
- Brennan, R.J.B.; Glaze-Corcoran, S.; Wick, R.; Hashemi, M. Biofumigation: An alternative strategy for the control of plant parasitic nematodes. J. Integr. Agric. 2020, 19, 1680–1690. [Google Scholar] [CrossRef]
- Galletti, S.; Fornasier, F.; Cianchetta, S.; Lazzeri, L. Soil incorporation of brassica materials and seed treatment with Trichoderma harzianum: Effects on melon growth and soil microbial activity. Ind. Crops Prod. 2015, 75, 73–78. [Google Scholar] [CrossRef]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil Biol. Biochem. 2004, 37, 1215–1227. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Ward, J.; Barbella, A.; Solares, N.; Izyumin, D.; Burman, P.; Chellemi, D.O.; Subbarao, K.V. Soil microbiomes associated with verticillium wilt-suppressive broccoli and chitin amendments are enriched with potential biocontrol agents. Phytopathology 2018, 108, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Wu, L.; Hollister, E.B.; Wang, A.S.; Somenahally, A.C.; Hons, F.M.; Gentry, T.J. Fungal Community Structural and Microbial Functional Pattern Changes After Soil Amendments by Oilseed Meals of Jatropha curcas and Camelina sativa: A Microcosm Study. Front. Microbiol. 2019, 10, 537. [Google Scholar] [CrossRef]
- Prasad, P.; Kumar, J.; Pandey, S. Investigating Disease Controlling Ability of Brassica Volatiles and Their Compatibility with Trichoderma harzianum. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2018, 88, 887–896. [Google Scholar] [CrossRef]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, T.; Jett, L.; Kotcon, J. Biocontrol agent, biofumigation, and grafting with resistant rootstock suppress soil-borne disease and improve yield of tomato in West Virginia. Crop Prot. 2021, 145, 105630. [Google Scholar] [CrossRef]
- Haipeng, W.; Guangming, Z.; Jie, L.; Jin, C.; Jijun, X.; Juan, D.; Xiaodong, L.; Ming, C.; Piao, X.; Yaoyu, Z.; et al. Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl. Microbiol. Biotechnol. 2016, 100, 8583–8591. [Google Scholar]
- Klose, S.; Acosta-Martínez, V.; Ajwa, H.A. Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides. Soil Biol. Biochem. 2006, 38, 1243–1254. [Google Scholar] [CrossRef]
- Tianzhu, L.; Tongtong, L.; Chengyu, Z.; Chunsheng, K.; Zichao, Y.; Xiaotong, Y.; Fengbin, S.; Runzhi, Z.; Xuerong, W.; Ning, X.; et al. Changes in soil bacterial community structure as a result of incorporation of Brassica plants compared with continuous planting eggplant and chemical disinfection in greenhouses. PLoS ONE 2017, 12, e0173923. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; Agriculture Press: Beijing, China, 2000; p. 495. (In Chinese) [Google Scholar]
- Wu, M.; Zhang, H.; Li, X.; Su, Z.; Zhang, C. An extraction method of fungal DNA from soils in North China. Chin. J. Ecol. 2007, 26, 611–616. [Google Scholar]
- Bilodeau, G.J.; Koike, S.T.; Uribe, P.; Martin, F.N. Development of an assay for rapid detection and quantification of Verticillium dahliae in soil. Phytopathology 2012, 102, 331–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogundeji, A.O.; Li, Y.; Liu, X.; Meng, L.; Sang, P.; Mu, Y.; Wu, H.; Ma, Z.; Hou, J.; Li, S. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Li, S.; Schonhof, I.; Krumbein, A.; Li, L.; Stützel, H.; Schreiner, M. Glucosinolate Concentration in Turnip (Brassica rapa ssp. rapifera L.) Roots as Affected by Nitrogen and Sulfur Supply. J. Agric. Food Chem. 2007, 55, 8452–8457. [Google Scholar] [PubMed]
- Lee, M.K.; Chun, J.H.; Byeon, D.H.; Chung, S.O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.P.; Kim, S.J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp pekinensis) and their antioxidant activity. LWT-Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Electrost. 2006, 65, 156–161. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Mwanza, C.; Blanco-López, M.A. Reduction of Verticillium dahliae microsclerotia viability in soil by dried plant residues. Crop Prot. 2006, 26, 127–133. [Google Scholar] [CrossRef]
- Meng, L.; Yao, X.; Yang, Z.; Zhang, R.; Zhang, C.; Wang, X.; Xu, N.; Li, S.; Liu, T.; Zheng, C. Changes in soil microbial diversity and control of Fusarium oxysporum in continuous cropping cucumber greenhouses following biofumigation. Emir. J. Food Agric. 2018, 30, 644–653. [Google Scholar] [CrossRef]
- Chung, W.C.; Huang, H.C.; Chiang, B.T.; Huang, H.C.; Huang, J.W. Inhibition of soil-borne plant pathogens by the treatment of sinigrin and myrosinases released from reconstructed Escherichia coli and Pichia pastoris. Biocontrol Sci. Technol. 2005, 15, 455–465. [Google Scholar] [CrossRef]
- Mazzola, M.; Granatstein, D.M.; Elfving, D.C.; Mullinix, K. Suppression of Specific Apple Root Pathogens by Brassica napus Seed Meal Amendment Regardless of Glucosinolate Content. Phytopathology 2001, 91, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoroge, S.M.C.; Vallad, G.E.; Park, S.Y.; Kang, S.; Koike, S.T.; Bolda, M.; Burman, P.; Polonik, W.; Subbarao, K.V. Phenological and phytochemical changes correlate with differential interactions of Verticillium dahliae with broccoli and cauliflower. Phytopathology 2011, 101, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kumar, J. Management of Fusarium wilt of chickpea using brassicas as biofumigants. Legume Res.—Int. J. 2017, 40, 178–182. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of plant developmental stage on microbial community structure and activity in the rhizosphere of three field crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.G.; Iida, T.; Uwagaki, Y.; Otani, Y.; Nakaho, K.; Ohkuma, M. Comparison of Prokaryotic and Eukaryotic Communities in Soil Samples with and without Tomato Bacterial Wilt Collected from Different Fields. Microbes Environ. 2017, 32, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Jun, Z.; Ruifu, Z.; Chao, X.; Weibing, X.; Li, S.; Yangchun, X.; Qirong, S. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 2014, 67, 443–453. [Google Scholar]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Martínez, V.; Dowd, S.; Sun, Y.; Allen, V. Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol. Biochem. 2008, 40, 2762–2770. [Google Scholar] [CrossRef]
- Roesch, L.F.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Triplett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ruan, Y.; Xue, C.; Zhong, S.; Li, R.; Shen, Q. Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 2015, 393, 21–33. [Google Scholar] [CrossRef]
- Rosenzweig, N.; Tiedje, J.M.; Quensen, J.F., III; Meng, Q.; Hao, J.J. Microbial Communities Associated with Potato Common Scab-Suppressive Soil Determined by Pyrosequencing Analyses. Plant Dis. 2012, 96, 718–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, R.; Groulx, E.; Defilippi, S.; Erak, T.; Tambong, J.T.; Tweddell, R.J.; Tsopmo, A.; Avis, T.J. Physiological and molecular characterization of compost bacteria antagonistic to soil-borne plant pathogens. Can. J. Microbiol. 2017, 63, 411–426. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Hashem, A.; Abd_Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Zongzhuan, S.; Ryan, P.C.; Nana, L.; Chao, X.; Xianfu, Y.; Yunze, R.; Rong, L.; Qirong, S. Banana Fusarium Wilt Disease Incidence Is Influenced by Shifts of Soil Microbial Communities Under Different Monoculture Spans. Microb. Ecol. 2018, 75, 739–750. [Google Scholar]
- Hollister, E.B.; Hu, P.; Wang, A.S.; Hons, F.M.; Gentry, T.J. Differential impacts of Brassicaceous and non Brassicaceous oilseed meals on soil bacterial and fungal communities. FEMS Microbiol. Ecol. 2013, 83, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Z.; Jian, S.; Songzhong, L.; Qinping, W. Manure refinement affects apple rhizosphere bacterial community structure: A study in sandy soil. PLoS ONE 2013, 8, e76937. [Google Scholar]
- Sherry, A.; Gray, N.D.; Ditchfield, A.K.; Aitken, C.M.; Jones, D.M.; Roling, W.F.M.; Hallmann, C.; Larter, S.R.; Bowler, B.F.J.; Head, I.M. Anaerobic biodegradation of crude oil under sulphate-reducing conditions leads to only modest enrichment of recognized sulphate-reducing taxa. Int. Biodeterior. Biodegrad. 2013, 81, 105–113. [Google Scholar] [CrossRef]
- Leo, V.O.; Dirk, V.E.J. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.). FEMS Microbiol. Ecol. 2008, 64, 283–296. [Google Scholar]
- Na, X.; Li, X.; Zhang, Z.; Li, M.; Kardol, P.; Xu, T.T.; Wang, M.; Cao, X.; Ma, F. Bacterial community dynamics in the rhizosphere of a long-lived, leguminous shrub across a 40-year age sequence. J. Soils Sediments 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Leoni, C.; Vries, M.; Braak, C.J.F.; Bruggen, A.H.C.; Rossing, W.A.H. Fusarium oxysporum f.sp. cepae dynamics: In-plant multiplication and crop sequence simulations. Eur. J. Plant Pathol. 2013, 137, 545–561. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation potential of brassicas: I. Variation in glucosinolate profiles of diverse field-grown brassicas. Plant Soil 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Omirou, M.; Rousidou, C.; Bekris, F.; Papadopoulou, K.K.; Menkissoglou-Spiroudi, U.; Ehaliotis, C.; Karpouzas, D.G. The impact of biofumigation and chemical fumigation methods on the structure and function of the soil microbial community. Microb. Ecol. 2011, 61, 201–213. [Google Scholar] [CrossRef]
- Friberg, H.; Edel-Hermann, V.; Faivre, C.; Gautheron, N.; Fayolle, L.; Faloya, V.; Montfort, F.; Steinberg, C. Cause and duration of mustard incorporation effects on soil-borne plant pathogenic fungi. Soil Biol. Biochem. 2009, 41, 2075–2084. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Deng, S.; Dong, X.; Song, A.; Yao, J.; Fang, W.; Chen, F. The Effects of Fungicide, Soil Fumigant, Bio-Organic Fertilizer and Their Combined Application on Chrysanthemum Fusarium Wilt Controlling, Soil Enzyme Activities and Microbial Properties. Molecules 2016, 21, 526. [Google Scholar] [CrossRef]
| Treatments | Number of V. dahlia (×105 Gene Copies g−1 DW) | Incidence Rate (%) | Disease Index | Disease Prevention (%) | Yield (×103 kg·hm−2) |
|---|---|---|---|---|---|
| CN | 35.04 ± 2.9 a 1 | 41.35 ± 1.3 a | 14.54 ± 0.4 a | - | 18.78 ± 0.2 b |
| BFN | 3.74 ± 0.2 cd | 25.19 ± 1.2 bc | 8.82 ± 0.3 c | 39.32 ± 2.1 a | 25.43 ± 1.0 a |
| BFC | 10.61 ± 0.8 b | 29.73 ± 1.6 b | 11.94 ± 0.2 b | 17.93 ± 1.6 b | 20.23 ± 1.7 b |
| CF | 5.22 ± 0.5 c | 24.12 ± 1.5 c | 8.45 ± 0.1 c | 41.89 ± 0.9 a | 23.94 ± 0.1 a |
| Trivial Name | Chemical Name | Mustard | Oilseed Rape | |
|---|---|---|---|---|
| Aliphatic GS | progoitrin | 2-hydroxy-3-butenyl GS | 0.76 ± 0.03 a 1 | 0.21 ± 0.02 b |
| pi-progoitrin | 2-hydroxy-3-butenyl GS | 0.32 ± 0.02 a | 0.29 ± 0.02 a | |
| sinigrin | 2-propenyl GS | 29.56 ± 0.41 a | — | |
| glucoraphanin | 4-methylsulfinylbutyl GS | 0.10 ± 0.03 b | 0.27 ± 0.01 a | |
| gluconapoleiferin | 2-hydroxy-4-pentenyl GS | 0.52 ± 0.07 a | — | |
| glucoalyssin | 5-methylsulfinylpentyl GS | 0.02 ± 0.01 b | 0.47 ± 0.03 a | |
| gluconapin | 3-butenyl GS | 1.53 ± 0.02 b | 4.21 ± 0.23 a | |
| glucoerucin | 4-methylthiobutyl GS | 0.16 ± 0.02 a | 0.02 ± 0.01 b | |
| Glucobrassicanapin | 4-pentenyl GS | 0.11 ± 0.01 b | 0.38 ± 0.01 a | |
| 5-methyl GS | 5- methyl GS | 0.04 ± 0.01 c | 0.17 ± 0.01 a | |
| Indolyl GS | 4-hydroxyglucobrassicin | 4-hydroxyindol-3- methyl GS | 0.81 ± 0.04 a | 0.02 ± 0.00 b |
| glucobrassicin | Indol-3-methyl GS | 0.01 ± 0.003 a | 0.03 ± 0.006 b | |
| 4-methoxyglucobrassicin | 4-methoxyindol-3- methyl GS | 0.05 ± 0.006 b | 0.16 ± 0.01 a | |
| neoglucobrassicin | 1-methoxyindol-3- methyl GS | 0.40 ± 0.006 a | 0.02 ± 0.006 c | |
| BenzenicGS | glucotropaeolin | benzyl GS | 0.05 ± 0.06 a | 0.09 ± 0.01 a |
| gluconasturtiin | 2-phenethyl GS | 0.31 ± 0.03 b | 0.21 ± 0.03 b |
| Treatment | Urease (NH3-N mg·g−1) | Invertase (Glucose mg·g−1·24 h−1) | ||||
|---|---|---|---|---|---|---|
| Flower Stage | Early Fruit Stage | Full Fruit Stage | Flower Stage | Early Fruit Stage | Full Fruit Stage | |
| CN | 0.33 ± 0.002 c 1 | 0.29 ± 0.001 d | 0.23 ± 0.002 d | 12.85 ± 0.177 d | 12.09 ± 0.143 c | 10.89 ± 0.116 b |
| BFN | 0.38 ± 0.004 a | 0.39 ± 0.002 a | 0.28 ± 0.002 a | 14.94 ± 0.130 a | 16.46 ± 0.106 a | 12.09 ± 0.124 a |
| BFC | 0.35 ± 0.005 b | 0.37 ± 0.004 b | 0.27 ± 0.002 b | 14.33 ± 0.186 b | 15.03 ± 0.082 b | 11.89 ± 0.130 a |
| CF | 0.34 ± 0.003 bc | 0.31 ± 0.004 c | 0.24 ± 0.002 c | 13.47 ± 0.124 c | 12.46 ± 0.117 c | 11.13 ± 0.129 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Zhang, Y.; Yu, S.; Ogundeji, A.O.; Zhang, S.; Li, S. Temporal Assessment of Biofumigation Using Mustard and Oilseed Rape Tissues on Verticillium dahliae, Soil Microbiome and Yield of Eggplant. Agronomy 2022, 12, 2963. https://doi.org/10.3390/agronomy12122963
Meng L, Zhang Y, Yu S, Ogundeji AO, Zhang S, Li S. Temporal Assessment of Biofumigation Using Mustard and Oilseed Rape Tissues on Verticillium dahliae, Soil Microbiome and Yield of Eggplant. Agronomy. 2022; 12(12):2963. https://doi.org/10.3390/agronomy12122963
Chicago/Turabian StyleMeng, Lingbo, Yuhang Zhang, Shaopeng Yu, Abiola O. Ogundeji, Shu Zhang, and Shumin Li. 2022. "Temporal Assessment of Biofumigation Using Mustard and Oilseed Rape Tissues on Verticillium dahliae, Soil Microbiome and Yield of Eggplant" Agronomy 12, no. 12: 2963. https://doi.org/10.3390/agronomy12122963





