Identification of Key Regulatory Factors of Molecular Marker TGS377 on Chromosome 1 and Its Response to Cold Stress in Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Chilling Injury Index
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Bioinformatics Analysis of Genes within 50 kb Upstream and Downstream of Tomato Molecular Marker TGS377
2.5. RT-qPCR Analysis
2.6. Gene Coexpression Network Analysis
2.7. Statistical Analysis
3. Results
3.1. Determination of Chilling Injury Index
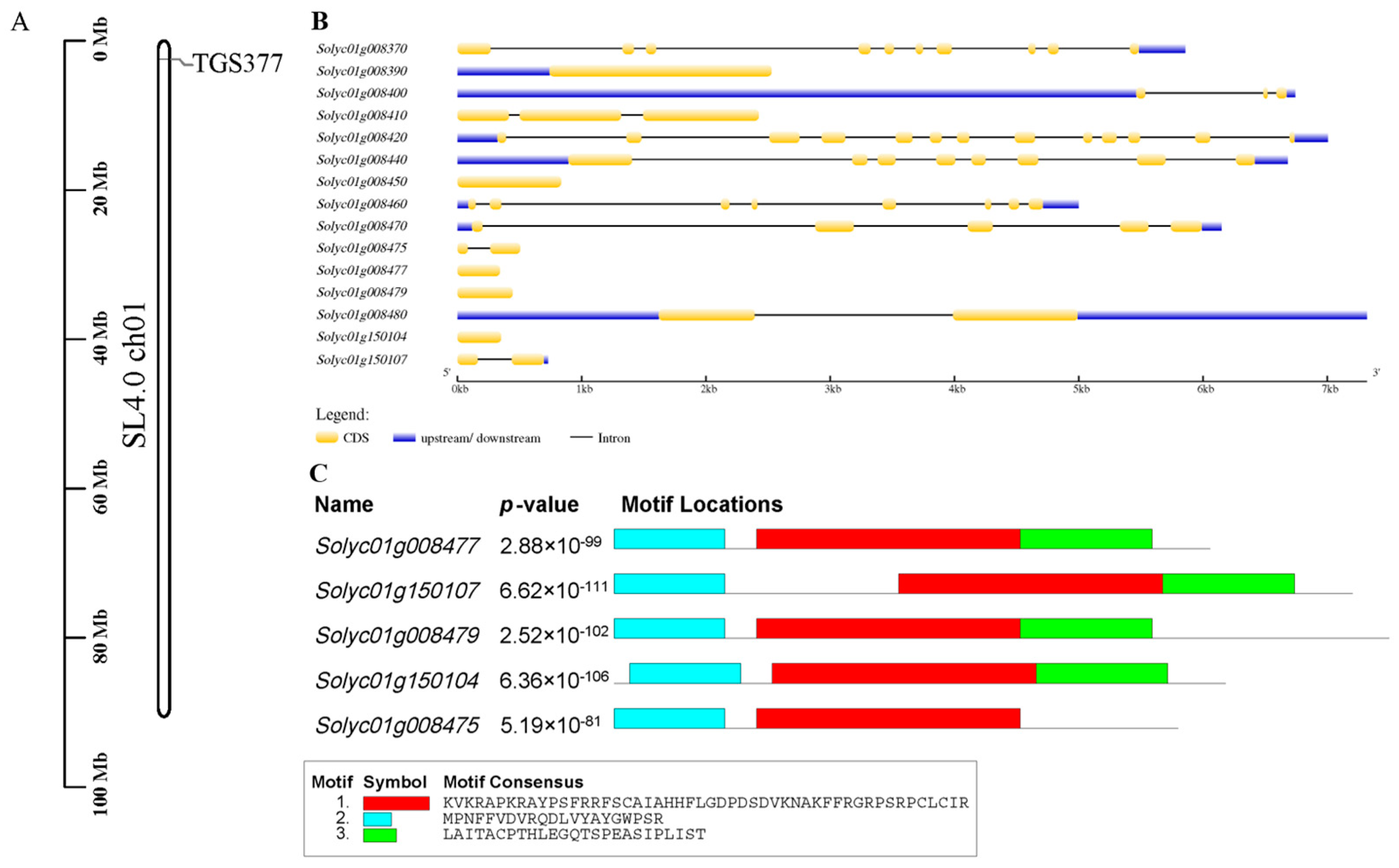
3.2. Gene Structure and Motif Analysis of Tomato Molecular Marker TGS377 within 50 kb Upstream and Downstream
3.3. Protein Information Prediction Analysis of Tomato Molecular Marker TGS377 within 50 kb Upstream and Downstream
3.4. Analysis of Gene Expression in Different Tissues of Heinz Tomato
3.5. GO Term Analysis of Genes within 50 KB Upstream and Downstream of Tomato Molecular Marker TGS377
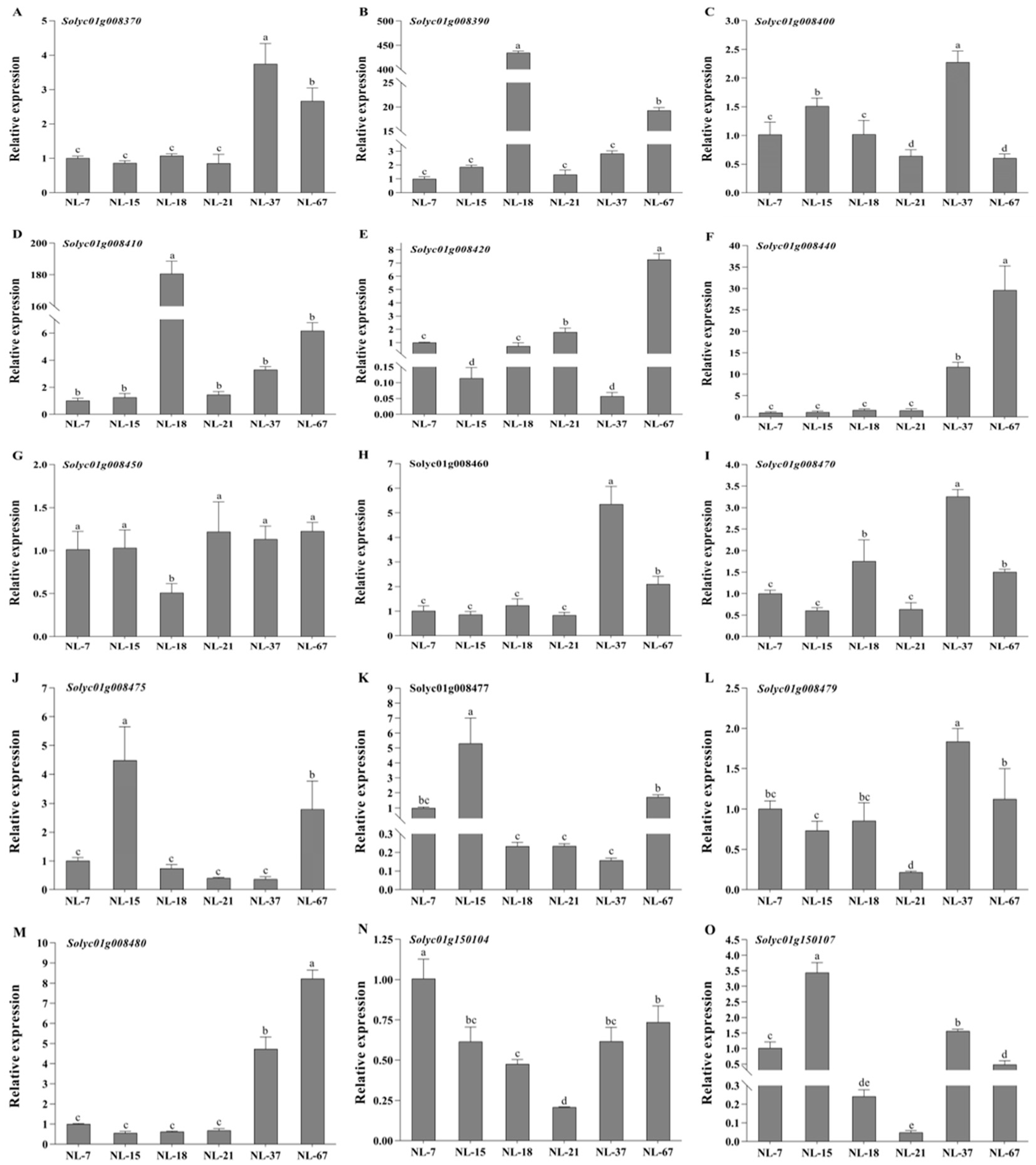
3.6. Expression Profiles of Predicted Genes under Low Temperature Treatment in Tomatoes
3.7. Gene Coexpression Network Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altieri, M.A.; Nicholls, C.I. The adaptation and mitigation potential of traditional agriculture in a changing climate. Clim. Chang. 2017, 140, 33–45. [Google Scholar] [CrossRef]
- Menon H, K.D.; Mishra, D.; Deepa, D. Automation and integration of growth monitoring in plants (with disease prediction) and crop prediction. Mater. Today Proc. 2021, 43, 3922–3927. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.R.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef]
- Kenchanmane Raju, S.K.; Barnes, A.C.; Schnable, J.C.; Roston, R.L. Low-temperature tolerance in land plants: Are transcript and membrane responses conserved? Plant Sci. 2018, 276, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, H.S.; Yang, S.; Tang, X.F.; Duan, M.; Meng, Q.W. Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol. Biochem. 2009, 47, 1102–1112. [Google Scholar] [CrossRef]
- Yoshida, S. A prefatory note on responses of plants to low temperature-stress. J. Plant Res. 1999, 112, 223–224. [Google Scholar] [CrossRef]
- Heidari, P.; Reza Amerian, M.; Barcaccia, G. Hormone profiles and antioxidant activity of cultivated and wild tomato seedlings under low-temperature stress. Agronomy 2021, 11, 1146. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Changes in activity of antioxidative enzyme in wheat (Triticum aestivum) seedlings under cold acclimation. Physiol. Plantarum. 1998, 104, 747–752. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, H.; Peng, S.M.; Korpelainen, H.; Li, C.Y. Sex-related differences in morphological, physiological, and ultrastructural responses of Populus cathayana to chilling. J. Exp. Bot. 2010, 62, 675–686. [Google Scholar] [CrossRef]
- Gu, K.Y.; Hou, S.; Chen, J.F.; Guo, J.G.; Wang, F.F.; He, C.G.; Zou, C.M.; Xie, X.Y. The physiological response of different tobacco varieties to chilling stress during the vigorous growing period. Sci. Rep. 2021, 11, 22136. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M. Molecular marker technology for crop improvement. Agronomy 2020, 10, 1462. [Google Scholar] [CrossRef]
- Amiteye, S. Basic concepts and methodologies of DNA marker systems in plant molecular breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Akbar Anjum, M.; Naz, S.; Mukhtar Balal, R. Applications of molecular markers in fruit crops for breeding programs—A review. Phyton-Int. J. Exp. Bot. 2021, 90, 17–34. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Champagne, G.; Hubert, N.; Landry, B.S. Comparison of the genetic maps of Brassica napus and Brassica oleracea. Theor. Appl. Genet. 1997, 94, 569–582. [Google Scholar] [CrossRef]
- Yang, X.; Quiros, C. Identification and classification of celery cultivars with RAPD markers. Theor. Appl. Genet. 1993, 86, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mcgregor, C.E.; Lambert, C.A.; Greyling, M.M.; Louw, J.H.; Warnich, L. A comparative assessment of DNA fingerprinting techniques (RAPD, ISSR, AFLP and SSR) in tetraploid potato (Solanum tuberosum L.) Germplasm. Euphytica 2000, 113, 135–144. [Google Scholar] [CrossRef]
- Lefebvre, V.; Palloix, A.; Caranta, C.; Pochard, E. Construction of an intraspecific integrated linkage map of pepper using molecular markers and doubled-haploid progenies. Genome 1995, 38, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Egashira, H.; Ishihara, H.; Takashina, T.; Imanishi, S. Genetic diversity of the ‘peruvianum-complex’ (Lycopersicon peruvianum (L.) Mill, and L. Chilense dun.) Revealed by RAPD analysis. Euphytica 2000, 116, 23–31. [Google Scholar] [CrossRef]
- Foolad, M.R.; Chen, F.Q.; Lin, G.Y. RFLP mapping of QTLs conferring cold tolerance during seed germination in an interspecific cross of tomato. Mol. Breed. 1998, 4, 519–529. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.X.; Ge, H.Y.; Pang, W.; Gao, L.J.; Ren, L.; Chen, H.Y. SSR mapping of QTLs conferring cold tolerance in an interspecific cross of tomato. Int. J. Genom. 2016, 2016, 3219276. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S.R. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Gao, L.H.; Qu, M.; Ren, H.Z.; Sui, X.L.; Zhang, Z.X. Structure, function, application, and ecological benefit of a single-slope, energy-efficient solar greenhouse in china. HortTechnology 2010, 20, 626–631. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Kläring, H.; Schwarz, D. Growth, yield, and metabolic responses of temperature-stressed tomato to grafting onto rootstocks differing in cold tolerance. J. Am. Soc. Hortic. Sci. 2014, 139, 230–243. [Google Scholar] [CrossRef]
- Barrero-Gil, J.; Huertas, R.; Rambla, J.L.; Granell, A.; Salinas, J. Tomato plants increase their tolerance to low temperature in a chilling acclimation process entailing comprehensive transcriptional and metabolic adjustments. Plant Cell Environ. 2016, 39, 2303–2318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.J.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Effects of genotype and production system on quality of tomato fruits and in vitro bile acids binding capacity. J. Food Sci. 2020, 85, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shao, G.C.; Gao, Y.; Zhang, K.; Wei, Q.; Cheng, J.F. Effects of water deficit combined with soil texture, soil bulk density and tomato variety on tomato fruit quality: A meta-analysis. Agric. Water Manag. 2021, 243, 106427. [Google Scholar] [CrossRef]
- Han, N.N.; Fan, S.Y.; Zhang, T.T.; Sun, H.; Zhu, Y.X.; Gong, H.J.; Guo, J. SlHY5 is a necessary regulator of the cold acclimation response in tomato. Plant Growth Regul. 2020, 91, 1–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Yuan, L.; Zhou, F.; Gao, Y.; Kang, Z.; Li, T.; Hu, X. Exogenous 5-aminolevulinic acid alleviates low-temperature injury by regulating glutathione metabolism and β-alanine metabolism in tomato seedling roots. Ecotox. Environ. Safe. 2022, 245, 114112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Ding, J.P.; Ibrahim, M.; Jiao, X.C.; Song, X.M.; Bai, P.; Li, J.M. Effects of the interaction between vapor-pressure deficit and potassium on the photosynthesis system of tomato seedlings under low temperature. Sci. Hortic. 2021, 283, 110089. [Google Scholar] [CrossRef]
- Fung, R.W.M.; Wang, C.Y.; Smith, D.L.; Gross, K.C.; Tao, Y.; Tian, M.S. Characterization of alternative oxidase (AOX) gene expression in response to methyl salicylate and methyl jasmonate pre-treatment and low temperature in tomatoes. J. Plant Physiol. 2006, 163, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.X. Studies on Cold Tolerance Evaluation in Recombinant Inbred Lines (RILs) and QTL Detection for Cold Tolerance of Tomato (Solamum lycopersicum). Master’s Thesis, Shanghai Jiao Tong University, Shanghai, China, 2016. (In Chinese). [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.; Xu, Z.S.; Wang, F.; Xiong, A.S. Six NAC transcription factors involved in response to TVLCV infection in resistant and susceptible tomato cultivars. Plant Physiol. Biochem. 2017, 120, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Sherzod, R.; Yang, E.Y.; Cho, M.C.; Chae, S.Y.; Kim, J.H.; Nam, C.W.; Chae, W.B. Traits affecting low temperature tolerance in tomato and its application to breeding program. Plant Breed. Biotechnol. 2019, 7, 350–359. [Google Scholar] [CrossRef]
- Criddle, R.S.; Smith, B.N.; Hansen, L.D. A respiration based description of plant growth rate responses to temperature. Planta 1997, 201, 441–445. [Google Scholar] [CrossRef]
- Venema, J.H.; Posthumus, F.; van Hasselt, P.R. Impact of suboptimal temperature on growth, photosynthesis, leaf pigments and carbohydrates of domestic and high-altitude wild lycopersicon species. J. Plant Physiol. 1999, 155, 711–718. [Google Scholar] [CrossRef]
- Xiaoa, F.; Yang, Z.Q.; Zhua, L.Y. Low temperature and weak light affect greenhouse tomato growth and fruit quality. J. Plant Sci. 2018, 1, 16–24. [Google Scholar] [CrossRef]
- Van Ploeg, D.; Heuvelink, E. Influence of sub-optimal temperature on tomato growth and yield: A review. J. Hortic. Sci. Biotechnol. 2015, 80, 652–659. [Google Scholar] [CrossRef]
- Brüggemann, W.; van der Kooij, T.A.W.; van Hasselt, P.R. Long-term chilling of young tomato plants under low light and subsequent recovery: I. Growth, development and photosynthesis. Planta 1992, 186, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, X.L.; Chen, D.; Li, J.F.; Zhang, Y.; Wang, A.X. A comparison of the low temperature transcriptomes of two tomato genotypes that differ in freezing tolerance: Solanum lycopersicum and Solanum habrochaites. BMC Plant Biol. 2015, 15, 132. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, X.C.; Yang, S.C.; Zhang, Z.D.; Ye, X.L.; Li, J.M.; Qi, H.Y.; Hu, X.H. Crosstalk between GABA and ALA to improve antioxidation and cell expansion of tomato seedling under cold stress. Environ. Exp. Bot. 2020, 180, 104228. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khara, J.; Heydari, R. Alleviating effects of exogenous gamma-aminobutiric acid on tomato seedling under chilling stress. Physiol. Mol. Biol. Plants 2014, 20, 133–137. [Google Scholar] [CrossRef]
- Ding, F.; Liu, B.; Zhang, S.X. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Vannozzi, A.; Palumbo, F.; Barcaccia, G. Exogenous EBR ameliorates endogenous hormone contents in tomato species under low-temperature stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
- Hightower, R.; Baden, C.; Penzes, E.; Lund, P.; Dunsmuir, P. Expression of antifreeze proteins in transgenic plants. Plant Mol. Biol. 1991, 17, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Li, B.; Zhang, J.T.; Li, Y.Z.; Zhang, H.X. Generation of selectable marker-free transgenic tomato resistant to drought, cold and oxidative stress using the Cre/loxP DNA excision system. Transgenic Res. 2009, 18, 607–619. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Geetha, M.; Subramanyam, K.; Girija, S. Ectopic expression of Arabidopsis RCI2A gene contributes to cold tolerance in tomato. Transgenic Res. 2015, 24, 237–251. [Google Scholar] [CrossRef]
- Liang, G.P.; He, H.H.; Nai, G.J.; Feng, L.D.; Li, Y.M.; Zhou, Q.; Ma, Z.H.; Yue, Y.; Chen, B.H.; Mao, J. Genome-wide identification of BAM genes in grapevine (Vitis vinifera L.) and ectopic expression of VvBAM1 modulating soluble sugar levels to improve low-temperature tolerance in tomato. BMC Plant Biol. 2021, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, C.E.; Tanksley, S.D. Segregation of isozyme markers and cold tolerance in an interspecific backcross of tomato. Theor. Appl. Genet. 1983, 66, 241–247. [Google Scholar] [CrossRef]
- Truco, M.J.; Randall, L.B.; Bloom, A.J.; Clair, D.A.S. Detection of QTLs associated with shoot wilting and root ammonium uptake under chilling temperatures in an interspecific backcross population from Lycopersicon esculentum × L. hirsutum. Theor. Appl. Genet. 2000, 101, 1082–1092. [Google Scholar] [CrossRef]
- Ma, X.C.; Chen, C.; Yang, M.M.; Dong, X.C.; Lv, W.; Meng, Q.W. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F.; Gilmour, S.J.; Stockinger, E.J.; Jaglo-Ottosen, K.R.; Zarka, D.G.; Daniel, G.Z. Role of the Arabidopsis CBF transcriptional activators in cold acclimation. Physiol. Plant. 2001, 112, 171–175. [Google Scholar] [CrossRef]
- Weiss, J.; Egea-Cortines, M. Transcriptomic analysis of cold response in tomato fruits identifies dehydrin as a marker of cold stress. J. Appl. Genet. 2009, 50, 311–319. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | Primer Sequence 5′→3′ | |
|---|---|---|
| Solyc01g008370 | F | AATTGTTCACCCGCTTGTGC |
| R | CTCCTCCTCAAAGGGCACTG | |
| Solyc01g008390 | F | CGACAAAGGGACTGGAGCTT |
| R | GTACGTGACCTTCCAAGCGA | |
| Solyc01g008400 | F | GCTTCTGTGGCTGTGCAATC |
| R | GCACACGCACAAGCTGATAG | |
| Solyc01g008410 | F | TACCAGCATCACTGCACCAG |
| R | CATCGCCGCCACAATCTTTT | |
| Solyc01g008420 | F | CCCTGGTCAGCGTAACAACT |
| R | CAATGCAGATGACGCGGATG | |
| Solyc01g008440 | F | AAACATGCCTCTGGGAGTGG |
| R | CCCTTGCCTGTGACACATCT | |
| Solyc01g008450 | F | GCATACGACGATGCTGCTCA |
| R | TTTCTGCCCTCCAACCCTTG | |
| Solyc01g008460 | F | CCGGCTATGTTTCACGCTCT |
| R | GACATGTGGAACAGGCAAGC | |
| Solyc01g008470 | F | ACCCCTGTGGCAAACACTTC |
| R | GCTCGCCTTCATCCTTCTGA | |
| Solyc01g008477 | F | GGATGATCCGGATTCCGACG |
| R | ATGCTCTCTTCAGGGCTTGT | |
| Solyc01g150107 | F | CAACGATTTCGTGTTTTATTGC |
| R | TTGGCATTTTTGACGTCGG | |
| Solyc01g008479 | F | AGCTCCGAAGCGAGCA |
| R | GACGGACGTCCACGAAAAA | |
| Solyc01g150104 | F | GGGTGATCCGAATTTCGACG |
| R | CTCGCTTCGGGGCTCG | |
| Solyc01g008475 | F | GGGTGATCTGGATTCCGACG |
| R | ATGGGTCGGACATAACCGTG | |
| Solyc01g008480 | F | GCAAAGCTGGGGAAACACAG |
| R | CCCAGCCATCTTCAACGTCT | |
| Solyc04g077020 [33] | F | TGACGAAGTCAGGACAGGAA |
| (Tubulin) | R | CTGCATCTTCTTTGCCACTG |
| Tomato Accessions | Chilling Injury Index | Cold Resistance Type |
|---|---|---|
| ‘NL-7’ | 0.43 | Cold Sensitive |
| ‘NL-15’ | 0.28 | Cold Tolerance |
| ‘NL-18’ | 0.17 | Cold Tolerance |
| ‘NL-21’ | 0.13 | Cold Tolerance |
| ‘NL-37’ | 0.60 | Cold Sensitive |
| ‘NL-67’ | 0.57 | Cold Sensitive |
| Gene ID | Chromosomal Location | Protein Length (aa) | Molecular Weight (Da) | Theoretical Isoelectric Point | Grand Average of Hydropathicity |
|---|---|---|---|---|---|
| Solyc01g008370 | chr1:2424882-2430739 | 337 | 38560.23 | 5.74 | −0.126 |
| Solyc01g008390 | chr1:2434836-2437364 | 595 | 65833.15 | 5.33 | −0.047 |
| Solyc01g008400 | chr1:2433343-2440084 | 64 | 7491.06 | 7.71 | 0.595 |
| Solyc01g008410 | chr1:2440518-2442943 | 720 | 80748.07 | 5.51 | −0.090 |
| Solyc01g008420 | chr1:2452193-2459198 | 524 | 56135.78 | 6.96 | 0.609 |
| Solyc01g008440 | chr1:2469910-2476591 | 533 | 59987.63 | 6.55 | −0.491 |
| Solyc01g008450 | chr1:2484922-2485756 | 277 | 32020.33 | 5.04 | −0.796 |
| Solyc01g008460 | chr1:2489427-2494425 | 207 | 23197.49 | 5.26 | −0.236 |
| Solyc01g008470 | chr1:2495684-2501832 | 359 | 41177.26 | 6.57 | −0.205 |
| Solyc01g008475 | chr1:2506199-2506703 | 107 | 12454.45 | 9.62 | −0.337 |
| Solyc01g008477 | chr1:2510337-2510679 | 113 | 13012.11 | 8.91 | 0.004 |
| Solyc01g008479 | chr1:2512135-2512579 | 147 | 16568.21 | 9.61 | −0.161 |
| Solyc01g008480 | chr1:2515370-2522690 | 591 | 67332.11 | 8.88 | −0.180 |
| Solyc01g150104 | chr1:2504047-2504398 | 116 | 13371.65 | 9.98 | −0.067 |
| Solyc01g150107 | chr1:2510879-2511607 | 140 | 16269.22 | 10.30 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-Q.; Tao, J.-P.; Song, L.-X.; Zhang, R.-R.; Liu, H.; Zhao, T.-M.; Zhu, W.-M.; Xiong, A.-S. Identification of Key Regulatory Factors of Molecular Marker TGS377 on Chromosome 1 and Its Response to Cold Stress in Tomato. Agronomy 2022, 12, 2985. https://doi.org/10.3390/agronomy12122985
Zhang J-Q, Tao J-P, Song L-X, Zhang R-R, Liu H, Zhao T-M, Zhu W-M, Xiong A-S. Identification of Key Regulatory Factors of Molecular Marker TGS377 on Chromosome 1 and Its Response to Cold Stress in Tomato. Agronomy. 2022; 12(12):2985. https://doi.org/10.3390/agronomy12122985
Chicago/Turabian StyleZhang, Jia-Qi, Jian-Ping Tao, Liu-Xia Song, Rong-Rong Zhang, Hui Liu, Tong-Min Zhao, Wei-Min Zhu, and Ai-Sheng Xiong. 2022. "Identification of Key Regulatory Factors of Molecular Marker TGS377 on Chromosome 1 and Its Response to Cold Stress in Tomato" Agronomy 12, no. 12: 2985. https://doi.org/10.3390/agronomy12122985
APA StyleZhang, J.-Q., Tao, J.-P., Song, L.-X., Zhang, R.-R., Liu, H., Zhao, T.-M., Zhu, W.-M., & Xiong, A.-S. (2022). Identification of Key Regulatory Factors of Molecular Marker TGS377 on Chromosome 1 and Its Response to Cold Stress in Tomato. Agronomy, 12(12), 2985. https://doi.org/10.3390/agronomy12122985









