Preliminary Studies on How to Reduce the Effects of Salinity
Abstract
:1. Introduction
2. Materials and Methods
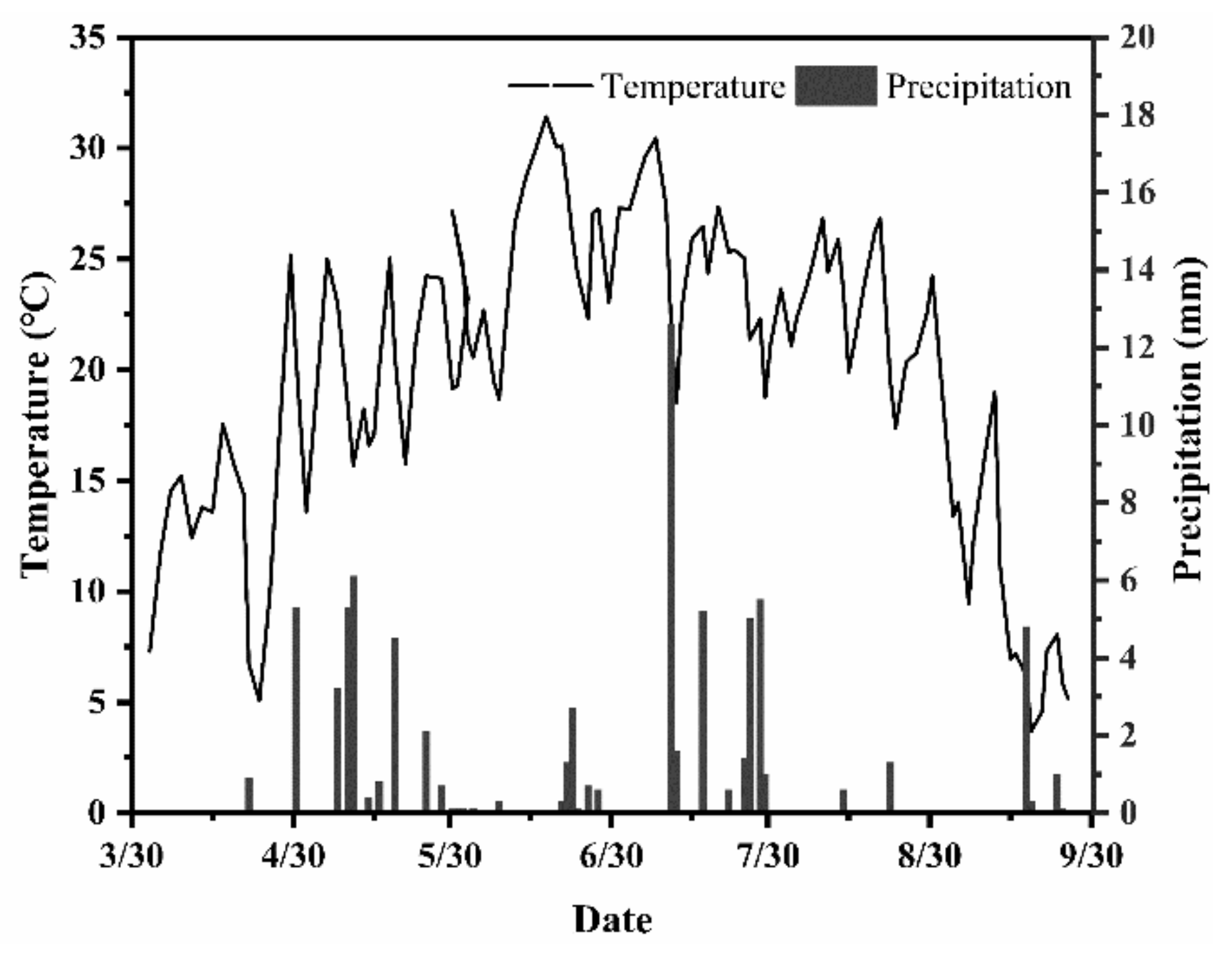
2.1. Experimental Site
2.2. Experimental Design
2.3. Sample Collection and Measurement
2.3.1. Soil Water Stable Macroaggregates Determination
2.3.2. Soil Moisture Determination
2.3.3. Soil Salinity Determination
2.3.4. Soil pH Value Determination
2.3.5. Determination of Soil Organic Matter Content
2.3.6. Determination of Carbon Content in Each Component of Soil Humus
2.3.7. Determination of Leaf Area Index (LAI), Stem Diameter and Plant Height in Cotton
2.3.8. Cotton Yield Index
2.4. Statistical Analysis
3. Results
3.1. Effects of Fulvic Acid on Soil Physicochemical Properties
3.1.1. Water-Stable Macroaggregates (>0.25 mm) (R0.25)
3.1.2. Soil Moisture Distribution
3.1.3. Distribution of Soil Salinity
3.1.4. Distribution of Soil pH
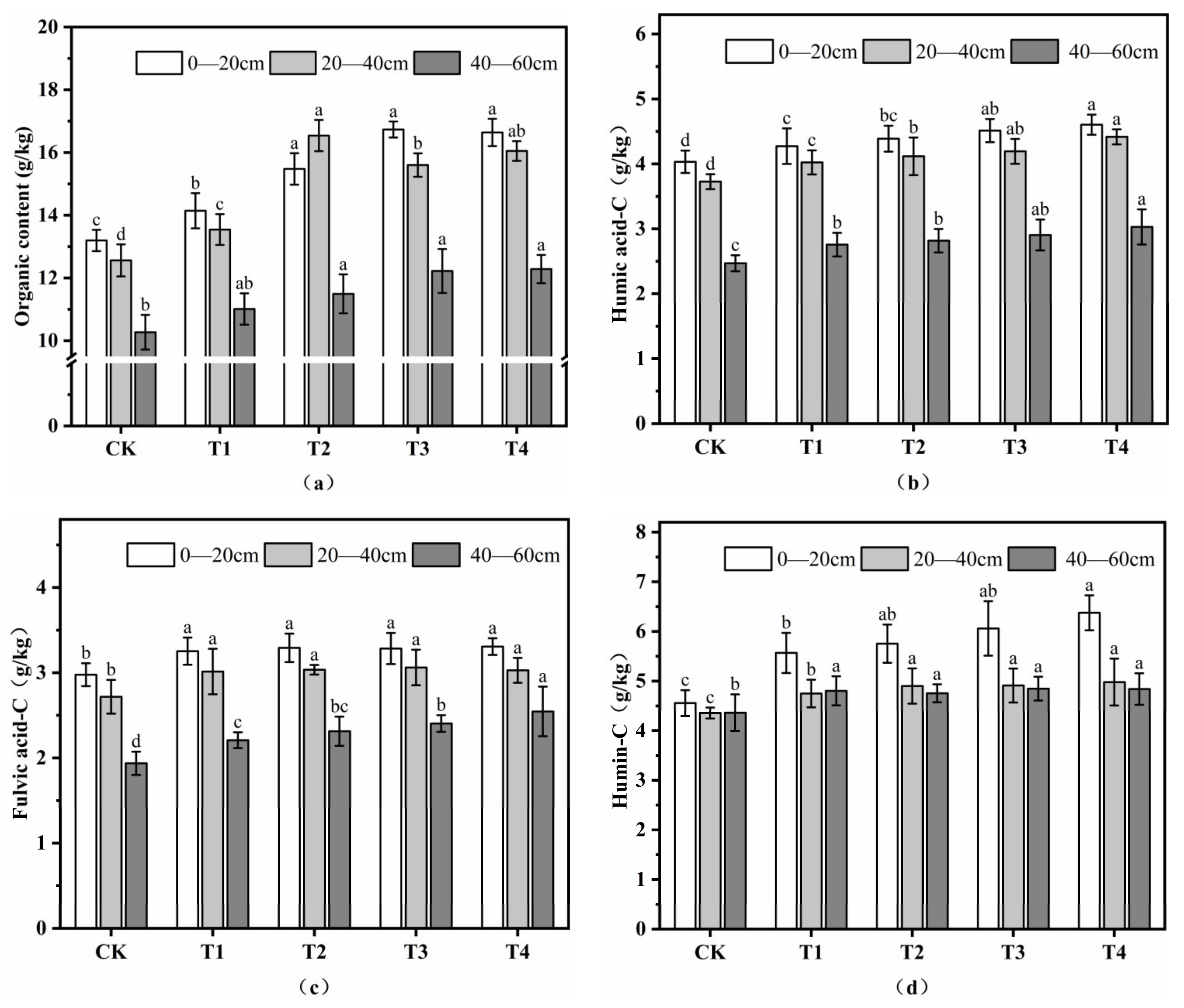
3.1.5. Soil Organic Matter
3.2. Effects of Fulvic Acid on Soil Content of Humus Component
3.2.1. Content of Humic Acid (CHA)
3.2.2. Content of Fulvic Acid (CFA)
3.2.3. Content of Humin (CHM)
3.2.4. Changes in Soil Humus Composition
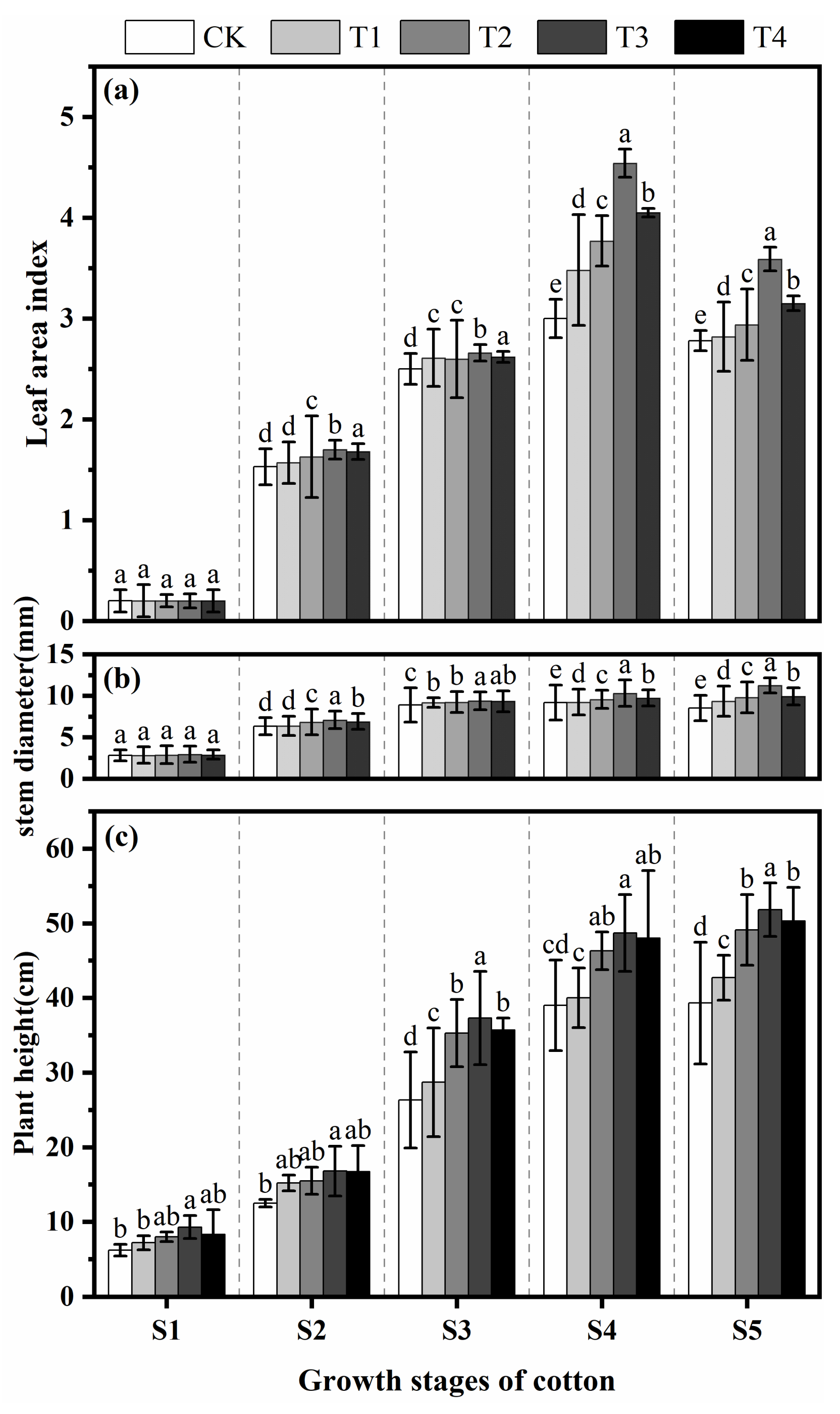
3.3. Effects of Fulvic Acid on Growth
3.3.1. Leaf Area Index (LAI), Stem Diameter and Plant Height
3.3.2. Cotton Yield
3.3.3. Correlation of Cotton Yield with Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Lu, P.N.; Bainard, L.D.; Ma, B.; Liu, J.H. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline-alkaline environment. Sci. Rep. 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Mao, W.; Kang, S.; Wan, Y.; Sun, Y.; Li, X.; Wang, Y. Yellow river sediment as a soil amendment for amelioration of saline land in the yellow river delta. Land Degrad. Dev. 2016, 27, 1595–1602. [Google Scholar] [CrossRef]
- Yang, G.; Li, F.; Tian, L.; He, X.; Gao, Y.; Wang, Z.; Ren, F. Soil physicochemical properties and cotton (Gossypium hirsutum L.) yield under brackish water mulched drip irrigation. Soil Tillage Res. 2020, 199, 104592. [Google Scholar] [CrossRef]
- Wang, R.; Kang, Y.; Wan, S.; Hu, W.; Liu, S.; Jiang, S.; Liu, S. Influence of different amounts of irrigation water on salt leaching and cotton growth under drip irrigation in an arid and saline area. Agric. Water Manag. 2012, 110, 109–117. [Google Scholar] [CrossRef]
- Han, Z.; Hu, Y.; Tian, Q.; Cao, Y.; Si, A.; Si, Z.; Zang, Y.; Xu, C.; Shen, W.; Dai, F. Genomic signatures and candidate genes of lint yield and fibre quality improvement in Upland cotton in Xinjiang. Plant Biotechnol. J. 2020, 18, 2002–2014. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.S.; Peng, J.; Biswas, A.; Hu, J.; Zhao, R.Y.; He, K.; Shi, Z. Characterising dryland salinity in three dimensions. Sci. Total Environ. 2019, 682, 190–199. [Google Scholar] [CrossRef]
- Hai-Bin, G.U.; Sheng, J.D.; Hong-Qi, W.U.; Zhang, L.; Wang, Z. Survey and evaluation on soil salinization of irrigation area scale—A case study of irrigation area in Shihhotze and Manas. J. Xinjiang Agric. Univ. 2010, 33, 95–100. [Google Scholar]
- Wang, R.; Wan, S.; Sun, J.; Xiao, H. Soil salinity, sodicity and cotton yield parameters under different drip irrigation regimes during saline wasteland reclamation. Agric. Water Manag. 2018, 209, 20–31. [Google Scholar] [CrossRef]
- Qadir, M.; Noble, A.; Schubert, S.; Thomas, R.J.; Arslan, A. Sodicity-induced land degradation and its sustainable management: Problems and prospects. Land Degrad. Dev. 2006, 17, 661–676. [Google Scholar] [CrossRef]
- Rashad, M.; Hafez, M.; Popov, A.I.; Gaber, H. Toward sustainable agriculture using extracts of natural materials for transferring organic wastes to environmental-friendly ameliorants in Egypt. Int. J. Environ. Sci. Technol. 2022, 1–16. [Google Scholar] [CrossRef]
- Rashad, M.; Hafez, M.; Popov, A.I. Humic substances composition and properties as an environmentally sustainable system: A review and way forward to soil conservation. J. Plant Nutr. 2022, 45, 1072–1122. [Google Scholar] [CrossRef]
- Bai, Y.; Xue, W.; Yan, Y.; Zuo, W.; Shan, Y.; Feng, K. The challenge of improving coastal mudflat soil: Formation and stability of organo-mineral complexes. Land Degrad. Dev. 2018, 29, 1074–1080. [Google Scholar] [CrossRef]
- Cai, W.K.; Liu, J.H.; Zhou, C.H.; Keeling, J.; Glasmacher, U.A. Structure, genesis and resources efficiency of dolomite: New insights and remaining enigmas. Chem. Geol. 2021, 573, 120191. [Google Scholar] [CrossRef]
- Małek, S.; Ważny, R.; Błońska, E.; Jasik, M.; Lasota, J. Soil fungal diversity and biological activity as indicators of fertilization strategies in a forest ecosystem after spruce disintegration in the Karpaty Mountains. Sci. Total Environ. 2021, 751, 142335. [Google Scholar] [CrossRef]
- Bandiera, M.; Mosca, G.; Vamerali, T. Humic acids affect root characteristics of fodder radish (Raphanus sativus L. var. oleiformis Pers.) in metal-polluted wastes. Desalination 2009, 246, 78–91. [Google Scholar] [CrossRef]
- Daur, I.; Bakhashwain, A.A. Effect of humic acid on growth and quality of maize fodder production. Pak. J. Bot. 2013, 45, 21–25. [Google Scholar]
- Saruhan, V.; Kuvuran, A.; Babat, S. The effect of different humic acid fertilization on yield and yield components performances of common millet (Panicum miliaceum L.). Sci. Res. Essays 2011, 6, 663–669. [Google Scholar]
- Hafez, M.; Abo El-Ezz, S.F.; Popov, A.I.; Rashad, M. Organic Amendments Combined with Plant Growth-Promoting Rhizobacteria (Azospirillum brasilense) as an Eco-Friendly By-Product to Remediate and Enhance the Fertility of Saline Sodic-Soils in Egypt. Commun. Soil Sci. Plant Anal. 2021, 52, 1416–1433. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, C.; Xie, Z.; Li, Y.; Zhang, X.; Wang, G.; Jin, J.; Ding, G.; Liu, X. Humic substances and distribution in Mollisols affected by six-year organic amendments. Agron. J. 2020, 112, 4723–4740. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Basis of a humeomics science: Chemical fractionation and molecular characterization of humic biosuprastructures. Biomacromolecules 2011, 12, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.E. Organic matter, humus, humate, humic acid, fulvic acid and humin: Their importance in soil fertility and plant health. CTI Res. 2008, 10, 1–17. [Google Scholar]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.R.; Naseri, L.A. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Khaleda, L.; Park, H.J.; Yun, D.J.; Jeon, J.R.; Kim, W.Y. Humic acid confers high-affinity K+ transporter 1-mediated salinity stress tolerance in Arabidopsis. Mol. Cells 2017, 40, 966. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.A.; Wright, S.F. Carbon and nitrogen in operationally defined soil organic matter pools. Biol. Fertil. Soils 2006, 43, 215–220. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z.; Tang, C.; Chu, G. Influences of the combination using of silicon, selenium, fulvic acid, nitrapyrin on cotton growth and cotton plant salt-resistant physiological characteristics. Ecol. Environ. Sci. 2016, 25, 1671–1677. [Google Scholar]
- Ferrari, E.; Francioso, O.; Nardi, S.; Saladini, M.; Ferro, N.D.; Morari, F. DRIFT and HR MAS NMR characterization of humic substances from a soil treated with different organic and mineral fertilizers. J. Mol. Struct. 2011, 998, 216–224. [Google Scholar] [CrossRef]
- Siwik, A.; Pensini, E.; Rodriguez, B.M.; Marangoni, A.G.; Collier, C.M.; Sleep, B. Effect of rheology and humic acids on the transport of environmental fluids: Potential implications for soil remediation revealed through microfluidics. J. Appl. Polym. Sci. 2020, 137, 48465. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distributions; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Bao, S.; Qin, H.; Lao, J. Soil Agricultural Chemistry Analysis; China Agriculture Press: Beijing China, 2000. [Google Scholar]
- Kumada, K.; Sato, O.; Ohsumi, Y.; Ohta, S. Humus composition of mountain soils in central Japan with special reference to the distribution of P-type humic acid. Soil Sci. Plant Nutr. 1967, 13, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Dou, S.; Zhang, J.; Ping, L.; Guan, S.; Li, K. Effects of different oxygen concentrations on formation of humic substances during corn stalk decomposition. J. Jilin Agric. Univ. 2005, 27, 6. [Google Scholar]
- Dou, S.; Xiao, Y.; Zhang, J. Quantities and structural characteristics of various fractions of soil humin. Acta Pedol. Sin. 2006, 43, 934–940. [Google Scholar]
- Li, X.X.; Liu, H.G.; He, X.L.; Gong, P.; Lin, E. Water-Nitrogen Coupling and Multi-Objective Optimization of Cotton under Mulched Drip Irrigation in Arid Northwest China. Agronomy 2019, 9, 894. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, Z.; Zhang, J.; Cao, W. Effects of different salt stress on physiological growth and yield of drip irrigation cotton (Gossypium hirsutum L.). Intell. Autom. Soft Comput. 2020, 26, 949–959. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Yuan, Q.; Lu, Y.; Chidthaisong, A. Stable carbon isotope fractionation, carbon flux partitioning and priming effects in anoxic soils during methanogenic degradation of straw and soil organic matter. Soil Biol. Biochem. 2012, 49, 193–199. [Google Scholar] [CrossRef]
- Zheng, M.; Liang, X.; Han, Z.; Kang, J.; Chen, Y. Effects of Different Measures on Soil Salinity Leaching Characteristics in Saline-alkali Soil. J. Shanxi Agric. Sci. 2021, 49, 318–323. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yao, R. Synergistic effects of fertilizer reduction and fulvic acid application on decreasing NaCl content and N, P availability of salinized soil. J. Plant Nutr. Fertil. 2021, 27, 1339–1350. [Google Scholar] [CrossRef]
- Hui, Z.; Li, C.; Shi, W.; Zhang, J.; Wang, D. A study on the use of fulvic acid to improve growth and resistance in continuous cropping of potato. Acta Pratacult. Sin. 2013, 22, 7. [Google Scholar]
- Liu, J.; Jing, F.; Li, T.; Huang, J.; Tan, J.; Cao, J.; Liu, J. Effects of returning stalks into field on soil humus composition of continuous cropping cotton field. Sci. Agric. Sin. 2015, 48, 293–302. [Google Scholar] [CrossRef]
- Lv, Y.; Li, B. Experimental Course of Soil Science; China Agriculture Press: Beijing, China, 2010; Available online: http://www.tushu007.com/download/ISBN-9787109151253.html (accessed on 4 October 2022).
- Suh, H.Y.; Yoo, K.S.; Suh, S.G. Tuber growth and quality of potato (Solanum tuberosum L.) as affected by foliar or soil application of fulvic and humic acids. Hortic. Environ. Biotechnol. 2014, 55, 183–189. [Google Scholar] [CrossRef]
- Zhao, X.M.; He, L.; Zhang, Z.D.; Wang, H.B.; Zhao, L.P. Simulation of accumulation and mineralization (CO2 release) of organic carbon in chernozem under different straw return ways after corn harvesting. Soil Tillage Res. 2016, 156, 148–154. [Google Scholar] [CrossRef]
- Bai, L.; Li, Q.; Deng, Y.; Huang, Z.; Xie, L.; Ruan, W. Humification process of biogas residue combined with food waste and cattle manure co-composting. Trans. Chin. Soc. Agric. Mach. 2019, 50, 8. [Google Scholar]
- Zhang, X.; Li, B.; Liu, G.; Sun, J.; Lu, X.; Wang, X. Effect of composite soil improvement agents on soil amendment and salt reduction in coastal saline soil. Chin. J. Eco-Agric. 2019, 27, 11. [Google Scholar]
- Liang, A.; Zhang, Y.; Zhang, X.; Yang, X.; McLaughlin, N.; Chen, X.; Guo, Y.; Jia, S.; Zhang, S.; Wang, L. Investigations of relationships among aggregate pore structure, microbial biomass, and soil organic carbon in a Mollisol using combined non-destructive measurements and phospholipid fatty acid analysis. Soil Tillage Res. 2019, 185, 94–101. [Google Scholar] [CrossRef]
- Miao, S.; Qiao, Y.; Li, P.; Han, X.; Tang, C. Fallow associated with autumn-plough favors structure stability and storage of soil organic carbon compared to continuous maize cropping in Mollisols. Plant Soil 2017, 416, 27–38. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Wang, Q.; Qu, Z.; Wang, C.; Zhang, X. Effect of biochemical fulvic acid on water and salt transport characteristics in saline-alkali soil. Trans. Chin. Soc. Agric. Mach. 2022, 53, 9. [Google Scholar]
- Yu, J.; Zhu, C.; Guo, P.; Zhao, Y. Effect of Bio-active Humic Substance on the Biomass of Glycyrrhiza uralensis. Soil Humus Compos. Enzym. Act. 2011, 19, 68–74. [Google Scholar]
- Navarrete, I.A.; Tsutsuki, K.; Navarrete, R.A. Humus composition and the structural characteristics of humic substances in soils under different land uses in Leyte, Philippines. Soil Sci. Plant Nutr. 2010, 56, 289–296. [Google Scholar] [CrossRef]
- Anderson, D.W.; Paul, E. Organo-mineral complexes and their study by radiocarbon dating. Soil Sci. Soc. Am. J. 1984, 48, 298–301. [Google Scholar] [CrossRef] [Green Version]
- Senesi, N.; Plaza, C.; Brunetti, G.; Polo, A. A comparative survey of recent results on humic-like fractions in organic amendments and effects on native soil humic substances. Soil Biol. Biochem. 2007, 39, 1244–1262. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Zandonadi, D.B.; Santos, M.P.; Busato, J.G.; Peres, L.; Façanha, A. Plant physiology as affected by humified organic matter. Theor. Exp. Plant Physiol. 2013, 25, 13–25. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Zhang, S.; Yao, R.; Xie, W. Effects of different amendments application on cotton growth and soil properties in arid areas. Ecol. Environ. Sci. 2020, 29, 757–762. [Google Scholar] [CrossRef]
- Kumar, S.M.; Zeng, X.; Su, S.; Wang, Y.; Bai, L.; Zhang, Y.; Li, T.; Zhang, X. The effect of fulvic acids derived from different materials on changing properties of albic black soil in the northeast plain of China. Molecules 2019, 24, 1535. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jin, Q.; Lu, G.; Wang, X. Effect of the soil modifier of biochemical fulvic acid on saline land. J. Anhui Agri 2010, 38, 1931–1932. [Google Scholar] [CrossRef]
- Pettigrew, W.T. Physiological consequences of moisture deficit stress in cotton. Crop Sci. 2004, 44, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Yang, G.; Li, W.; He, X.; Gao, Y.; Tian, L.; Li, F.; Wang, Z.; Liu, S. Yield-compatible salinity level for growing cotton (Gossypium hirsutum L.) under mulched drip irrigation using saline water. Agric. Water Manag. 2021, 250, 106859. [Google Scholar] [CrossRef]



| Items | Values |
|---|---|
| Soil texture | Sandy loam |
| Soil holding capacity/(%) | 20.6 |
| Initial soil electrical conductivity/(μS·cm−1) | 2245.31 |
| Initial soil salt content/(g·kg−1) | 8.56 |
| Initial soil volumetric water content/(m3·m−3) | 17.83 |
| Soil organic matter/(g·kg−1) | 13.75 |
| Humic acid/(g·kg−1) | 3.77 |
| Fulvic acid/(g·kg−1) | 2.55 |
| Humin/(g·kg−1) | 3.97 |
| Total nitrogen/(g·kg−1) | 0.78 |
| Alkaline hydrolysis nitrogen/(mg·kg−1) | 51.38 |
| Available P/(mg·kg−1) | 48.12 |
| Available K/(mg·kg−1) | 551.29 |
| pH | 8.55 |
| Cation exchange capacity/(cmol·kg−1) | 5.08 |
| Growth Period | Dates (2021) | Irrigation | Fertilization | |||
|---|---|---|---|---|---|---|
| Irrigation Quota/(m3·ha−1) | Irrigation Times | CO(NH2)2/(kg·ha−1) | KH2PO4/(kg·ha−1) | Fertilization Times | ||
| Seeding | 04.20–04.30 | 350 | 1 | — | — | — |
| Seeding stage | 05.01–06.15 | 600 | 2 | 30 | 15 | 1 |
| Bud stage | 06.16–07.03 | 730 | 2 | 90 | 40 | 2 |
| Blossoming and boll stage | 07.04–08.18 | 2070 | 5 | 450 | 230 | 5 |
| Boll opening stage | 08.19–10.16 | 2500 | 1 | 30 | 15 | 1 |
| Whole growth period | 167 days | 4000 | 11 | 600 | 300 | 9 |
| Table | Soil Depth/(cm) | |||||
|---|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | |
| T1 | 4.10 ± 0.42 d | 3.04 ± 0.38 d | 4.19 ± 0.33 d | 4.75 ± 0.39 b | 9.72 ± 0.31 a | 0.37 ± 0.28 d |
| T2 | 11.41 ± 0.40 b | 8.46 ± 0.46 b | 8.74 ± 0.40 c | 5.15 ± 0.30 b | 7.75 ± 0.67 c | 8.53 ± 0.48 a |
| T3 | 13.65 ± 0.34 a | 9.85 ± 0.37 a | 13.19 ± 0.33 a | 8.13 ± 0.53 a | 8.74 ± 0.23 b | 1.82 ± 0.30 c |
| T4 | 9.63 ± 0.53 c | 5.04 ± 0.36 c | 12.18 ± 0.39 b | 3.23 ± 0.22 c | 6.85 ± 0.29 d | 5.58 ± 0.46 b |
| Treatment | Seed Cotton Yield per Boll/(g) | Number of Bolls per Plant | Seed Cotton Yield/(g/plant) | Relative Production (%) |
|---|---|---|---|---|
| CK | 4.83 ± 0.24 c | 5.22 ± 0.14 c | 25.25 ± 1.16 d | 100 |
| T1 | 4.88 ± 0.11 c | 5.36 ± 0.26 b | 26.17 ± 2.35 c | 103.64 |
| T2 | 4.91 ± 0.22 bc | 5.44 ± 0.31 a | 26.71 ± 2.06 b | 105.78 |
| T3 | 4.98 ± 0.16 a | 5.47 ± 0.22 a | 27.26 ± 1.21 a | 107.96 |
| T4 | 4.93 ± 0.36 ab | 5.44 ± 0.13 ab | 26.82 ± 3.44 ab | 106.22 |
| Index | Seed Cotton Yield | Aggregates | Moisture Content | Salt Content | pH | Organic Matter |
|---|---|---|---|---|---|---|
| Aggregate | 0.92 * | |||||
| Moisture content | 0.92 * | 0.98 ** | ||||
| Salt content | −0.93 * | −0.97 ** | −0.99 ** | |||
| pH | −0.89 * | −0.93 * | −0.99 ** | 0.97 ** | ||
| Organic matter | 0.97 ** | 0.92 * | 0.91 * | −0.95 * | −0.87 | |
| Humus composition | 0.96 ** | 0.81 | 0.77 | −0.79 | −0.73 | 0.90 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, H.; Gong, P.; Li, P.; Tian, R.; Zhang, Y.; Xu, Y.; Xue, B. Preliminary Studies on How to Reduce the Effects of Salinity. Agronomy 2022, 12, 3006. https://doi.org/10.3390/agronomy12123006
Guo Y, Liu H, Gong P, Li P, Tian R, Zhang Y, Xu Y, Xue B. Preliminary Studies on How to Reduce the Effects of Salinity. Agronomy. 2022; 12(12):3006. https://doi.org/10.3390/agronomy12123006
Chicago/Turabian StyleGuo, Yaru, Hongguang Liu, Ping Gong, Pengfei Li, Rumeng Tian, Yao Zhang, Yibin Xu, and Bao Xue. 2022. "Preliminary Studies on How to Reduce the Effects of Salinity" Agronomy 12, no. 12: 3006. https://doi.org/10.3390/agronomy12123006
APA StyleGuo, Y., Liu, H., Gong, P., Li, P., Tian, R., Zhang, Y., Xu, Y., & Xue, B. (2022). Preliminary Studies on How to Reduce the Effects of Salinity. Agronomy, 12(12), 3006. https://doi.org/10.3390/agronomy12123006






